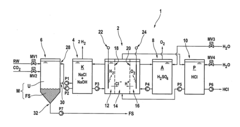
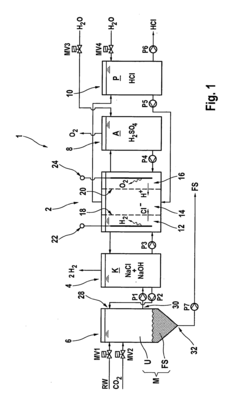
How to Deliver Resultative Hydrochloric Acid Applications?
JUL 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HCl Applications Background and Objectives
Hydrochloric acid (HCl) applications have been a cornerstone in various industries for decades, playing a crucial role in numerous processes and products. The evolution of HCl usage has been driven by advancements in chemical engineering, material science, and environmental considerations. As industries continue to seek more efficient and sustainable solutions, the demand for innovative HCl applications has grown significantly.
The primary objective of this technical research is to explore and evaluate cutting-edge methods for delivering resultative hydrochloric acid applications across diverse sectors. This involves investigating novel techniques, materials, and processes that can enhance the effectiveness, safety, and environmental compatibility of HCl usage. By examining the current state of the art and emerging trends, we aim to identify potential breakthroughs that could revolutionize HCl applications in the coming years.
One of the key drivers behind this research is the increasing emphasis on sustainability and environmental stewardship. As regulatory frameworks become more stringent, industries are under pressure to adopt cleaner and more efficient processes. This has led to a growing interest in developing HCl applications that minimize waste, reduce energy consumption, and mitigate environmental impacts. Our investigation will focus on identifying technologies and methodologies that align with these sustainability goals while maintaining or improving process efficiency.
Another critical aspect of this research is the exploration of HCl applications in emerging fields such as nanotechnology, advanced materials, and biotechnology. These rapidly evolving domains present new opportunities for HCl usage, potentially opening up entirely new markets and applications. By examining the intersection of HCl chemistry with these cutting-edge fields, we aim to uncover innovative approaches that could lead to significant advancements in various industries.
Furthermore, this technical research will delve into the challenges associated with HCl handling, storage, and transportation. As industries seek to optimize their supply chains and reduce operational risks, there is a growing need for improved methods of managing HCl throughout its lifecycle. This includes investigating novel containment systems, corrosion-resistant materials, and advanced monitoring technologies that can enhance safety and reliability in HCl-related processes.
Lastly, our research will explore the potential for digitalization and automation in HCl applications. With the advent of Industry 4.0 and the increasing adoption of smart manufacturing principles, there are opportunities to integrate advanced control systems, predictive analytics, and artificial intelligence into HCl-based processes. By examining these technological trends, we aim to identify ways to improve process control, optimize resource utilization, and enhance overall operational efficiency in HCl applications.
The primary objective of this technical research is to explore and evaluate cutting-edge methods for delivering resultative hydrochloric acid applications across diverse sectors. This involves investigating novel techniques, materials, and processes that can enhance the effectiveness, safety, and environmental compatibility of HCl usage. By examining the current state of the art and emerging trends, we aim to identify potential breakthroughs that could revolutionize HCl applications in the coming years.
One of the key drivers behind this research is the increasing emphasis on sustainability and environmental stewardship. As regulatory frameworks become more stringent, industries are under pressure to adopt cleaner and more efficient processes. This has led to a growing interest in developing HCl applications that minimize waste, reduce energy consumption, and mitigate environmental impacts. Our investigation will focus on identifying technologies and methodologies that align with these sustainability goals while maintaining or improving process efficiency.
Another critical aspect of this research is the exploration of HCl applications in emerging fields such as nanotechnology, advanced materials, and biotechnology. These rapidly evolving domains present new opportunities for HCl usage, potentially opening up entirely new markets and applications. By examining the intersection of HCl chemistry with these cutting-edge fields, we aim to uncover innovative approaches that could lead to significant advancements in various industries.
Furthermore, this technical research will delve into the challenges associated with HCl handling, storage, and transportation. As industries seek to optimize their supply chains and reduce operational risks, there is a growing need for improved methods of managing HCl throughout its lifecycle. This includes investigating novel containment systems, corrosion-resistant materials, and advanced monitoring technologies that can enhance safety and reliability in HCl-related processes.
Lastly, our research will explore the potential for digitalization and automation in HCl applications. With the advent of Industry 4.0 and the increasing adoption of smart manufacturing principles, there are opportunities to integrate advanced control systems, predictive analytics, and artificial intelligence into HCl-based processes. By examining these technological trends, we aim to identify ways to improve process control, optimize resource utilization, and enhance overall operational efficiency in HCl applications.
Market Analysis for HCl Usage
The global hydrochloric acid (HCl) market has shown steady growth in recent years, driven by increasing demand across various industries. The market size was valued at over $7 billion in 2020 and is projected to expand at a compound annual growth rate (CAGR) of 5.2% from 2021 to 2028. This growth is primarily attributed to the rising applications of HCl in diverse sectors such as chemicals, steel pickling, oil well acidizing, and food processing.
In the chemical industry, HCl plays a crucial role as a raw material for producing various chemicals, including vinyl chloride, chlorinated solvents, and water treatment chemicals. The expanding chemical sector, particularly in emerging economies, is expected to fuel the demand for HCl. The steel industry represents another significant market for HCl, where it is used in the pickling process to remove rust and scale from steel surfaces. As global steel production continues to rise, the demand for HCl in this sector is anticipated to grow correspondingly.
The oil and gas industry utilizes HCl for well acidizing, a process that enhances the production of oil and gas wells. With the increasing exploration and production activities in unconventional oil and gas reserves, the demand for HCl in this sector is projected to witness substantial growth. Additionally, the food processing industry employs HCl in various applications, including the production of high fructose corn syrup and gelatin. The growing food and beverage sector, driven by changing consumer preferences and increasing population, is expected to contribute to the rising demand for HCl.
Geographically, Asia Pacific dominates the global HCl market, accounting for the largest share of consumption. This can be attributed to the rapid industrialization and urbanization in countries like China and India, which have led to increased demand across multiple end-use industries. North America and Europe follow as significant markets, with steady demand from established industrial sectors.
The market for HCl is characterized by the presence of both large multinational corporations and regional players. Key market participants include Dow Chemical Company, BASF SE, Olin Corporation, and Covestro AG. These companies are focusing on expanding their production capacities and developing innovative applications to maintain their market positions and cater to the growing demand.
In the chemical industry, HCl plays a crucial role as a raw material for producing various chemicals, including vinyl chloride, chlorinated solvents, and water treatment chemicals. The expanding chemical sector, particularly in emerging economies, is expected to fuel the demand for HCl. The steel industry represents another significant market for HCl, where it is used in the pickling process to remove rust and scale from steel surfaces. As global steel production continues to rise, the demand for HCl in this sector is anticipated to grow correspondingly.
The oil and gas industry utilizes HCl for well acidizing, a process that enhances the production of oil and gas wells. With the increasing exploration and production activities in unconventional oil and gas reserves, the demand for HCl in this sector is projected to witness substantial growth. Additionally, the food processing industry employs HCl in various applications, including the production of high fructose corn syrup and gelatin. The growing food and beverage sector, driven by changing consumer preferences and increasing population, is expected to contribute to the rising demand for HCl.
Geographically, Asia Pacific dominates the global HCl market, accounting for the largest share of consumption. This can be attributed to the rapid industrialization and urbanization in countries like China and India, which have led to increased demand across multiple end-use industries. North America and Europe follow as significant markets, with steady demand from established industrial sectors.
The market for HCl is characterized by the presence of both large multinational corporations and regional players. Key market participants include Dow Chemical Company, BASF SE, Olin Corporation, and Covestro AG. These companies are focusing on expanding their production capacities and developing innovative applications to maintain their market positions and cater to the growing demand.
Current HCl Technology Challenges
The application of hydrochloric acid (HCl) in various industries faces several significant challenges that hinder its effective and safe utilization. One of the primary issues is the corrosive nature of HCl, which poses risks to equipment, infrastructure, and personnel safety. This corrosiveness necessitates the use of specialized materials and protective measures, increasing operational costs and complexity.
Another major challenge is the precise control of HCl concentration and delivery. Many applications require specific concentrations of HCl, and maintaining these levels consistently can be difficult due to the acid's volatility and reactivity. This challenge is particularly acute in industries such as semiconductor manufacturing, where even slight variations in acid concentration can significantly impact product quality.
Environmental concerns also present a substantial hurdle in HCl applications. The release of HCl vapors and waste streams containing the acid can have detrimental effects on the environment and human health. Stringent regulations governing the handling, storage, and disposal of HCl necessitate sophisticated containment and treatment systems, adding to the overall complexity and cost of its use.
The transportation and storage of HCl present additional challenges. Due to its hazardous nature, special containers and handling procedures are required, which can be logistically complex and expensive. Moreover, the risk of accidental spills or leaks during transport or storage remains a constant concern, requiring robust safety protocols and emergency response plans.
In certain applications, such as oil and gas well stimulation, the interaction of HCl with formation minerals can lead to unwanted side effects. These include the precipitation of insoluble compounds, which can reduce the effectiveness of the treatment and potentially damage the formation. Developing formulations that mitigate these issues while maintaining the desired acid functionality is an ongoing challenge.
The health and safety risks associated with HCl exposure continue to be a significant concern. Despite advances in personal protective equipment and safety protocols, the potential for acid burns, respiratory issues, and other health hazards remains. This necessitates continuous improvement in safety measures and training programs for personnel working with HCl.
Lastly, the pursuit of more sustainable and environmentally friendly alternatives to HCl in various applications is an ongoing challenge. While HCl remains essential in many processes, there is growing pressure to develop less hazardous substitutes or to minimize its use where possible. This drive for sustainability requires significant research and development efforts to find viable alternatives that can match the performance of HCl without its associated risks.
Another major challenge is the precise control of HCl concentration and delivery. Many applications require specific concentrations of HCl, and maintaining these levels consistently can be difficult due to the acid's volatility and reactivity. This challenge is particularly acute in industries such as semiconductor manufacturing, where even slight variations in acid concentration can significantly impact product quality.
Environmental concerns also present a substantial hurdle in HCl applications. The release of HCl vapors and waste streams containing the acid can have detrimental effects on the environment and human health. Stringent regulations governing the handling, storage, and disposal of HCl necessitate sophisticated containment and treatment systems, adding to the overall complexity and cost of its use.
The transportation and storage of HCl present additional challenges. Due to its hazardous nature, special containers and handling procedures are required, which can be logistically complex and expensive. Moreover, the risk of accidental spills or leaks during transport or storage remains a constant concern, requiring robust safety protocols and emergency response plans.
In certain applications, such as oil and gas well stimulation, the interaction of HCl with formation minerals can lead to unwanted side effects. These include the precipitation of insoluble compounds, which can reduce the effectiveness of the treatment and potentially damage the formation. Developing formulations that mitigate these issues while maintaining the desired acid functionality is an ongoing challenge.
The health and safety risks associated with HCl exposure continue to be a significant concern. Despite advances in personal protective equipment and safety protocols, the potential for acid burns, respiratory issues, and other health hazards remains. This necessitates continuous improvement in safety measures and training programs for personnel working with HCl.
Lastly, the pursuit of more sustainable and environmentally friendly alternatives to HCl in various applications is an ongoing challenge. While HCl remains essential in many processes, there is growing pressure to develop less hazardous substitutes or to minimize its use where possible. This drive for sustainability requires significant research and development efforts to find viable alternatives that can match the performance of HCl without its associated risks.
Existing HCl Delivery Solutions
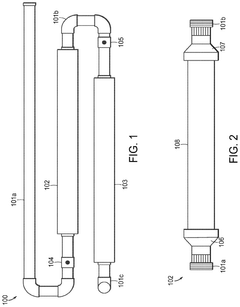
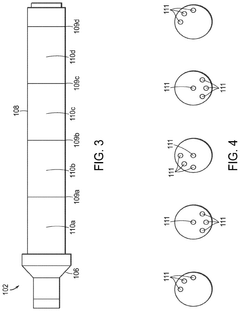
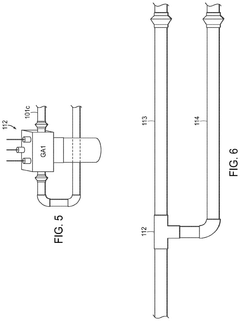
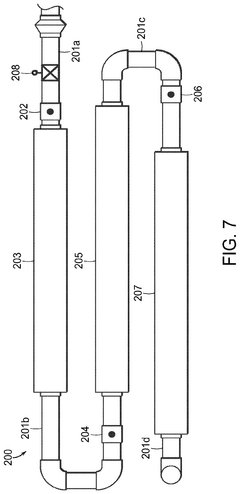
01 Production of hydrochloric acid
Various methods and processes for producing hydrochloric acid, including industrial-scale production techniques and chemical reactions that result in the formation of hydrochloric acid as a product or by-product.- Production of hydrochloric acid: Various methods and processes for producing hydrochloric acid, including reactions involving chlorine and hydrogen, as well as the recovery of hydrochloric acid from industrial waste streams. These processes aim to improve efficiency and yield in hydrochloric acid production.
- Purification and concentration of hydrochloric acid: Techniques for purifying and concentrating hydrochloric acid, including distillation, membrane separation, and other chemical processes. These methods are designed to remove impurities and increase the concentration of hydrochloric acid for various industrial applications.
- Applications of hydrochloric acid in chemical processes: Utilization of hydrochloric acid in various chemical processes and reactions, such as metal treatment, pH adjustment, and as a catalyst in organic synthesis. The acid's properties are leveraged to achieve specific chemical transformations and industrial outcomes.
- Handling and storage of hydrochloric acid: Equipment and methods for safely handling, storing, and transporting hydrochloric acid. This includes specialized containers, safety measures, and systems designed to prevent corrosion and ensure worker safety when dealing with this highly corrosive substance.
- Environmental and safety considerations in hydrochloric acid use: Techniques and systems for mitigating the environmental impact of hydrochloric acid use, including waste treatment, emissions control, and safety protocols. These measures aim to reduce the risks associated with hydrochloric acid in industrial settings and minimize its ecological footprint.
02 Purification and treatment of hydrochloric acid
Techniques and systems for purifying and treating hydrochloric acid, including removal of impurities, concentration adjustment, and preparation for specific industrial applications.Expand Specific Solutions03 Applications of hydrochloric acid in chemical processes
Utilization of hydrochloric acid in various chemical processes and industrial applications, such as metal treatment, synthesis of organic compounds, and pH adjustment in chemical reactions.Expand Specific Solutions04 Handling and storage of hydrochloric acid
Equipment, containers, and safety measures for handling, storing, and transporting hydrochloric acid, including corrosion-resistant materials and specialized storage systems.Expand Specific Solutions05 Environmental and safety considerations
Methods and systems for mitigating environmental impact and ensuring safety in processes involving hydrochloric acid, including emission control, waste treatment, and worker protection measures.Expand Specific Solutions
Key HCl Industry Players
The hydrochloric acid applications market is in a growth phase, driven by increasing demand across various industries. The global market size is projected to expand significantly, with key players like Industrie De Nora SpA, Covestro Deutschland AG, and Fluid Energy Group Ltd. leading innovation. These companies are developing advanced technologies to enhance the efficiency and safety of hydrochloric acid applications. The market is characterized by a mix of established firms and emerging players, such as WIAB WATER INNOVATION AB and Aquaox, Inc., focusing on eco-friendly solutions. As the technology matures, we're seeing a shift towards more sustainable and cost-effective methods, with companies like Australian BioRefining Pty Ltd. and Jiangyin Runma Electronic Material Co., Ltd. contributing to the development of specialized applications in water treatment and electronics manufacturing.
Industrie De Nora SpA
Technical Solution: Industrie De Nora SpA has developed advanced electrochemical technologies for hydrochloric acid applications. Their approach involves using dimensionally stable anodes (DSA) in electrolysis processes to produce high-purity hydrochloric acid on-site[1]. This method allows for precise control of acid concentration and purity, reducing transportation costs and safety risks associated with bulk acid handling. The company has also implemented membrane cell technology, which enables the production of ultra-pure hydrochloric acid with minimal impurities[2]. Additionally, De Nora has integrated IoT and automation systems to optimize acid production and delivery processes, ensuring consistent quality and efficiency[3].
Strengths: On-site production reduces transportation costs and risks; high purity acid production; advanced process control. Weaknesses: Higher initial investment costs; requires specialized technical expertise for operation and maintenance.
Covestro Deutschland AG
Technical Solution: Covestro Deutschland AG has developed innovative approaches to hydrochloric acid applications in the polymer and materials science sectors. Their focus has been on utilizing HCl as a key raw material in the production of high-performance plastics and coatings. Covestro has implemented a closed-loop chlorine recycling system that captures and reuses HCl generated as a by-product in their manufacturing processes[13]. This not only improves resource efficiency but also reduces waste and environmental impact. The company has also developed advanced catalysts that enable more efficient conversion of HCl into valuable chlorine-based products[14]. Additionally, Covestro has invested in digitalization and process intensification technologies to optimize HCl usage across their production facilities, resulting in improved yield and reduced energy consumption[15].
Strengths: Circular economy approach to HCl usage; efficient conversion of HCl to value-added products; optimized production processes. Weaknesses: Limited to specific industry applications; high capital investment required for implementation.
Innovative HCl Application Methods
Compositions of hypochlorous acid and methods of manufacture thereof
PatentActiveUS12115185B2
Innovation
- An air-free mixing method producing stable HOCl by combining a compound that generates protons (H+) with one that generates hypochlorite anions (OCl-) in water, without using chlorine gas or electrolysis, maintaining a controlled pH and using buffering agents to stabilize the product.
Method for producing hydrogen chloride or an aqueous solution thereof using untreated salt water, thus produced product, use of the product and electrodialysis system
PatentActiveUS20130272952A1
Innovation
- Producing hydrogen chloride or an aqueous hydrochloric acid solution on-site using untreated saline water through an electrodialysis method, which involves furnishing a chloride-ion-containing electrolyte, subjecting it to cathodic reduction, processing the catholyte with untreated saline water to increase chloride ions and decrease hydroxide ions, and reusing the catholyte as the electrolyte, thereby avoiding costly disposal and additional chemical use.
Safety and Handling Protocols
Safety and handling protocols are paramount when working with hydrochloric acid (HCl) due to its corrosive and hazardous nature. Proper personal protective equipment (PPE) is essential, including chemical-resistant gloves, goggles, face shields, and acid-resistant clothing. Respiratory protection may be necessary depending on the concentration and application method. All personnel involved in handling HCl must be thoroughly trained in safety procedures and emergency response protocols.
Storage of hydrochloric acid requires specialized containers made of corrosion-resistant materials such as high-density polyethylene or glass-lined steel. These containers should be kept in well-ventilated areas, away from incompatible substances and heat sources. Proper labeling and segregation from other chemicals are crucial to prevent accidental mixing and potential reactions.
When transferring or applying HCl, closed systems and proper ventilation are vital to minimize exposure risks. Spill containment measures, such as secondary containment systems and neutralization materials, should be readily available. Regular equipment inspections and maintenance are necessary to prevent leaks and ensure the integrity of acid-handling systems.
Emergency response procedures must be clearly defined and communicated to all personnel. This includes evacuation plans, spill response protocols, and first aid measures. Eyewash stations and safety showers should be easily accessible in all areas where HCl is handled or stored. A comprehensive safety data sheet (SDS) must be available and regularly updated.
Waste management is another critical aspect of HCl handling. Proper neutralization and disposal methods must be employed to comply with environmental regulations. This may involve treatment facilities or specialized waste management services capable of handling hazardous materials.
Regular safety audits and risk assessments should be conducted to identify potential hazards and improve safety measures. This includes reviewing handling procedures, updating safety protocols, and ensuring compliance with local and international safety standards. Continuous training and education of personnel on the latest safety practices and technologies are essential for maintaining a safe working environment.
Implementing a robust monitoring system for air quality and potential leaks can provide early warning of potential hazards. This may include the use of HCl vapor detectors and pH monitors in critical areas. Additionally, establishing clear communication channels for reporting safety concerns and near-miss incidents can help prevent accidents and improve overall safety culture.
Storage of hydrochloric acid requires specialized containers made of corrosion-resistant materials such as high-density polyethylene or glass-lined steel. These containers should be kept in well-ventilated areas, away from incompatible substances and heat sources. Proper labeling and segregation from other chemicals are crucial to prevent accidental mixing and potential reactions.
When transferring or applying HCl, closed systems and proper ventilation are vital to minimize exposure risks. Spill containment measures, such as secondary containment systems and neutralization materials, should be readily available. Regular equipment inspections and maintenance are necessary to prevent leaks and ensure the integrity of acid-handling systems.
Emergency response procedures must be clearly defined and communicated to all personnel. This includes evacuation plans, spill response protocols, and first aid measures. Eyewash stations and safety showers should be easily accessible in all areas where HCl is handled or stored. A comprehensive safety data sheet (SDS) must be available and regularly updated.
Waste management is another critical aspect of HCl handling. Proper neutralization and disposal methods must be employed to comply with environmental regulations. This may involve treatment facilities or specialized waste management services capable of handling hazardous materials.
Regular safety audits and risk assessments should be conducted to identify potential hazards and improve safety measures. This includes reviewing handling procedures, updating safety protocols, and ensuring compliance with local and international safety standards. Continuous training and education of personnel on the latest safety practices and technologies are essential for maintaining a safe working environment.
Implementing a robust monitoring system for air quality and potential leaks can provide early warning of potential hazards. This may include the use of HCl vapor detectors and pH monitors in critical areas. Additionally, establishing clear communication channels for reporting safety concerns and near-miss incidents can help prevent accidents and improve overall safety culture.
Environmental Impact Assessment
The environmental impact assessment of hydrochloric acid applications is a critical aspect that requires thorough evaluation. Hydrochloric acid, while essential in various industrial processes, poses significant environmental risks if not properly managed. The primary concern is its corrosive nature and potential for acidification of soil and water bodies.
When released into aquatic ecosystems, hydrochloric acid can drastically alter pH levels, leading to detrimental effects on aquatic life. Fish, amphibians, and other water-dwelling organisms are particularly vulnerable to these changes. The acid can also dissolve heavy metals in sediments, increasing their bioavailability and toxicity to aquatic organisms.
In terrestrial environments, hydrochloric acid can cause soil acidification, affecting plant growth and soil microbial communities. This can lead to reduced agricultural productivity and ecosystem disruption. Additionally, the acid can react with soil minerals, potentially releasing toxic elements into the environment.
Air pollution is another significant concern. When hydrochloric acid vaporizes, it can form acid mist or aerosols, contributing to air quality degradation. These airborne particles can cause respiratory issues in humans and animals, as well as contribute to acid rain formation.
To mitigate these environmental impacts, stringent control measures are essential. Proper storage, handling, and disposal protocols must be implemented to prevent accidental releases. Neutralization techniques should be employed before discharging any acid-containing waste streams. Advanced air filtration systems are crucial in industrial settings to capture acid vapors and prevent atmospheric emissions.
Monitoring programs are vital to assess the long-term environmental effects of hydrochloric acid applications. Regular soil and water quality testing in areas surrounding industrial sites can help detect and address any contamination issues promptly. Ecological surveys can provide insights into the impact on local flora and fauna, allowing for adaptive management strategies.
Sustainable alternatives and process optimizations should be explored to reduce the overall use of hydrochloric acid where possible. This may include developing closed-loop systems that minimize waste generation or investigating less harmful substitutes for specific applications.
In conclusion, while hydrochloric acid remains an indispensable chemical in many industries, its environmental impact must be carefully managed. A comprehensive approach involving prevention, mitigation, and monitoring is essential to ensure the sustainable use of this powerful acid while safeguarding ecosystems and human health.
When released into aquatic ecosystems, hydrochloric acid can drastically alter pH levels, leading to detrimental effects on aquatic life. Fish, amphibians, and other water-dwelling organisms are particularly vulnerable to these changes. The acid can also dissolve heavy metals in sediments, increasing their bioavailability and toxicity to aquatic organisms.
In terrestrial environments, hydrochloric acid can cause soil acidification, affecting plant growth and soil microbial communities. This can lead to reduced agricultural productivity and ecosystem disruption. Additionally, the acid can react with soil minerals, potentially releasing toxic elements into the environment.
Air pollution is another significant concern. When hydrochloric acid vaporizes, it can form acid mist or aerosols, contributing to air quality degradation. These airborne particles can cause respiratory issues in humans and animals, as well as contribute to acid rain formation.
To mitigate these environmental impacts, stringent control measures are essential. Proper storage, handling, and disposal protocols must be implemented to prevent accidental releases. Neutralization techniques should be employed before discharging any acid-containing waste streams. Advanced air filtration systems are crucial in industrial settings to capture acid vapors and prevent atmospheric emissions.
Monitoring programs are vital to assess the long-term environmental effects of hydrochloric acid applications. Regular soil and water quality testing in areas surrounding industrial sites can help detect and address any contamination issues promptly. Ecological surveys can provide insights into the impact on local flora and fauna, allowing for adaptive management strategies.
Sustainable alternatives and process optimizations should be explored to reduce the overall use of hydrochloric acid where possible. This may include developing closed-loop systems that minimize waste generation or investigating less harmful substitutes for specific applications.
In conclusion, while hydrochloric acid remains an indispensable chemical in many industries, its environmental impact must be carefully managed. A comprehensive approach involving prevention, mitigation, and monitoring is essential to ensure the sustainable use of this powerful acid while safeguarding ecosystems and human health.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!