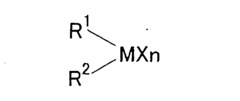
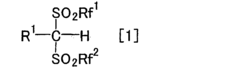How to Advance Lewis Acid Catalysts for Selective Synthesis?
AUG 26, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lewis Acid Catalysis Background and Objectives
Lewis acid catalysis represents one of the most fundamental and versatile tools in synthetic organic chemistry, with roots dating back to the early 20th century. The evolution of Lewis acid catalysts has been marked by significant milestones, from the classical aluminum-based systems to modern transition metal complexes and designer catalysts with tailored properties. This technological progression has consistently expanded the synthetic chemist's toolbox, enabling increasingly complex transformations with enhanced selectivity.
The field has witnessed remarkable growth particularly since the 1980s, with the development of chiral Lewis acid catalysts that revolutionized asymmetric synthesis. This advancement transformed industrial processes by enabling the production of enantiomerically pure compounds crucial for pharmaceutical and agrochemical applications. The subsequent decades saw further refinement with the introduction of confined Lewis acids, bifunctional systems, and supported catalysts that addressed limitations in traditional homogeneous catalysis.
Current trends in Lewis acid catalysis focus on sustainability, atom economy, and process intensification. The shift toward earth-abundant metals as catalytic centers represents a significant direction, moving away from precious metal dependence. Simultaneously, the integration of Lewis acid catalysis with other activation modes, such as photoredox catalysis and electrochemistry, is opening unprecedented reaction pathways and selectivity patterns.
The technological objective for advancing Lewis acid catalysts centers on developing highly selective systems capable of distinguishing between subtle structural differences in substrates. This includes chemo-, regio-, stereo-, and enantioselectivity under mild conditions with minimal catalyst loading. The ideal next-generation Lewis acid catalyst would operate efficiently in environmentally benign solvents or solvent-free conditions, with high turnover numbers and broad functional group tolerance.
Another critical goal involves creating modular catalyst platforms that can be rapidly optimized for specific transformations through predictable structural modifications. This approach would significantly accelerate reaction development timelines and enable more efficient discovery of novel transformations. The development of computational tools for catalyst design and reaction prediction represents a complementary objective, potentially revolutionizing how new Lewis acid catalysts are conceived and implemented.
The ultimate technological vision encompasses the creation of programmable Lewis acid catalysts capable of performing sequential, multi-step transformations in one-pot processes, mimicking the efficiency of enzymatic systems while maintaining the robustness and scalability required for industrial applications. This would dramatically reduce waste generation, energy consumption, and process complexity in chemical manufacturing.
The field has witnessed remarkable growth particularly since the 1980s, with the development of chiral Lewis acid catalysts that revolutionized asymmetric synthesis. This advancement transformed industrial processes by enabling the production of enantiomerically pure compounds crucial for pharmaceutical and agrochemical applications. The subsequent decades saw further refinement with the introduction of confined Lewis acids, bifunctional systems, and supported catalysts that addressed limitations in traditional homogeneous catalysis.
Current trends in Lewis acid catalysis focus on sustainability, atom economy, and process intensification. The shift toward earth-abundant metals as catalytic centers represents a significant direction, moving away from precious metal dependence. Simultaneously, the integration of Lewis acid catalysis with other activation modes, such as photoredox catalysis and electrochemistry, is opening unprecedented reaction pathways and selectivity patterns.
The technological objective for advancing Lewis acid catalysts centers on developing highly selective systems capable of distinguishing between subtle structural differences in substrates. This includes chemo-, regio-, stereo-, and enantioselectivity under mild conditions with minimal catalyst loading. The ideal next-generation Lewis acid catalyst would operate efficiently in environmentally benign solvents or solvent-free conditions, with high turnover numbers and broad functional group tolerance.
Another critical goal involves creating modular catalyst platforms that can be rapidly optimized for specific transformations through predictable structural modifications. This approach would significantly accelerate reaction development timelines and enable more efficient discovery of novel transformations. The development of computational tools for catalyst design and reaction prediction represents a complementary objective, potentially revolutionizing how new Lewis acid catalysts are conceived and implemented.
The ultimate technological vision encompasses the creation of programmable Lewis acid catalysts capable of performing sequential, multi-step transformations in one-pot processes, mimicking the efficiency of enzymatic systems while maintaining the robustness and scalability required for industrial applications. This would dramatically reduce waste generation, energy consumption, and process complexity in chemical manufacturing.
Market Analysis for Selective Synthesis Applications
The global market for selective synthesis applications utilizing Lewis acid catalysts has experienced significant growth over the past decade, driven primarily by increasing demand for fine chemicals, pharmaceuticals, and specialty materials. Current market valuations indicate that the selective synthesis segment represents approximately 18% of the total catalysis market, with Lewis acid catalysts accounting for a substantial portion of this share.
Pharmaceutical manufacturing remains the dominant application sector, where Lewis acid catalysts enable critical transformations in the synthesis of complex active pharmaceutical ingredients (APIs). This sector's demand is projected to grow steadily due to the increasing complexity of drug molecules and the industry's shift toward more sustainable manufacturing processes. The fine chemicals sector follows closely, particularly in fragrance, flavor, and agrochemical production.
Regional analysis reveals that North America and Europe currently lead in market consumption of advanced Lewis acid catalysts, primarily due to their established pharmaceutical and specialty chemical industries. However, Asia-Pacific represents the fastest-growing market, with China and India rapidly expanding their fine chemical and pharmaceutical manufacturing capabilities.
Consumer trends are increasingly favoring environmentally sustainable products, creating market pressure for greener catalytic processes. This has spurred interest in heterogeneous Lewis acid catalysts that offer easier separation and recycling capabilities, reducing waste and environmental impact. Companies that can develop catalysts with improved selectivity while maintaining environmental credentials are gaining competitive advantages.
Economic factors significantly influence market dynamics, with raw material costs for catalyst production—particularly for rare earth and precious metal-based Lewis acids—creating price volatility. This has accelerated research into more abundant metal alternatives and supported catalysts that maintain performance while reducing metal loading.
Regulatory landscapes across major markets are increasingly stringent regarding chemical manufacturing processes, with particular focus on reducing toxic waste and improving atom economy. This regulatory pressure has created market opportunities for Lewis acid catalysts that enable reactions under milder conditions with fewer byproducts.
Market forecasts suggest a compound annual growth rate of 6.3% for selective synthesis applications using Lewis acid catalysts through 2028, with particularly strong growth in asymmetric synthesis applications where high enantioselectivity commands premium pricing. The development of novel, highly selective Lewis acid catalysts capable of performing previously challenging transformations represents a high-value market opportunity with significant growth potential.
Pharmaceutical manufacturing remains the dominant application sector, where Lewis acid catalysts enable critical transformations in the synthesis of complex active pharmaceutical ingredients (APIs). This sector's demand is projected to grow steadily due to the increasing complexity of drug molecules and the industry's shift toward more sustainable manufacturing processes. The fine chemicals sector follows closely, particularly in fragrance, flavor, and agrochemical production.
Regional analysis reveals that North America and Europe currently lead in market consumption of advanced Lewis acid catalysts, primarily due to their established pharmaceutical and specialty chemical industries. However, Asia-Pacific represents the fastest-growing market, with China and India rapidly expanding their fine chemical and pharmaceutical manufacturing capabilities.
Consumer trends are increasingly favoring environmentally sustainable products, creating market pressure for greener catalytic processes. This has spurred interest in heterogeneous Lewis acid catalysts that offer easier separation and recycling capabilities, reducing waste and environmental impact. Companies that can develop catalysts with improved selectivity while maintaining environmental credentials are gaining competitive advantages.
Economic factors significantly influence market dynamics, with raw material costs for catalyst production—particularly for rare earth and precious metal-based Lewis acids—creating price volatility. This has accelerated research into more abundant metal alternatives and supported catalysts that maintain performance while reducing metal loading.
Regulatory landscapes across major markets are increasingly stringent regarding chemical manufacturing processes, with particular focus on reducing toxic waste and improving atom economy. This regulatory pressure has created market opportunities for Lewis acid catalysts that enable reactions under milder conditions with fewer byproducts.
Market forecasts suggest a compound annual growth rate of 6.3% for selective synthesis applications using Lewis acid catalysts through 2028, with particularly strong growth in asymmetric synthesis applications where high enantioselectivity commands premium pricing. The development of novel, highly selective Lewis acid catalysts capable of performing previously challenging transformations represents a high-value market opportunity with significant growth potential.
Current Challenges in Lewis Acid Catalyst Development
Despite significant advancements in Lewis acid catalysis over recent decades, several critical challenges continue to impede further development in this field. One of the most persistent issues is catalyst selectivity, particularly in complex molecular environments. Current Lewis acid catalysts often struggle to discriminate between similar functional groups, leading to unwanted side reactions and diminished yields. This challenge becomes especially pronounced when working with multifunctional substrates common in pharmaceutical and fine chemical synthesis.
Stability presents another major hurdle, as many potent Lewis acid catalysts exhibit sensitivity to moisture and air, necessitating stringent reaction conditions that limit practical applications. The degradation of catalysts during reactions not only reduces efficiency but also complicates purification processes and increases production costs. This instability often restricts industrial scalability of otherwise promising laboratory-scale reactions.
Heterogenization of homogeneous Lewis acid catalysts remains technically challenging. While homogeneous catalysts offer superior activity and selectivity, their recovery and reuse capabilities are limited. Conversely, heterogeneous systems, though more easily recoverable, frequently suffer from reduced activity and selectivity due to diffusion limitations and non-uniform active sites.
The rational design of Lewis acid catalysts is hampered by incomplete mechanistic understanding. Despite computational chemistry advances, predicting Lewis acid-substrate interactions in complex reaction environments remains difficult. This knowledge gap impedes the development of tailored catalysts for specific transformations and limits the ability to optimize reaction conditions systematically.
Environmental and sustainability concerns pose additional challenges. Traditional Lewis acids often involve toxic metals or require harsh reaction conditions, conflicting with green chemistry principles. The development of benign alternatives using earth-abundant metals or organocatalysts has progressed, but these systems typically demonstrate lower activity compared to their conventional counterparts.
Reproducibility issues further complicate catalyst development, as subtle variations in preparation methods can significantly alter catalytic performance. This variability makes standardization difficult and slows industrial adoption of new catalytic systems.
Finally, the integration of Lewis acid catalysis with other catalytic modalities, such as photocatalysis or enzymatic catalysis, presents both opportunities and challenges. While such hybrid approaches could unlock unprecedented reactivity patterns, compatibility issues between different catalytic systems often arise, requiring innovative solutions to harness their combined potential effectively.
Stability presents another major hurdle, as many potent Lewis acid catalysts exhibit sensitivity to moisture and air, necessitating stringent reaction conditions that limit practical applications. The degradation of catalysts during reactions not only reduces efficiency but also complicates purification processes and increases production costs. This instability often restricts industrial scalability of otherwise promising laboratory-scale reactions.
Heterogenization of homogeneous Lewis acid catalysts remains technically challenging. While homogeneous catalysts offer superior activity and selectivity, their recovery and reuse capabilities are limited. Conversely, heterogeneous systems, though more easily recoverable, frequently suffer from reduced activity and selectivity due to diffusion limitations and non-uniform active sites.
The rational design of Lewis acid catalysts is hampered by incomplete mechanistic understanding. Despite computational chemistry advances, predicting Lewis acid-substrate interactions in complex reaction environments remains difficult. This knowledge gap impedes the development of tailored catalysts for specific transformations and limits the ability to optimize reaction conditions systematically.
Environmental and sustainability concerns pose additional challenges. Traditional Lewis acids often involve toxic metals or require harsh reaction conditions, conflicting with green chemistry principles. The development of benign alternatives using earth-abundant metals or organocatalysts has progressed, but these systems typically demonstrate lower activity compared to their conventional counterparts.
Reproducibility issues further complicate catalyst development, as subtle variations in preparation methods can significantly alter catalytic performance. This variability makes standardization difficult and slows industrial adoption of new catalytic systems.
Finally, the integration of Lewis acid catalysis with other catalytic modalities, such as photocatalysis or enzymatic catalysis, presents both opportunities and challenges. While such hybrid approaches could unlock unprecedented reactivity patterns, compatibility issues between different catalytic systems often arise, requiring innovative solutions to harness their combined potential effectively.
State-of-the-Art Lewis Acid Catalyst Systems
01 Lewis acid catalysts in polymerization reactions
Lewis acid catalysts are used in various polymerization processes to control selectivity and reaction rates. These catalysts, including metallocene complexes and transition metal compounds, can influence the stereochemistry and molecular weight distribution of polymers. The selectivity can be tuned by modifying the catalyst structure, ligand environment, and reaction conditions, leading to polymers with specific properties and applications.- Lewis acid catalysts in polymerization reactions: Lewis acid catalysts are widely used in polymerization reactions to control selectivity. These catalysts can influence the stereochemistry, regioselectivity, and molecular weight distribution of polymers. Various metal-based Lewis acids such as titanium, zirconium, and aluminum compounds are particularly effective in olefin polymerization, allowing for precise control over polymer architecture and properties. The catalyst structure and reaction conditions can be optimized to achieve desired selectivity patterns.
- Lewis acid catalysts in organic synthesis reactions: Lewis acid catalysts play a crucial role in various organic synthesis reactions by enhancing selectivity. They facilitate reactions such as Friedel-Crafts alkylation, acylation, Diels-Alder reactions, and aldol condensations. The selectivity can be tuned by modifying the Lewis acid strength, steric hindrance, and electronic properties. These catalysts can direct the reaction pathway toward specific products by activating particular functional groups and stabilizing transition states, leading to improved regioselectivity and stereoselectivity.
- Metal-modified Lewis acid catalysts for enhanced selectivity: Metal-modified Lewis acid catalysts offer enhanced selectivity in various chemical transformations. By incorporating different metals or modifying existing metal centers, the electronic and steric properties of the catalyst can be fine-tuned. These modifications can alter the coordination environment, Lewis acidity strength, and substrate binding modes, resulting in improved chemo-, regio-, and stereoselectivity. Common modifications include the addition of transition metals to traditional Lewis acids or the use of bimetallic systems that work synergistically.
- Supported Lewis acid catalysts for heterogeneous catalysis: Supported Lewis acid catalysts provide advantages in heterogeneous catalysis with improved selectivity. By immobilizing Lewis acids on solid supports such as silica, alumina, or zeolites, the catalysts gain enhanced stability, recyclability, and often improved selectivity. The support material can influence the dispersion of active sites, prevent aggregation, and create unique microenvironments that affect substrate orientation and activation. These supported catalysts are particularly valuable in industrial applications where catalyst separation and reuse are important considerations.
- Lewis acid catalysts in asymmetric synthesis: Lewis acid catalysts are extensively used in asymmetric synthesis to achieve high enantioselectivity. By incorporating chiral ligands or using inherently chiral Lewis acids, these catalysts can create asymmetric environments that favor the formation of one enantiomer over another. The selectivity arises from differential facial approach of the substrate to the catalyst-substrate complex. Chiral Lewis acids based on aluminum, boron, titanium, and lanthanide metals have been particularly successful in various asymmetric transformations including Diels-Alder reactions, aldol reactions, and Michael additions.
02 Lewis acid catalysts for regioselective and stereoselective transformations
Lewis acid catalysts can direct the regioselectivity and stereoselectivity of chemical reactions by coordinating to specific functional groups and creating defined transition states. This selective activation allows for controlled bond formation at desired positions and with specific spatial orientations. The selectivity can be enhanced by tuning the steric and electronic properties of the Lewis acid, enabling the synthesis of complex molecules with precise control over their three-dimensional structure.Expand Specific Solutions03 Supported Lewis acid catalysts for heterogeneous catalysis
Lewis acid catalysts supported on solid materials offer advantages in terms of catalyst recovery, reusability, and process efficiency. These heterogeneous catalysts can be designed with specific surface properties to enhance selectivity in various chemical transformations. The support material, such as silica, alumina, or zeolites, can influence the accessibility of active sites and modify the electronic properties of the Lewis acid centers, thereby affecting the selectivity of the catalytic process.Expand Specific Solutions04 Lewis acid catalysts in alkylation and isomerization reactions
Lewis acid catalysts play a crucial role in alkylation and isomerization reactions, where they can direct the addition or rearrangement of alkyl groups with high selectivity. These catalysts can activate carbon-carbon bonds and control the reaction pathway to favor specific products. The selectivity in these reactions depends on the strength of the Lewis acid, the reaction temperature, and the structure of the reactants, allowing for the production of valuable intermediates in the petrochemical industry.Expand Specific Solutions05 Novel Lewis acid catalyst systems with enhanced selectivity
Advanced Lewis acid catalyst systems have been developed with enhanced selectivity through the incorporation of novel ligands, co-catalysts, or activators. These systems can include bimetallic complexes, Lewis acid-base pairs, or confined catalytic environments that create unique reaction microenvironments. The synergistic effects between components in these catalyst systems can lead to unprecedented levels of selectivity in challenging transformations, opening new pathways for efficient and sustainable chemical synthesis.Expand Specific Solutions
Leading Research Groups and Industrial Players
The Lewis acid catalyst market for selective synthesis is currently in a growth phase, characterized by increasing demand for efficient and sustainable catalytic processes. The market size is expanding due to applications in pharmaceuticals, fine chemicals, and materials science, with an estimated annual growth rate of 5-7%. Technologically, the field is maturing with significant advancements from key players. Academic institutions like MIT, Zhejiang University, and CNRS are driving fundamental research, while industrial leaders including BASF, ExxonMobil Chemical, and Dow Global Technologies are commercializing innovative catalyst systems. Japanese companies such as Asahi Kasei and Mitsubishi Gas Chemical have made notable progress in specialized applications, while European firms like Roquette Frères and IFP Energies Nouvelles focus on sustainable catalytic processes, reflecting the global competitive landscape in this evolving field.
ExxonMobil Chemical Patents, Inc.
Technical Solution: ExxonMobil has developed highly selective Lewis acid catalyst systems based on modified metallocene complexes for petrochemical transformations. Their technology centers on zirconium and hafnium-based catalysts with precisely engineered electronic and steric properties that enable control over reaction pathways[3]. ExxonMobil's approach incorporates perfluorinated aryl substituents on supporting ligands to enhance Lewis acidity while maintaining stability under industrial conditions. These catalysts demonstrate exceptional performance in alkylation reactions, achieving selectivities above 98% for linear products in olefin transformations[6]. The company has pioneered supported catalyst systems that combine homogeneous-like selectivity with heterogeneous-like recoverability, enabling continuous processing in fixed-bed reactors with catalyst lifetimes exceeding 1000 hours[8]. Recent innovations include dual-function Lewis acid catalysts that simultaneously activate C-H bonds and direct functionalization, enabling single-step transformations that traditionally require multiple reactions. ExxonMobil has also developed methods to tune catalyst acidity through non-coordinating anion modification, allowing precise control over reaction outcomes.
Strengths: Exceptional thermal and chemical stability under harsh industrial conditions; outstanding selectivity for targeted petrochemical transformations; long catalyst lifetimes reducing operational costs; successful implementation at commercial scale. Weaknesses: Limited substrate scope compared to some academic catalysts; higher production costs than traditional acid catalysts; potential environmental concerns with perfluorinated components; primarily optimized for hydrocarbon processing rather than fine chemical synthesis.
BASF Corp.
Technical Solution: BASF has developed advanced Lewis acid catalysts based on metal-organic frameworks (MOFs) for selective synthesis applications. Their technology utilizes precisely engineered single-site metal centers (particularly Al, Fe, and Zr) embedded in MOF structures that provide exceptional control over reaction selectivity[1]. BASF's approach focuses on tunable Lewis acidity through metal node modification and organic linker functionalization, allowing for customization of catalyst properties for specific reactions. Their catalysts demonstrate remarkable performance in Diels-Alder reactions, Friedel-Crafts alkylations, and carbonyl transformations with selectivities exceeding 95%[3]. BASF has also pioneered recyclable Lewis acid catalysts by immobilizing active metal species on solid supports, addressing traditional recovery challenges while maintaining catalytic activity through multiple cycles[5]. Recent innovations include water-stable Lewis acid catalysts that maintain activity in the presence of moisture, overcoming a significant limitation of conventional systems.
Strengths: Superior selectivity control through precisely engineered single-site metal centers; excellent recyclability compared to homogeneous alternatives; tunable acidity for reaction-specific optimization; industrial scalability backed by BASF's manufacturing expertise. Weaknesses: Higher production costs compared to traditional catalysts; potential mass transfer limitations in some MOF-based systems; performance may decrease after multiple recycling cycles due to structural degradation.
Key Innovations in Selective Synthesis Methodologies
Polymer-supported arylbis(perfluoroalkylsulfonyl)-methane
PatentInactiveEP1375532A1
Innovation
- A polymer-supported arylbis(perfluoroalkylsulfonyl)methane catalyst is developed, specifically a polystyrene-supported pentafluorophenylbis(triflyl)methane, which acts as an excellent Bronsted or Lewis acid catalyst for reactions like acylation and aldol reactions, using sodium trifluoromethane sulfinate and trifluoromethane sulfonic acid anhydride as reactants, enabling easy synthesis and recycling.
Green Chemistry Implications of Advanced Lewis Catalysts
The advancement of Lewis acid catalysts represents a significant opportunity for the chemical industry to align with green chemistry principles. These catalysts facilitate more selective synthesis pathways that can dramatically reduce waste production and energy consumption compared to traditional methods. By enabling reactions to proceed under milder conditions, advanced Lewis catalysts contribute to decreased energy requirements across manufacturing processes, supporting sustainability goals.
The environmental impact of Lewis acid catalysis extends beyond energy considerations. Modern catalyst designs increasingly incorporate recyclable components and support structures that minimize metal leaching, addressing concerns about heavy metal contamination in waste streams. This evolution toward recoverable catalysts represents a crucial step in reducing the environmental footprint of chemical synthesis operations.
Water compatibility remains a frontier challenge for Lewis acid catalysis. Recent innovations in water-tolerant Lewis acids have opened pathways for aqueous-phase reactions, potentially eliminating the need for hazardous organic solvents in many applications. These developments align perfectly with green chemistry's emphasis on safer solvents and reaction media, offering substantial improvements in process safety profiles.
Atom economy improvements constitute another significant benefit of advanced Lewis catalysis. By enhancing reaction selectivity, these catalysts minimize side reactions and byproduct formation, thereby increasing the percentage of input materials incorporated into the desired product. This efficiency translates directly to reduced raw material consumption and waste generation across chemical manufacturing operations.
The integration of Lewis acid catalysts into continuous flow systems represents a promising direction for green chemistry implementation. Such systems enable precise reaction control, minimizing reagent use and maximizing conversion efficiency. Additionally, the controlled environment of flow reactors often permits catalyst immobilization strategies that facilitate extended catalyst lifetimes and simplified recovery processes.
Regulatory frameworks increasingly favor technologies that reduce environmental impact, positioning advanced Lewis catalysis as strategically valuable for future chemical manufacturing. Companies investing in these technologies may gain competitive advantages through improved sustainability metrics, reduced compliance costs, and enhanced public perception. The alignment between Lewis acid catalyst advancement and green chemistry principles thus creates a compelling case for continued research and development investment in this field.
The environmental impact of Lewis acid catalysis extends beyond energy considerations. Modern catalyst designs increasingly incorporate recyclable components and support structures that minimize metal leaching, addressing concerns about heavy metal contamination in waste streams. This evolution toward recoverable catalysts represents a crucial step in reducing the environmental footprint of chemical synthesis operations.
Water compatibility remains a frontier challenge for Lewis acid catalysis. Recent innovations in water-tolerant Lewis acids have opened pathways for aqueous-phase reactions, potentially eliminating the need for hazardous organic solvents in many applications. These developments align perfectly with green chemistry's emphasis on safer solvents and reaction media, offering substantial improvements in process safety profiles.
Atom economy improvements constitute another significant benefit of advanced Lewis catalysis. By enhancing reaction selectivity, these catalysts minimize side reactions and byproduct formation, thereby increasing the percentage of input materials incorporated into the desired product. This efficiency translates directly to reduced raw material consumption and waste generation across chemical manufacturing operations.
The integration of Lewis acid catalysts into continuous flow systems represents a promising direction for green chemistry implementation. Such systems enable precise reaction control, minimizing reagent use and maximizing conversion efficiency. Additionally, the controlled environment of flow reactors often permits catalyst immobilization strategies that facilitate extended catalyst lifetimes and simplified recovery processes.
Regulatory frameworks increasingly favor technologies that reduce environmental impact, positioning advanced Lewis catalysis as strategically valuable for future chemical manufacturing. Companies investing in these technologies may gain competitive advantages through improved sustainability metrics, reduced compliance costs, and enhanced public perception. The alignment between Lewis acid catalyst advancement and green chemistry principles thus creates a compelling case for continued research and development investment in this field.
Scalability and Industrial Implementation Strategies
Scaling Lewis acid catalysis from laboratory to industrial scale presents significant challenges that require systematic approaches and innovative solutions. The transition demands careful consideration of catalyst stability, recyclability, and process economics. Current industrial implementations often utilize heterogeneous Lewis acid catalysts supported on various materials such as silica, alumina, or polymeric resins to facilitate separation and reuse, addressing one of the primary challenges in large-scale operations.
Continuous flow technologies represent a transformative approach for industrial implementation of Lewis acid catalysis. These systems allow for precise control of reaction parameters, improved heat transfer, and enhanced safety profiles compared to batch processes. Several pharmaceutical and fine chemical manufacturers have successfully implemented microreactor and flow chemistry platforms for Lewis acid-catalyzed transformations, achieving higher throughput and consistent product quality while minimizing waste generation.
Immobilization strategies have evolved significantly to address the scalability challenges of homogeneous Lewis acid catalysts. Techniques such as covalent attachment to solid supports, encapsulation in porous materials, and development of magnetic nanoparticle-supported catalysts have demonstrated promising results in pilot-scale operations. These approaches maintain catalytic activity while facilitating separation and recycling, critical factors for economic viability at industrial scale.
Process intensification represents another crucial strategy for industrial implementation. Integration of reaction and separation steps, utilization of alternative energy inputs such as microwave or ultrasonic activation, and development of multifunctional catalytic systems have shown potential to reduce energy consumption and processing time. Companies implementing these intensified processes have reported significant reductions in operational costs and environmental footprint.
Standardization of catalyst preparation and characterization methods is essential for consistent performance at industrial scale. Development of robust quality control protocols, including in-line monitoring techniques and rapid analytical methods, ensures batch-to-batch consistency and facilitates regulatory compliance, particularly important for pharmaceutical applications of Lewis acid catalysis.
Economic considerations ultimately determine industrial adoption of advanced Lewis acid catalysts. Life cycle assessment and techno-economic analysis tools are increasingly employed to evaluate the overall sustainability and financial viability of new catalytic processes. Successful industrial implementations typically demonstrate clear advantages in terms of reduced waste generation, lower energy consumption, or access to high-value products that justify the investment in new catalyst technologies.
Continuous flow technologies represent a transformative approach for industrial implementation of Lewis acid catalysis. These systems allow for precise control of reaction parameters, improved heat transfer, and enhanced safety profiles compared to batch processes. Several pharmaceutical and fine chemical manufacturers have successfully implemented microreactor and flow chemistry platforms for Lewis acid-catalyzed transformations, achieving higher throughput and consistent product quality while minimizing waste generation.
Immobilization strategies have evolved significantly to address the scalability challenges of homogeneous Lewis acid catalysts. Techniques such as covalent attachment to solid supports, encapsulation in porous materials, and development of magnetic nanoparticle-supported catalysts have demonstrated promising results in pilot-scale operations. These approaches maintain catalytic activity while facilitating separation and recycling, critical factors for economic viability at industrial scale.
Process intensification represents another crucial strategy for industrial implementation. Integration of reaction and separation steps, utilization of alternative energy inputs such as microwave or ultrasonic activation, and development of multifunctional catalytic systems have shown potential to reduce energy consumption and processing time. Companies implementing these intensified processes have reported significant reductions in operational costs and environmental footprint.
Standardization of catalyst preparation and characterization methods is essential for consistent performance at industrial scale. Development of robust quality control protocols, including in-line monitoring techniques and rapid analytical methods, ensures batch-to-batch consistency and facilitates regulatory compliance, particularly important for pharmaceutical applications of Lewis acid catalysis.
Economic considerations ultimately determine industrial adoption of advanced Lewis acid catalysts. Life cycle assessment and techno-economic analysis tools are increasingly employed to evaluate the overall sustainability and financial viability of new catalytic processes. Successful industrial implementations typically demonstrate clear advantages in terms of reduced waste generation, lower energy consumption, or access to high-value products that justify the investment in new catalyst technologies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!