How to Facilitate Hydrochloric Acid Disposal Responsibly?
JUL 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HCl Disposal Background and Objectives
Hydrochloric acid (HCl) disposal has become a critical environmental and safety concern in various industries, including chemical manufacturing, metal processing, and pharmaceutical production. The responsible management of this corrosive substance is essential to mitigate potential hazards to human health and the environment. As industrial processes continue to evolve, the need for efficient and environmentally friendly HCl disposal methods has grown significantly.
The primary objective of this technical research report is to explore and evaluate current and emerging strategies for the responsible disposal of hydrochloric acid. We aim to identify best practices, innovative technologies, and potential areas for improvement in HCl disposal processes. This investigation will consider various aspects, including environmental impact, cost-effectiveness, regulatory compliance, and scalability of disposal methods.
Throughout the history of industrial chemistry, HCl disposal has presented numerous challenges. Early practices often involved direct discharge into water bodies or neutralization with basic substances, which proved inadequate in addressing long-term environmental concerns. As environmental awareness increased and regulations tightened, more sophisticated disposal techniques emerged, focusing on recycling, recovery, and neutralization processes.
The evolution of HCl disposal methods has been driven by a combination of factors, including stricter environmental regulations, advancements in chemical engineering, and a growing emphasis on sustainable industrial practices. Key milestones in this journey include the development of membrane-based recovery systems, the implementation of closed-loop recycling processes, and the adoption of advanced neutralization techniques using specialized reagents.
Current trends in HCl disposal technology are moving towards more sustainable and circular economy approaches. These include the development of on-site treatment systems, the integration of HCl recovery into production processes, and the exploration of novel applications for recovered HCl in other industries. Additionally, there is a growing interest in green chemistry principles that aim to reduce or eliminate the generation of HCl waste at the source.
As we look to the future, the technical goals for HCl disposal are centered around maximizing efficiency, minimizing environmental impact, and ensuring compliance with increasingly stringent regulations. This includes developing more energy-efficient recovery processes, exploring new materials for containment and treatment, and leveraging digital technologies for real-time monitoring and optimization of disposal systems.
The primary objective of this technical research report is to explore and evaluate current and emerging strategies for the responsible disposal of hydrochloric acid. We aim to identify best practices, innovative technologies, and potential areas for improvement in HCl disposal processes. This investigation will consider various aspects, including environmental impact, cost-effectiveness, regulatory compliance, and scalability of disposal methods.
Throughout the history of industrial chemistry, HCl disposal has presented numerous challenges. Early practices often involved direct discharge into water bodies or neutralization with basic substances, which proved inadequate in addressing long-term environmental concerns. As environmental awareness increased and regulations tightened, more sophisticated disposal techniques emerged, focusing on recycling, recovery, and neutralization processes.
The evolution of HCl disposal methods has been driven by a combination of factors, including stricter environmental regulations, advancements in chemical engineering, and a growing emphasis on sustainable industrial practices. Key milestones in this journey include the development of membrane-based recovery systems, the implementation of closed-loop recycling processes, and the adoption of advanced neutralization techniques using specialized reagents.
Current trends in HCl disposal technology are moving towards more sustainable and circular economy approaches. These include the development of on-site treatment systems, the integration of HCl recovery into production processes, and the exploration of novel applications for recovered HCl in other industries. Additionally, there is a growing interest in green chemistry principles that aim to reduce or eliminate the generation of HCl waste at the source.
As we look to the future, the technical goals for HCl disposal are centered around maximizing efficiency, minimizing environmental impact, and ensuring compliance with increasingly stringent regulations. This includes developing more energy-efficient recovery processes, exploring new materials for containment and treatment, and leveraging digital technologies for real-time monitoring and optimization of disposal systems.
Market Demand for Safe Acid Disposal Solutions
The market demand for safe hydrochloric acid disposal solutions has been steadily increasing due to growing environmental concerns and stricter regulations. Industries such as chemical manufacturing, metal processing, and semiconductor production generate significant amounts of hydrochloric acid waste, creating a pressing need for responsible disposal methods.
Environmental regulations, particularly in developed countries, have become more stringent regarding the handling and disposal of hazardous chemicals. This has led to a surge in demand for innovative and compliant acid disposal solutions. Companies are increasingly seeking cost-effective and environmentally friendly methods to manage their acid waste, driving the market for specialized disposal services and technologies.
The global market for hazardous waste management, including acid disposal, is experiencing robust growth. Factors contributing to this trend include rapid industrialization in emerging economies, increased awareness of environmental issues, and the implementation of more stringent waste management policies worldwide. Industries are recognizing the importance of proper acid disposal not only for regulatory compliance but also for maintaining a positive corporate image and social responsibility.
There is a growing demand for on-site treatment solutions that allow companies to neutralize or recycle hydrochloric acid waste at their facilities. This approach reduces transportation risks and costs associated with off-site disposal. Technologies such as acid neutralization systems, membrane filtration, and electrodialysis are gaining traction in various industries.
The market is also seeing increased interest in circular economy principles, with companies exploring ways to recover and reuse hydrochloric acid from waste streams. This trend is driven by both economic and environmental factors, as recycling acid can reduce raw material costs and minimize environmental impact.
Safety considerations are paramount in the acid disposal market. There is a strong demand for solutions that minimize human exposure to hazardous chemicals and reduce the risk of accidents during handling and transportation. This has led to the development of automated systems and remote monitoring technologies for acid management.
Geographically, the demand for safe acid disposal solutions is particularly high in regions with strict environmental regulations, such as North America and Europe. However, emerging economies in Asia-Pacific and Latin America are also showing significant growth potential as they adopt more stringent environmental standards and invest in industrial infrastructure.
The market is characterized by a mix of large waste management companies offering comprehensive services and specialized firms focusing on innovative acid treatment technologies. There is also a growing trend of partnerships between technology providers and waste management companies to offer integrated solutions to end-users.
Environmental regulations, particularly in developed countries, have become more stringent regarding the handling and disposal of hazardous chemicals. This has led to a surge in demand for innovative and compliant acid disposal solutions. Companies are increasingly seeking cost-effective and environmentally friendly methods to manage their acid waste, driving the market for specialized disposal services and technologies.
The global market for hazardous waste management, including acid disposal, is experiencing robust growth. Factors contributing to this trend include rapid industrialization in emerging economies, increased awareness of environmental issues, and the implementation of more stringent waste management policies worldwide. Industries are recognizing the importance of proper acid disposal not only for regulatory compliance but also for maintaining a positive corporate image and social responsibility.
There is a growing demand for on-site treatment solutions that allow companies to neutralize or recycle hydrochloric acid waste at their facilities. This approach reduces transportation risks and costs associated with off-site disposal. Technologies such as acid neutralization systems, membrane filtration, and electrodialysis are gaining traction in various industries.
The market is also seeing increased interest in circular economy principles, with companies exploring ways to recover and reuse hydrochloric acid from waste streams. This trend is driven by both economic and environmental factors, as recycling acid can reduce raw material costs and minimize environmental impact.
Safety considerations are paramount in the acid disposal market. There is a strong demand for solutions that minimize human exposure to hazardous chemicals and reduce the risk of accidents during handling and transportation. This has led to the development of automated systems and remote monitoring technologies for acid management.
Geographically, the demand for safe acid disposal solutions is particularly high in regions with strict environmental regulations, such as North America and Europe. However, emerging economies in Asia-Pacific and Latin America are also showing significant growth potential as they adopt more stringent environmental standards and invest in industrial infrastructure.
The market is characterized by a mix of large waste management companies offering comprehensive services and specialized firms focusing on innovative acid treatment technologies. There is also a growing trend of partnerships between technology providers and waste management companies to offer integrated solutions to end-users.
Current Challenges in HCl Disposal
The responsible disposal of hydrochloric acid (HCl) presents several significant challenges in today's industrial and environmental landscape. One of the primary issues is the corrosive nature of HCl, which poses risks to both human health and infrastructure. This characteristic necessitates specialized handling, storage, and transportation protocols, increasing the complexity and cost of disposal processes.
Environmental concerns also play a crucial role in HCl disposal challenges. The acid's potential to alter soil and water pH levels can have devastating effects on ecosystems if not properly managed. Regulatory bodies worldwide have implemented strict guidelines for HCl disposal, creating a complex compliance landscape that industries must navigate. These regulations often vary by region, adding another layer of difficulty for multinational corporations.
The volume of HCl waste generated by various industries presents a significant challenge. Sectors such as chemical manufacturing, metal processing, and oil refining produce large quantities of HCl as a byproduct. The sheer scale of waste generation necessitates efficient, large-scale disposal solutions that are both economically viable and environmentally sound.
Another critical challenge is the limited availability of suitable disposal facilities. Many waste management sites are not equipped to handle highly corrosive substances like HCl, leading to a shortage of disposal options. This scarcity can result in increased transportation distances and costs, as well as potential bottlenecks in the disposal process.
The neutralization of HCl before disposal is often necessary but presents its own set of challenges. The process requires precise control and monitoring to ensure complete neutralization without creating harmful byproducts. Additionally, the neutralization process itself consumes resources and may generate secondary waste streams that require further treatment.
Technological limitations also contribute to the challenges in HCl disposal. While advancements have been made in recycling and reuse technologies, these solutions are not universally applicable or economically feasible for all industries and scales of operation. The development of more efficient and cost-effective treatment technologies remains an ongoing challenge in the field.
Lastly, public perception and community concerns surrounding HCl disposal facilities can create obstacles in establishing or expanding disposal operations. The "Not In My Backyard" (NIMBY) phenomenon often leads to resistance against the siting of new disposal facilities, even when they adhere to strict safety and environmental standards. Addressing these concerns through community engagement and education is an ongoing challenge for both industry and regulatory bodies.
Environmental concerns also play a crucial role in HCl disposal challenges. The acid's potential to alter soil and water pH levels can have devastating effects on ecosystems if not properly managed. Regulatory bodies worldwide have implemented strict guidelines for HCl disposal, creating a complex compliance landscape that industries must navigate. These regulations often vary by region, adding another layer of difficulty for multinational corporations.
The volume of HCl waste generated by various industries presents a significant challenge. Sectors such as chemical manufacturing, metal processing, and oil refining produce large quantities of HCl as a byproduct. The sheer scale of waste generation necessitates efficient, large-scale disposal solutions that are both economically viable and environmentally sound.
Another critical challenge is the limited availability of suitable disposal facilities. Many waste management sites are not equipped to handle highly corrosive substances like HCl, leading to a shortage of disposal options. This scarcity can result in increased transportation distances and costs, as well as potential bottlenecks in the disposal process.
The neutralization of HCl before disposal is often necessary but presents its own set of challenges. The process requires precise control and monitoring to ensure complete neutralization without creating harmful byproducts. Additionally, the neutralization process itself consumes resources and may generate secondary waste streams that require further treatment.
Technological limitations also contribute to the challenges in HCl disposal. While advancements have been made in recycling and reuse technologies, these solutions are not universally applicable or economically feasible for all industries and scales of operation. The development of more efficient and cost-effective treatment technologies remains an ongoing challenge in the field.
Lastly, public perception and community concerns surrounding HCl disposal facilities can create obstacles in establishing or expanding disposal operations. The "Not In My Backyard" (NIMBY) phenomenon often leads to resistance against the siting of new disposal facilities, even when they adhere to strict safety and environmental standards. Addressing these concerns through community engagement and education is an ongoing challenge for both industry and regulatory bodies.
Existing HCl Neutralization Methods
01 Neutralization and treatment of hydrochloric acid
Hydrochloric acid can be neutralized using alkaline substances such as lime or sodium hydroxide. The resulting solution can then be treated further to remove any remaining contaminants before disposal. This process helps to reduce the acidity and make the waste safer for disposal.- Neutralization and treatment of hydrochloric acid: Hydrochloric acid can be neutralized using alkaline substances such as lime or sodium hydroxide. The resulting neutralized solution can then be further treated or disposed of safely. This process helps to reduce the acidity and harmful effects of the acid before disposal.
- Recycling and recovery of hydrochloric acid: Methods for recycling and recovering hydrochloric acid from waste streams or industrial processes. This can involve techniques such as distillation, membrane separation, or chemical reactions to purify and concentrate the acid for reuse, reducing the need for disposal.
- Specialized equipment for handling and disposal: Use of specialized equipment and systems designed for the safe handling, storage, and disposal of hydrochloric acid. This may include corrosion-resistant containers, automated dispensing systems, and specialized waste treatment facilities to ensure safe and efficient disposal.
- Conversion of hydrochloric acid into useful products: Processes for converting hydrochloric acid into useful products or less harmful substances. This can involve chemical reactions to produce chlorine gas, metal chlorides, or other valuable chemicals, effectively repurposing the acid rather than disposing of it as waste.
- Environmental considerations in disposal: Methods and systems that focus on minimizing the environmental impact of hydrochloric acid disposal. This includes techniques for reducing emissions, treating wastewater, and ensuring compliance with environmental regulations during the disposal process.
02 Recycling and reuse of hydrochloric acid
Instead of disposal, hydrochloric acid can be recycled and reused in various industrial processes. This approach involves purification techniques to remove impurities and concentration adjustments to meet the required specifications for reuse. Recycling reduces waste and minimizes environmental impact.Expand Specific Solutions03 Conversion of hydrochloric acid into useful products
Hydrochloric acid can be converted into useful products such as metal chlorides or hydrogen gas. This process not only disposes of the acid but also creates valuable materials for industrial use. The conversion process often involves chemical reactions with specific metals or compounds.Expand Specific Solutions04 Specialized equipment for hydrochloric acid disposal
Specialized equipment and systems have been developed for the safe handling and disposal of hydrochloric acid. These may include corrosion-resistant storage tanks, neutralization chambers, and automated disposal systems that ensure proper treatment and safe release of the processed waste.Expand Specific Solutions05 Environmental considerations in hydrochloric acid disposal
Proper disposal of hydrochloric acid must consider environmental impacts. This includes monitoring and controlling emissions, ensuring proper containment to prevent soil and water contamination, and adhering to local and international regulations for hazardous waste disposal. Advanced treatment methods may be employed to minimize environmental risks.Expand Specific Solutions
Key Players in Chemical Waste Management
The responsible disposal of hydrochloric acid presents a competitive landscape in a mature industry with established players. The market size is significant, driven by industrial demand and environmental regulations. Technologically, the field is well-developed, with companies like Arkema France SA, Covestro Deutschland AG, and LG Chem Ltd. offering advanced solutions. These firms, along with others such as Wacker Chemie AG and DuPont de Nemours, Inc., have invested in research and development to improve disposal methods, focusing on safety, efficiency, and environmental impact. The competition is characterized by a mix of global chemical conglomerates and specialized environmental technology firms, each striving to innovate and capture market share in this critical area of industrial waste management.
LG Chem Ltd.
Technical Solution: LG Chem has implemented a comprehensive hydrochloric acid management system in their production facilities. Their approach focuses on recycling and reuse within the manufacturing process, significantly reducing the need for disposal. The company employs a closed-loop system where HCl is recovered from waste streams and purified for reuse in various applications, such as chlor-alkali production[3]. Additionally, LG Chem has invested in advanced membrane technology for HCl recovery, which allows for the separation of HCl from other components in waste streams with high efficiency and purity[4]. For any residual acid that cannot be recycled, the company utilizes a neutralization process with careful pH control to ensure safe disposal.
Strengths: Minimizes waste through recycling and reuse, reduces raw material costs, and lowers environmental impact. Weaknesses: Requires sophisticated infrastructure and may not be suitable for smaller-scale operations.
AGC, Inc. (Japan)
Technical Solution: AGC has developed an innovative approach to hydrochloric acid disposal and recycling in their glass manufacturing processes. The company utilizes a proprietary ion exchange resin technology to selectively remove and concentrate HCl from waste acid streams[9]. This process allows for the recovery of high-purity HCl that can be reused in various applications within the plant or sold to other industries. For residual acid that cannot be recycled, AGC employs a two-stage neutralization process. The first stage uses limestone to neutralize the bulk of the acid, followed by a fine-tuning stage with sodium hydroxide to achieve precise pH control. The resulting salt solution is then treated in a zero-liquid discharge (ZLD) system, which evaporates the water and produces a dry salt cake for safe disposal or potential use as a raw material in other industries[10].
Strengths: High-purity acid recovery, zero-liquid discharge capability, and potential for by-product valorization. Weaknesses: High energy consumption for the ZLD process and potential scaling issues with ion exchange resins.
Innovative HCl Treatment Technologies
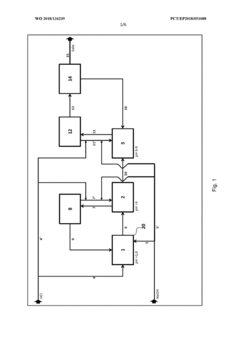
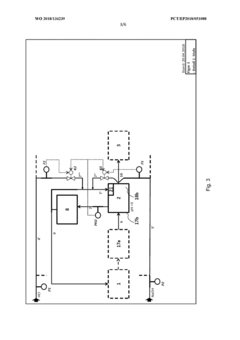
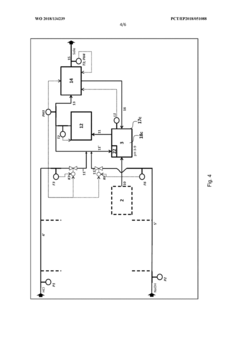
Method for flexibly controlling the use of hydrochloric acid from chemical production
PatentWO2018134239A1
Innovation
- A flexible control process for hydrochloric acid management involves neutralizing hydrochloric acid with concentrated alkali, specifically sodium hydroxide, in a multi-stage continuous process that adjusts pH values and compensates for flow and concentration variations, allowing for efficient handling and recycling of hydrochloric acid even when traditional acceptance points are unavailable.
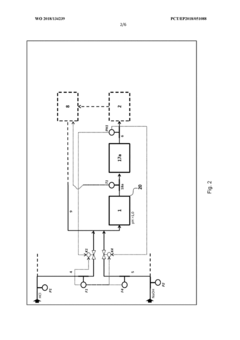
Method for absorbing chlorine from gas streams
PatentWO2009138401A1
Innovation
- A continuous process using controlled addition of hydrogen peroxide in water to suppress hypochlorous acid formation, allowing chlorine to react and form hydrochloric acid, which is then bound in the absorption medium, eliminating salt formation and enabling the production of usable hydrochloric acid.
Environmental Impact Assessment
The responsible disposal of hydrochloric acid requires a comprehensive environmental impact assessment to ensure minimal harm to ecosystems and human health. This assessment begins with an analysis of the acid's potential effects on soil and water systems. When improperly disposed of, hydrochloric acid can significantly alter soil pH, leading to decreased fertility and potential contamination of groundwater. In aquatic environments, even small quantities can cause severe disruptions to the ecosystem balance, affecting fish populations and other aquatic organisms.
Air quality is another critical consideration in the environmental impact assessment. While hydrochloric acid is not highly volatile, improper handling during disposal can lead to the release of acidic fumes. These emissions can contribute to acid rain formation, potentially impacting vegetation and infrastructure over a wider area. The assessment must also consider the potential for accidental releases during transportation and disposal processes, which could pose immediate risks to both environmental and human health.
Long-term ecological effects are a key focus of the environmental impact assessment. Chronic exposure to even low levels of hydrochloric acid can lead to cumulative damage in ecosystems, affecting biodiversity and ecological stability. This includes potential bioaccumulation in food chains and alterations to soil microbial communities, which play crucial roles in nutrient cycling and overall ecosystem health.
The assessment also evaluates the impact on local water treatment facilities. Improper disposal through sewage systems can overwhelm treatment plants, potentially leading to the release of inadequately treated wastewater into the environment. This not only poses risks to aquatic ecosystems but also to human health if it affects drinking water sources.
Human health considerations form a significant part of the environmental impact assessment. Direct exposure risks to workers involved in the disposal process must be thoroughly evaluated, including potential for skin burns, respiratory issues, and long-term health effects from chronic low-level exposure. The assessment also considers potential impacts on nearby communities, particularly in the event of accidental releases or improper disposal practices.
Lastly, the environmental impact assessment must consider the broader implications of hydrochloric acid disposal on climate change and global environmental issues. This includes evaluating the carbon footprint of different disposal methods and their potential contributions to greenhouse gas emissions. The assessment should also consider the lifecycle impact of hydrochloric acid, from production to disposal, to provide a comprehensive understanding of its environmental footprint.
Air quality is another critical consideration in the environmental impact assessment. While hydrochloric acid is not highly volatile, improper handling during disposal can lead to the release of acidic fumes. These emissions can contribute to acid rain formation, potentially impacting vegetation and infrastructure over a wider area. The assessment must also consider the potential for accidental releases during transportation and disposal processes, which could pose immediate risks to both environmental and human health.
Long-term ecological effects are a key focus of the environmental impact assessment. Chronic exposure to even low levels of hydrochloric acid can lead to cumulative damage in ecosystems, affecting biodiversity and ecological stability. This includes potential bioaccumulation in food chains and alterations to soil microbial communities, which play crucial roles in nutrient cycling and overall ecosystem health.
The assessment also evaluates the impact on local water treatment facilities. Improper disposal through sewage systems can overwhelm treatment plants, potentially leading to the release of inadequately treated wastewater into the environment. This not only poses risks to aquatic ecosystems but also to human health if it affects drinking water sources.
Human health considerations form a significant part of the environmental impact assessment. Direct exposure risks to workers involved in the disposal process must be thoroughly evaluated, including potential for skin burns, respiratory issues, and long-term health effects from chronic low-level exposure. The assessment also considers potential impacts on nearby communities, particularly in the event of accidental releases or improper disposal practices.
Lastly, the environmental impact assessment must consider the broader implications of hydrochloric acid disposal on climate change and global environmental issues. This includes evaluating the carbon footprint of different disposal methods and their potential contributions to greenhouse gas emissions. The assessment should also consider the lifecycle impact of hydrochloric acid, from production to disposal, to provide a comprehensive understanding of its environmental footprint.
Regulatory Framework for HCl Disposal
The regulatory framework for hydrochloric acid (HCl) disposal is a complex and multifaceted system designed to ensure the safe and responsible management of this hazardous substance. At the international level, the Basel Convention on the Control of Transboundary Movements of Hazardous Wastes and Their Disposal provides guidelines for the global management of hazardous wastes, including HCl. This convention establishes principles for environmentally sound management and restricts the movement of hazardous wastes across international borders.
In the United States, the Environmental Protection Agency (EPA) plays a crucial role in regulating HCl disposal through various legislative acts. The Resource Conservation and Recovery Act (RCRA) is the primary federal law governing the disposal of hazardous waste. Under RCRA, HCl is classified as a characteristic hazardous waste due to its corrosivity. This classification mandates specific handling, storage, and disposal procedures for generators, transporters, and treatment, storage, and disposal facilities.
The Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA), also known as Superfund, addresses the cleanup of hazardous waste sites and spills, including those involving HCl. This act establishes liability for responsible parties and provides a framework for site remediation.
At the state level, regulations may be more stringent than federal standards. Many states have their own environmental protection agencies that oversee HCl disposal within their jurisdictions. These agencies often require permits for facilities handling HCl and may impose additional requirements for treatment and disposal.
The Occupational Safety and Health Administration (OSHA) also plays a role in the regulatory framework by setting standards for worker safety in environments where HCl is present. These standards include requirements for personal protective equipment, handling procedures, and emergency response plans.
In the European Union, the regulation of HCl disposal falls under the broader framework of chemical management established by REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals). This regulation requires manufacturers and importers to register chemicals and provide safety information. The Classification, Labeling, and Packaging (CLP) Regulation complements REACH by ensuring that the hazards of chemicals are clearly communicated to workers and consumers.
Compliance with these regulations typically involves proper labeling, secure containment, neutralization processes, and documentation of disposal activities. Facilities handling HCl must often implement waste minimization strategies, maintain detailed records of waste generation and disposal, and undergo regular inspections to ensure adherence to regulatory standards.
As environmental concerns continue to grow, the regulatory framework for HCl disposal is likely to evolve. Emerging trends include increased focus on circular economy principles, which may lead to more emphasis on recycling and reuse of HCl where possible, as well as stricter emissions controls and more comprehensive lifecycle management approaches.
In the United States, the Environmental Protection Agency (EPA) plays a crucial role in regulating HCl disposal through various legislative acts. The Resource Conservation and Recovery Act (RCRA) is the primary federal law governing the disposal of hazardous waste. Under RCRA, HCl is classified as a characteristic hazardous waste due to its corrosivity. This classification mandates specific handling, storage, and disposal procedures for generators, transporters, and treatment, storage, and disposal facilities.
The Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA), also known as Superfund, addresses the cleanup of hazardous waste sites and spills, including those involving HCl. This act establishes liability for responsible parties and provides a framework for site remediation.
At the state level, regulations may be more stringent than federal standards. Many states have their own environmental protection agencies that oversee HCl disposal within their jurisdictions. These agencies often require permits for facilities handling HCl and may impose additional requirements for treatment and disposal.
The Occupational Safety and Health Administration (OSHA) also plays a role in the regulatory framework by setting standards for worker safety in environments where HCl is present. These standards include requirements for personal protective equipment, handling procedures, and emergency response plans.
In the European Union, the regulation of HCl disposal falls under the broader framework of chemical management established by REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals). This regulation requires manufacturers and importers to register chemicals and provide safety information. The Classification, Labeling, and Packaging (CLP) Regulation complements REACH by ensuring that the hazards of chemicals are clearly communicated to workers and consumers.
Compliance with these regulations typically involves proper labeling, secure containment, neutralization processes, and documentation of disposal activities. Facilities handling HCl must often implement waste minimization strategies, maintain detailed records of waste generation and disposal, and undergo regular inspections to ensure adherence to regulatory standards.
As environmental concerns continue to grow, the regulatory framework for HCl disposal is likely to evolve. Emerging trends include increased focus on circular economy principles, which may lead to more emphasis on recycling and reuse of HCl where possible, as well as stricter emissions controls and more comprehensive lifecycle management approaches.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!