How to Improve Electrochemical Cell Conductivity with Electrolytes
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electrochemical Cell Conductivity Enhancement Background
Electrochemical cells have been fundamental components in energy storage and conversion systems since their inception in the late 18th century with Alessandro Volta's pioneering work. These cells operate on the principle of converting chemical energy into electrical energy through redox reactions occurring at the electrodes. The conductivity of these cells, a critical parameter determining their efficiency and performance, has been a subject of continuous research and development over the decades.
The evolution of electrochemical cell technology has seen significant advancements, particularly in the understanding and enhancement of ionic conductivity mechanisms. Early systems relied on simple aqueous electrolytes, while modern applications employ sophisticated electrolyte formulations tailored for specific operational requirements. This technological progression has been driven by the growing demand for high-performance energy storage solutions across various sectors including automotive, consumer electronics, and renewable energy systems.
Electrolytes, as ion-conducting media between electrodes, play a pivotal role in determining cell conductivity. They facilitate the movement of ions between the anode and cathode, completing the electrical circuit and enabling energy conversion processes. The conductivity of an electrolyte is influenced by multiple factors including ion concentration, mobility, temperature, and the nature of the solvent or matrix in which ions are dispersed.
Traditional liquid electrolytes, while offering high ionic conductivity, present challenges related to safety, stability, and operational temperature range. This has led to the exploration of alternative electrolyte systems such as solid-state electrolytes, polymer electrolytes, and ionic liquids, each with unique conductivity characteristics and application potentials.
Recent research has focused on understanding the fundamental mechanisms of ion transport at the molecular level, employing advanced computational models and experimental techniques. This deeper understanding has enabled the development of novel electrolyte compositions with enhanced conductivity properties, addressing specific limitations of conventional systems.
The pursuit of improved electrochemical cell conductivity is driven by several technological imperatives: the need for faster charging capabilities in portable devices, extended operational range in electric vehicles, enhanced safety in energy storage systems, and improved efficiency in grid-scale energy storage solutions. These requirements have catalyzed innovation in electrolyte design, electrode materials, and cell architectures.
As we advance into an era increasingly dependent on efficient energy storage and conversion technologies, the enhancement of electrochemical cell conductivity through electrolyte optimization remains a critical research frontier with far-reaching implications for sustainable energy solutions and technological advancement.
The evolution of electrochemical cell technology has seen significant advancements, particularly in the understanding and enhancement of ionic conductivity mechanisms. Early systems relied on simple aqueous electrolytes, while modern applications employ sophisticated electrolyte formulations tailored for specific operational requirements. This technological progression has been driven by the growing demand for high-performance energy storage solutions across various sectors including automotive, consumer electronics, and renewable energy systems.
Electrolytes, as ion-conducting media between electrodes, play a pivotal role in determining cell conductivity. They facilitate the movement of ions between the anode and cathode, completing the electrical circuit and enabling energy conversion processes. The conductivity of an electrolyte is influenced by multiple factors including ion concentration, mobility, temperature, and the nature of the solvent or matrix in which ions are dispersed.
Traditional liquid electrolytes, while offering high ionic conductivity, present challenges related to safety, stability, and operational temperature range. This has led to the exploration of alternative electrolyte systems such as solid-state electrolytes, polymer electrolytes, and ionic liquids, each with unique conductivity characteristics and application potentials.
Recent research has focused on understanding the fundamental mechanisms of ion transport at the molecular level, employing advanced computational models and experimental techniques. This deeper understanding has enabled the development of novel electrolyte compositions with enhanced conductivity properties, addressing specific limitations of conventional systems.
The pursuit of improved electrochemical cell conductivity is driven by several technological imperatives: the need for faster charging capabilities in portable devices, extended operational range in electric vehicles, enhanced safety in energy storage systems, and improved efficiency in grid-scale energy storage solutions. These requirements have catalyzed innovation in electrolyte design, electrode materials, and cell architectures.
As we advance into an era increasingly dependent on efficient energy storage and conversion technologies, the enhancement of electrochemical cell conductivity through electrolyte optimization remains a critical research frontier with far-reaching implications for sustainable energy solutions and technological advancement.
Electrolyte Market Demand Analysis
The global electrolyte market has experienced significant growth in recent years, primarily driven by the expanding electric vehicle (EV) sector and portable electronics industry. Market research indicates that the electrolyte market was valued at approximately $6.5 billion in 2022 and is projected to reach $12.9 billion by 2028, growing at a CAGR of 12.1% during the forecast period.
The automotive sector represents the largest demand segment for advanced electrolytes, accounting for nearly 45% of the total market share. This is primarily attributed to the accelerating adoption of electric vehicles worldwide, with global EV sales surpassing 10 million units in 2022. Major automotive manufacturers have announced ambitious electrification plans, with companies like Volkswagen, GM, and Ford committing billions to EV development, further driving demand for high-performance electrolytes.
Consumer electronics constitutes the second-largest market segment, representing approximately 30% of electrolyte demand. The continuous innovation in smartphones, laptops, and wearable devices necessitates electrolytes with enhanced conductivity properties to support longer battery life and faster charging capabilities.
Geographically, Asia-Pacific dominates the electrolyte market with over 60% share, led by China, Japan, and South Korea. These countries host major battery manufacturers and have established robust supply chains for electrolyte production. North America and Europe are experiencing rapid growth rates of 15% and 17% respectively, driven by increasing investments in domestic battery production capabilities.
Market analysis reveals a growing demand for electrolytes with specific performance characteristics: higher ionic conductivity (>10 mS/cm), wider electrochemical stability windows (>4.5V), improved thermal stability, and enhanced safety profiles. Customers are increasingly willing to pay premium prices for electrolytes that can deliver these performance improvements, with price elasticity studies showing that a 20% improvement in conductivity can command up to 35% price premium in high-end applications.
The renewable energy storage sector represents an emerging market opportunity, with grid-scale energy storage installations growing at 32% annually. This application requires electrolytes with exceptional long-term stability and cost-effectiveness, creating a distinct market segment with specialized requirements.
Industry forecasts indicate that demand for advanced electrolytes will outpace supply by approximately 18% through 2025, creating significant market opportunities for companies that can develop innovative electrolyte solutions with superior conductivity properties. This supply-demand gap is particularly pronounced in the high-performance segment required for next-generation electric vehicles and advanced energy storage systems.
The automotive sector represents the largest demand segment for advanced electrolytes, accounting for nearly 45% of the total market share. This is primarily attributed to the accelerating adoption of electric vehicles worldwide, with global EV sales surpassing 10 million units in 2022. Major automotive manufacturers have announced ambitious electrification plans, with companies like Volkswagen, GM, and Ford committing billions to EV development, further driving demand for high-performance electrolytes.
Consumer electronics constitutes the second-largest market segment, representing approximately 30% of electrolyte demand. The continuous innovation in smartphones, laptops, and wearable devices necessitates electrolytes with enhanced conductivity properties to support longer battery life and faster charging capabilities.
Geographically, Asia-Pacific dominates the electrolyte market with over 60% share, led by China, Japan, and South Korea. These countries host major battery manufacturers and have established robust supply chains for electrolyte production. North America and Europe are experiencing rapid growth rates of 15% and 17% respectively, driven by increasing investments in domestic battery production capabilities.
Market analysis reveals a growing demand for electrolytes with specific performance characteristics: higher ionic conductivity (>10 mS/cm), wider electrochemical stability windows (>4.5V), improved thermal stability, and enhanced safety profiles. Customers are increasingly willing to pay premium prices for electrolytes that can deliver these performance improvements, with price elasticity studies showing that a 20% improvement in conductivity can command up to 35% price premium in high-end applications.
The renewable energy storage sector represents an emerging market opportunity, with grid-scale energy storage installations growing at 32% annually. This application requires electrolytes with exceptional long-term stability and cost-effectiveness, creating a distinct market segment with specialized requirements.
Industry forecasts indicate that demand for advanced electrolytes will outpace supply by approximately 18% through 2025, creating significant market opportunities for companies that can develop innovative electrolyte solutions with superior conductivity properties. This supply-demand gap is particularly pronounced in the high-performance segment required for next-generation electric vehicles and advanced energy storage systems.
Current Electrolyte Technology Challenges
Despite significant advancements in electrochemical cell technology, current electrolyte systems face several critical challenges that limit overall cell conductivity and performance. The primary obstacle remains the inherent trade-off between ionic conductivity and electrochemical stability. Conventional liquid electrolytes offering high conductivity typically suffer from narrow electrochemical windows, while more stable alternatives often demonstrate insufficient ionic transport properties.
Thermal stability presents another significant challenge, particularly in high-power applications where heat generation can trigger electrolyte degradation. Most commercial electrolytes experience performance deterioration at temperature extremes, with some becoming unstable above 60°C or losing functionality below -20°C, severely limiting their practical deployment in diverse environmental conditions.
Interface resistance between electrolytes and electrodes continues to impede efficient ion transfer. The formation of solid-electrolyte interphase (SEI) layers, while necessary for cell stability, often increases internal resistance and reduces conductivity over time. Current electrolyte formulations struggle to form optimal SEI layers that balance protection and conductivity requirements.
Concentration polarization during high-rate operation represents another substantial challenge. As ions are depleted near electrode surfaces during rapid charging or discharging, concentration gradients form within the electrolyte, significantly reducing effective conductivity and limiting power capabilities. Existing electrolyte systems lack sufficient mechanisms to mitigate these concentration gradients.
Safety concerns further complicate electrolyte development, as many high-conductivity formulations incorporate flammable organic solvents. The industry continues to search for non-flammable alternatives that maintain comparable conductivity metrics without compromising performance or increasing costs prohibitively.
Manufacturing scalability and cost factors present additional hurdles. Advanced electrolyte systems with superior conductivity properties often require complex synthesis procedures or expensive components, limiting their commercial viability. The challenge of developing high-performance electrolytes that remain economically competitive has slowed widespread adoption of novel formulations.
Environmental and regulatory considerations are increasingly influencing electrolyte development. Many traditional electrolyte components face scrutiny due to toxicity concerns or environmental persistence. Finding green alternatives that maintain or improve conductivity performance represents a growing challenge for researchers and manufacturers alike.
Lastly, long-term stability remains problematic, with many electrolytes experiencing conductivity degradation through mechanisms including solvent evaporation, salt decomposition, and impurity accumulation. Current technologies struggle to maintain consistent conductivity performance throughout the desired operational lifetime of electrochemical cells.
Thermal stability presents another significant challenge, particularly in high-power applications where heat generation can trigger electrolyte degradation. Most commercial electrolytes experience performance deterioration at temperature extremes, with some becoming unstable above 60°C or losing functionality below -20°C, severely limiting their practical deployment in diverse environmental conditions.
Interface resistance between electrolytes and electrodes continues to impede efficient ion transfer. The formation of solid-electrolyte interphase (SEI) layers, while necessary for cell stability, often increases internal resistance and reduces conductivity over time. Current electrolyte formulations struggle to form optimal SEI layers that balance protection and conductivity requirements.
Concentration polarization during high-rate operation represents another substantial challenge. As ions are depleted near electrode surfaces during rapid charging or discharging, concentration gradients form within the electrolyte, significantly reducing effective conductivity and limiting power capabilities. Existing electrolyte systems lack sufficient mechanisms to mitigate these concentration gradients.
Safety concerns further complicate electrolyte development, as many high-conductivity formulations incorporate flammable organic solvents. The industry continues to search for non-flammable alternatives that maintain comparable conductivity metrics without compromising performance or increasing costs prohibitively.
Manufacturing scalability and cost factors present additional hurdles. Advanced electrolyte systems with superior conductivity properties often require complex synthesis procedures or expensive components, limiting their commercial viability. The challenge of developing high-performance electrolytes that remain economically competitive has slowed widespread adoption of novel formulations.
Environmental and regulatory considerations are increasingly influencing electrolyte development. Many traditional electrolyte components face scrutiny due to toxicity concerns or environmental persistence. Finding green alternatives that maintain or improve conductivity performance represents a growing challenge for researchers and manufacturers alike.
Lastly, long-term stability remains problematic, with many electrolytes experiencing conductivity degradation through mechanisms including solvent evaporation, salt decomposition, and impurity accumulation. Current technologies struggle to maintain consistent conductivity performance throughout the desired operational lifetime of electrochemical cells.
Current Conductivity Enhancement Solutions
01 Electrolyte compositions for improved conductivity
Various electrolyte compositions can be formulated to enhance ionic conductivity in electrochemical systems. These compositions typically include specific salts, solvents, and additives that work synergistically to facilitate ion transport. The selection of appropriate electrolyte components can significantly impact the overall conductivity and performance of batteries, fuel cells, and other electrochemical devices.- Electrolyte compositions for improved conductivity: Various electrolyte compositions can be formulated to enhance ionic conductivity in electrochemical systems. These compositions typically include specific salts, solvents, and additives that work synergistically to facilitate ion transport. Optimized electrolyte formulations can significantly improve the performance of batteries, fuel cells, and other electrochemical devices by reducing internal resistance and enhancing power output.
- Measurement and testing of electrolyte conductivity: Various methods and devices have been developed for measuring and testing the conductivity of electrolytes. These include specialized sensors, probes, and analytical instruments that can accurately determine ionic conductivity under different conditions. Such measurements are crucial for quality control in manufacturing processes, monitoring the state of health of batteries, and optimizing electrolyte formulations for specific applications.
- Polymer electrolytes with enhanced conductivity: Polymer-based electrolytes offer advantages in terms of safety and flexibility compared to liquid electrolytes. Research focuses on enhancing the ionic conductivity of these materials through various approaches, including the incorporation of plasticizers, nanofillers, and specialized polymer architectures. These polymer electrolytes find applications in solid-state batteries, electrochromic devices, and other advanced electrochemical systems.
- Temperature effects on electrolyte conductivity: The conductivity of electrolytes is significantly influenced by temperature variations. Understanding these temperature dependencies is crucial for designing electrochemical systems that operate reliably across different environmental conditions. Various approaches have been developed to stabilize conductivity across temperature ranges or to exploit temperature effects for specific applications in energy storage and conversion technologies.
- Additives for enhancing electrolyte conductivity: Specific additives can be incorporated into electrolyte formulations to enhance ionic conductivity. These include ionic liquids, conductive nanoparticles, and specialized organic compounds that can modify the solvation structure or reduce ion pairing. The strategic use of additives allows for tailored electrolyte properties that meet the requirements of specific electrochemical applications while addressing challenges related to stability, safety, and performance.
02 Measurement techniques for electrolyte conductivity
Different methods and devices have been developed to accurately measure the conductivity of electrolytes. These techniques include impedance spectroscopy, conductivity cells with specific electrode configurations, and specialized sensors that can provide real-time monitoring of electrolyte properties. Advanced measurement systems often incorporate temperature compensation and calibration features to ensure precise conductivity readings across various operating conditions.Expand Specific Solutions03 Polymer electrolytes with enhanced conductivity
Polymer-based electrolytes offer advantages in terms of mechanical stability and safety compared to liquid electrolytes. Research has focused on improving the ionic conductivity of these materials through various approaches, including the incorporation of plasticizers, nanofillers, and cross-linking agents. Polymer electrolytes with optimized compositions can achieve conductivity levels suitable for practical applications while maintaining their structural integrity.Expand Specific Solutions04 Solid-state electrolytes for high-temperature applications
Solid-state electrolytes designed for high-temperature operations require specific material compositions to maintain conductivity under extreme conditions. Ceramic-based electrolytes, glass-ceramic composites, and specialized inorganic compounds have been developed to provide stable ionic transport at elevated temperatures. These materials often contain specific dopants that create oxygen vacancies or other defects that facilitate ion movement through the crystal lattice.Expand Specific Solutions05 Electrolyte additives for conductivity enhancement
Various additives can be incorporated into electrolyte systems to improve their conductivity properties. These include ionic liquids, conductivity enhancers, and stabilizing agents that modify the solvation structure around charge carriers. Some additives work by reducing ion pairing, while others create additional pathways for ion transport. The careful selection of additives can lead to significant improvements in electrolyte performance without compromising other important properties such as electrochemical stability.Expand Specific Solutions
Leading Electrolyte Manufacturers and Research Institutions
The electrochemical cell conductivity enhancement market is currently in a growth phase, with increasing demand driven by electric vehicle adoption and energy storage applications. The market size is projected to reach significant scale as battery technology becomes critical for global energy transition. Leading players demonstrate varying levels of technical maturity: established manufacturers like LG Energy Solution, BASF, and Panasonic hold advanced electrolyte formulations, while innovative companies such as Sion Power, EnPower, and Form Energy are developing breakthrough technologies with novel electrolyte systems. Research institutions including Kyushu University and CNRS collaborate with industrial partners like Bosch and BMW to bridge fundamental science with commercial applications. The competitive landscape features both traditional chemical companies and specialized battery technology firms working to overcome conductivity limitations through advanced electrolyte engineering.
LG Energy Solution Ltd.
Technical Solution: LG Energy Solution has developed advanced composite electrolytes that combine conventional liquid electrolytes with ceramic fillers to enhance ionic conductivity. Their proprietary technology incorporates nano-sized ceramic particles (such as Li7La3Zr2O12 or LLZO) uniformly dispersed within organic liquid electrolytes, creating pathways for improved lithium-ion transport. The company has also pioneered the use of high-concentration electrolytes with optimized salt concentrations (>3M) and solvent mixtures that minimize unwanted side reactions while maintaining high ionic conductivity. Their recent innovations include fluorinated electrolyte additives that form stable solid electrolyte interphase (SEI) layers, effectively preventing continuous electrolyte decomposition while facilitating ion transport at the electrode-electrolyte interface[1][3]. LG's approach also includes temperature-responsive electrolyte systems that maintain conductivity across wide operating ranges (-30°C to 60°C).
Strengths: Superior thermal stability and wide operating temperature range; excellent compatibility with high-voltage cathode materials; enhanced safety through reduced flammability. Weaknesses: Higher production costs compared to standard electrolytes; potential for increased viscosity affecting low-temperature performance; requires specialized manufacturing processes for uniform ceramic dispersion.
BASF Corp.
Technical Solution: BASF has developed a comprehensive electrolyte enhancement strategy centered on their "HiTEC" (High-performance Tailored Electrolyte Components) platform. This approach focuses on custom-designed electrolyte salts and solvents that significantly improve ionic conductivity while addressing stability issues. Their proprietary lithium bis(fluorosulfonyl)imide (LiFSI) and lithium difluoro(oxalato)borate (LiDFOB) salts demonstrate superior conductivity compared to conventional LiPF6, particularly at lower temperatures. BASF's electrolyte formulations incorporate multi-functional additives that simultaneously enhance conductivity and form protective interfaces on electrodes. Their recent breakthrough involves using ether-based solvents with optimized molecular structures that coordinate effectively with lithium ions while maintaining low viscosity, resulting in conductivity improvements of up to 40% compared to carbonate-based systems[2][5]. Additionally, BASF has pioneered ionic liquid-based electrolytes that offer non-flammability alongside high ionic conductivity.
Strengths: Exceptional low-temperature performance; reduced interfacial resistance leading to improved power capabilities; enhanced cycle life through superior chemical stability. Weaknesses: Higher cost structure compared to traditional electrolyte systems; some formulations may have compatibility issues with certain electrode materials; potential for increased gas generation during long-term cycling.
Key Electrolyte Innovation Patents
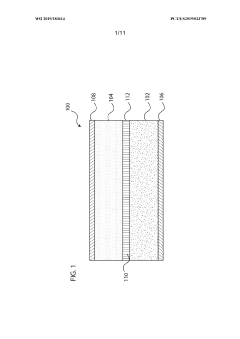
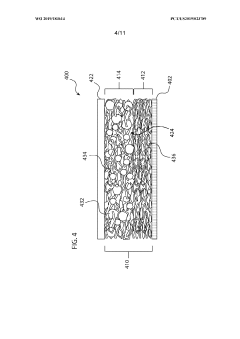
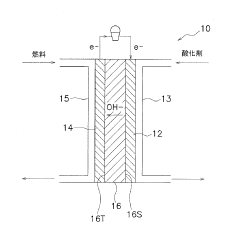
Electrochemical cells having improved ionic conductivity
PatentWO2019183614A1
Innovation
- The implementation of a multilayer electrode structure with a first layer adjacent to the current collector and a second layer farther from the separator, where the second layer includes non-active ceramic particles with higher hardness, allowing for differential compression during calendering to create a favorable porosity profile that enhances ionic conductivity.
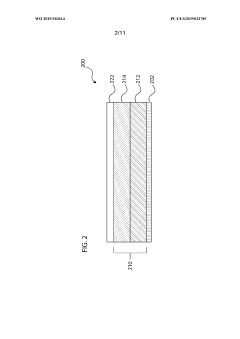
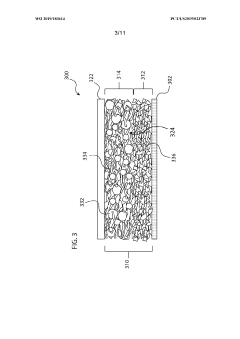
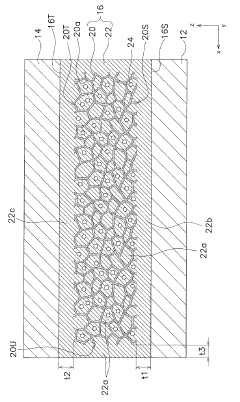
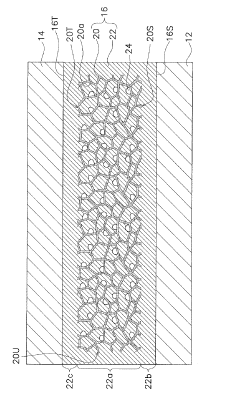
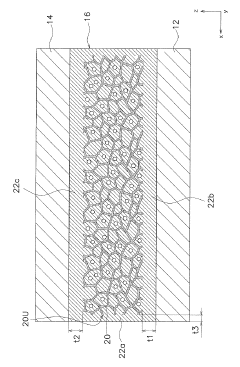
Electrolyte for electrochemical cell, and electrochemical cell
PatentInactiveJP2019220461A
Innovation
- The electrolyte comprises a porous substrate with an ionic conductor covering its sides and membrane-like parts on major surfaces, utilizing a three-dimensional network structure and inorganic solid electrolyte body with closed pores to enhance ionic conductivity and stability.
Safety Standards for Advanced Electrolytes
The development of advanced electrolytes for improving electrochemical cell conductivity necessitates comprehensive safety standards to mitigate potential hazards. Current regulatory frameworks, including IEC 62133 and UL 1642, establish baseline requirements for lithium-ion batteries but require updates to address emerging electrolyte technologies specifically.
Safety standards for advanced electrolytes must address thermal stability concerns, as many high-conductivity formulations exhibit lower thermal decomposition thresholds. Testing protocols should include accelerated thermal runaway assessments, differential scanning calorimetry (DSC) analysis, and isothermal calorimetry to quantify heat generation during normal and abuse conditions.
Chemical compatibility represents another critical safety dimension. Advanced electrolytes often incorporate novel salts, solvents, and additives that may react unpredictably with electrode materials or separator components. Standardized compatibility testing across operational temperature ranges (-40°C to 80°C) should be mandatory, with particular attention to gas generation and interface stability during extended cycling.
Toxicity and environmental impact considerations must be integrated into safety standards for next-generation electrolytes. Many high-conductivity formulations contain fluorinated compounds or organic solvents with significant health and environmental hazards. Standards should require comprehensive toxicological assessments, including acute toxicity, chronic exposure limits, and environmental persistence evaluations.
Transportation safety represents a particular challenge for advanced electrolytes. UN 38.3 testing protocols should be expanded to address the unique properties of novel electrolyte systems, including pressure accumulation tests, vibration resistance under various state-of-charge conditions, and leakage assessments under simulated transportation scenarios.
Manufacturing safety standards must evolve to address production-specific hazards associated with advanced electrolytes. This includes specifications for handling equipment, ventilation requirements, personal protective equipment, and emergency response protocols tailored to the chemical properties of high-conductivity electrolyte components.
Implementation of these safety standards requires international harmonization efforts. Organizations including ISO, IEC, and ASTM should collaborate to develop consistent testing methodologies and acceptance criteria that can be universally applied across different markets and applications, facilitating global adoption of safer advanced electrolyte technologies while maintaining innovation momentum in the field.
Safety standards for advanced electrolytes must address thermal stability concerns, as many high-conductivity formulations exhibit lower thermal decomposition thresholds. Testing protocols should include accelerated thermal runaway assessments, differential scanning calorimetry (DSC) analysis, and isothermal calorimetry to quantify heat generation during normal and abuse conditions.
Chemical compatibility represents another critical safety dimension. Advanced electrolytes often incorporate novel salts, solvents, and additives that may react unpredictably with electrode materials or separator components. Standardized compatibility testing across operational temperature ranges (-40°C to 80°C) should be mandatory, with particular attention to gas generation and interface stability during extended cycling.
Toxicity and environmental impact considerations must be integrated into safety standards for next-generation electrolytes. Many high-conductivity formulations contain fluorinated compounds or organic solvents with significant health and environmental hazards. Standards should require comprehensive toxicological assessments, including acute toxicity, chronic exposure limits, and environmental persistence evaluations.
Transportation safety represents a particular challenge for advanced electrolytes. UN 38.3 testing protocols should be expanded to address the unique properties of novel electrolyte systems, including pressure accumulation tests, vibration resistance under various state-of-charge conditions, and leakage assessments under simulated transportation scenarios.
Manufacturing safety standards must evolve to address production-specific hazards associated with advanced electrolytes. This includes specifications for handling equipment, ventilation requirements, personal protective equipment, and emergency response protocols tailored to the chemical properties of high-conductivity electrolyte components.
Implementation of these safety standards requires international harmonization efforts. Organizations including ISO, IEC, and ASTM should collaborate to develop consistent testing methodologies and acceptance criteria that can be universally applied across different markets and applications, facilitating global adoption of safer advanced electrolyte technologies while maintaining innovation momentum in the field.
Environmental Impact of Electrolyte Materials
The environmental impact of electrolyte materials in electrochemical cells represents a critical consideration in the sustainable development of energy storage technologies. Traditional electrolytes, particularly those containing fluorinated compounds such as lithium hexafluorophosphate (LiPF6), pose significant environmental concerns throughout their lifecycle. These materials can release toxic hydrogen fluoride when exposed to moisture, contributing to air pollution and potential groundwater contamination during production, usage, and disposal phases.
Manufacturing processes for conventional electrolytes often involve energy-intensive procedures and hazardous chemicals, resulting in substantial carbon footprints. The extraction of raw materials for these electrolytes, including lithium and various organic solvents, frequently leads to habitat disruption, water pollution, and soil degradation in mining regions. Furthermore, the limited recyclability of many current electrolyte formulations exacerbates waste management challenges.
Recent environmental impact assessments have revealed that electrolyte materials contribute approximately 15-20% of the total environmental burden of lithium-ion batteries. This significant proportion underscores the urgent need for developing more environmentally benign alternatives that maintain or enhance conductivity performance. Water-based electrolytes represent a promising direction, offering reduced toxicity and fire hazards, though they typically suffer from narrower electrochemical stability windows.
Ionic liquid-based electrolytes present another environmentally favorable option, characterized by negligible vapor pressure, non-flammability, and excellent thermal stability. However, their widespread adoption remains constrained by high production costs and viscosity issues that can limit ionic conductivity. Solid polymer electrolytes and composite gel electrolytes also demonstrate environmental advantages through reduced leakage risks and improved safety profiles.
Life cycle analysis (LCA) studies indicate that transitioning to bio-derived solvents and naturally abundant salt components could reduce the environmental impact of electrolytes by up to 40% compared to conventional formulations. Additionally, designing electrolyte systems with end-of-life considerations, including biodegradability and recyclability, represents a crucial strategy for minimizing long-term environmental consequences.
Regulatory frameworks worldwide are increasingly addressing the environmental implications of electrolyte materials. The European Union's Battery Directive and similar regulations in North America and Asia are establishing more stringent requirements for the environmental performance of battery components, including electrolytes. These evolving standards are accelerating research into green chemistry approaches for electrolyte development, emphasizing renewable feedstocks and benign synthesis routes.
Manufacturing processes for conventional electrolytes often involve energy-intensive procedures and hazardous chemicals, resulting in substantial carbon footprints. The extraction of raw materials for these electrolytes, including lithium and various organic solvents, frequently leads to habitat disruption, water pollution, and soil degradation in mining regions. Furthermore, the limited recyclability of many current electrolyte formulations exacerbates waste management challenges.
Recent environmental impact assessments have revealed that electrolyte materials contribute approximately 15-20% of the total environmental burden of lithium-ion batteries. This significant proportion underscores the urgent need for developing more environmentally benign alternatives that maintain or enhance conductivity performance. Water-based electrolytes represent a promising direction, offering reduced toxicity and fire hazards, though they typically suffer from narrower electrochemical stability windows.
Ionic liquid-based electrolytes present another environmentally favorable option, characterized by negligible vapor pressure, non-flammability, and excellent thermal stability. However, their widespread adoption remains constrained by high production costs and viscosity issues that can limit ionic conductivity. Solid polymer electrolytes and composite gel electrolytes also demonstrate environmental advantages through reduced leakage risks and improved safety profiles.
Life cycle analysis (LCA) studies indicate that transitioning to bio-derived solvents and naturally abundant salt components could reduce the environmental impact of electrolytes by up to 40% compared to conventional formulations. Additionally, designing electrolyte systems with end-of-life considerations, including biodegradability and recyclability, represents a crucial strategy for minimizing long-term environmental consequences.
Regulatory frameworks worldwide are increasingly addressing the environmental implications of electrolyte materials. The European Union's Battery Directive and similar regulations in North America and Asia are establishing more stringent requirements for the environmental performance of battery components, including electrolytes. These evolving standards are accelerating research into green chemistry approaches for electrolyte development, emphasizing renewable feedstocks and benign synthesis routes.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!