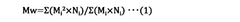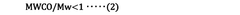How to Improve Rejection Rates for Emerging Contaminants Using Ultrafiltration
SEP 22, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ultrafiltration Technology Background and Objectives
Ultrafiltration (UF) technology has evolved significantly since its inception in the 1960s, transitioning from laboratory-scale applications to widespread industrial implementation. Initially developed for protein concentration and fractionation in the biochemical industry, UF has expanded into water and wastewater treatment, becoming a cornerstone technology in membrane-based separation processes. The evolution of UF membranes has seen remarkable improvements in materials science, moving from cellulose acetate to advanced polymeric materials such as polysulfone, polyethersulfone, and polyvinylidene fluoride, which offer enhanced chemical resistance and mechanical stability.
The emergence of contaminants of concern (EOCs) including pharmaceuticals, personal care products, endocrine-disrupting compounds, and microplastics has created new challenges for conventional water treatment technologies. Traditional UF systems, while effective for removing particulates, bacteria, and viruses, demonstrate limited capability in rejecting these low-molecular-weight organic compounds, which typically range from 100-1000 Daltons—often smaller than the pore size of standard UF membranes (10-100 nm).
Current rejection rates for many EOCs using conventional UF systems remain suboptimal, typically below 50% for compounds smaller than the membrane's molecular weight cut-off. This technological gap has spurred research into enhancing UF performance specifically targeting these emerging contaminants, with the objective of achieving rejection rates exceeding 90% without significantly compromising permeate flux or increasing energy consumption.
The primary technical objectives for improving UF rejection of emerging contaminants include: developing novel membrane materials with tailored surface properties; optimizing membrane pore size distribution and morphology; incorporating functional additives or modifiers that enhance selectivity; and exploring hybrid processes that combine UF with complementary technologies such as adsorption or advanced oxidation.
Recent technological trends indicate growing interest in surface-modified UF membranes, particularly those incorporating nanomaterials or specific functional groups designed to interact with target contaminants. Additionally, the development of responsive or "smart" membranes capable of adapting their properties based on environmental conditions represents a promising frontier in UF technology evolution.
The ultimate goal of this technological advancement is to position UF as a more comprehensive barrier against the full spectrum of water contaminants, including emerging micropollutants, while maintaining the operational and economic advantages that have made UF a preferred technology in water treatment applications. This would significantly expand UF's role in ensuring water security and public health protection in an era of increasingly complex water quality challenges.
The emergence of contaminants of concern (EOCs) including pharmaceuticals, personal care products, endocrine-disrupting compounds, and microplastics has created new challenges for conventional water treatment technologies. Traditional UF systems, while effective for removing particulates, bacteria, and viruses, demonstrate limited capability in rejecting these low-molecular-weight organic compounds, which typically range from 100-1000 Daltons—often smaller than the pore size of standard UF membranes (10-100 nm).
Current rejection rates for many EOCs using conventional UF systems remain suboptimal, typically below 50% for compounds smaller than the membrane's molecular weight cut-off. This technological gap has spurred research into enhancing UF performance specifically targeting these emerging contaminants, with the objective of achieving rejection rates exceeding 90% without significantly compromising permeate flux or increasing energy consumption.
The primary technical objectives for improving UF rejection of emerging contaminants include: developing novel membrane materials with tailored surface properties; optimizing membrane pore size distribution and morphology; incorporating functional additives or modifiers that enhance selectivity; and exploring hybrid processes that combine UF with complementary technologies such as adsorption or advanced oxidation.
Recent technological trends indicate growing interest in surface-modified UF membranes, particularly those incorporating nanomaterials or specific functional groups designed to interact with target contaminants. Additionally, the development of responsive or "smart" membranes capable of adapting their properties based on environmental conditions represents a promising frontier in UF technology evolution.
The ultimate goal of this technological advancement is to position UF as a more comprehensive barrier against the full spectrum of water contaminants, including emerging micropollutants, while maintaining the operational and economic advantages that have made UF a preferred technology in water treatment applications. This would significantly expand UF's role in ensuring water security and public health protection in an era of increasingly complex water quality challenges.
Market Analysis for Advanced Contaminant Rejection Systems
The global market for advanced contaminant rejection systems is experiencing robust growth, driven by increasing concerns over water quality and the emergence of new contaminants. The market was valued at approximately $8.2 billion in 2022 and is projected to reach $14.5 billion by 2028, representing a compound annual growth rate (CAGR) of 9.8%. This growth trajectory is supported by stringent regulatory frameworks across developed economies and growing awareness in developing regions.
Ultrafiltration technology specifically holds a significant market share within this sector, accounting for roughly 27% of the total advanced water treatment technologies market. The ultrafiltration segment is expected to grow at a higher rate than the overall market, with projections indicating a 11.3% CAGR through 2028, primarily due to its versatility and cost-effectiveness compared to other membrane technologies.
Demand for improved rejection rates for emerging contaminants is particularly strong in municipal water treatment, which represents approximately 42% of the market application. Industrial applications follow closely at 38%, with pharmaceutical and food & beverage industries showing the fastest growth rates at 13.2% and 12.7% respectively. These industries require increasingly sophisticated solutions for removing contaminants of emerging concern (CECs) such as pharmaceuticals, personal care products, and industrial chemicals.
Geographically, North America dominates the market with a 34% share, followed by Europe (28%) and Asia-Pacific (25%). However, the Asia-Pacific region is expected to witness the highest growth rate at 14.2% annually, driven by rapid industrialization, urbanization, and increasing regulatory pressure in countries like China and India.
Customer willingness to pay for advanced ultrafiltration solutions varies significantly by sector. Municipal utilities demonstrate price sensitivity but are increasingly mandated by regulations to adopt advanced technologies. Industrial end-users, particularly in pharmaceuticals and electronics manufacturing, show greater willingness to invest in premium solutions that offer higher rejection rates for specific contaminants critical to their operations.
Market research indicates that customers prioritize four key factors when selecting advanced contaminant rejection systems: rejection efficiency (cited by 87% of surveyed customers), operational costs (82%), system lifespan (76%), and ease of integration with existing infrastructure (71%). Solutions that can demonstrate superior performance in rejecting emerging contaminants while maintaining reasonable operational costs are positioned to capture premium market segments.
Competition in this space is intensifying, with over 200 companies globally offering various ultrafiltration solutions. Market consolidation is evident, with the top five players controlling approximately 43% of the market share. Technological differentiation, particularly in membrane materials and module designs that enhance rejection rates for emerging contaminants, represents the primary competitive advantage in this evolving marketplace.
Ultrafiltration technology specifically holds a significant market share within this sector, accounting for roughly 27% of the total advanced water treatment technologies market. The ultrafiltration segment is expected to grow at a higher rate than the overall market, with projections indicating a 11.3% CAGR through 2028, primarily due to its versatility and cost-effectiveness compared to other membrane technologies.
Demand for improved rejection rates for emerging contaminants is particularly strong in municipal water treatment, which represents approximately 42% of the market application. Industrial applications follow closely at 38%, with pharmaceutical and food & beverage industries showing the fastest growth rates at 13.2% and 12.7% respectively. These industries require increasingly sophisticated solutions for removing contaminants of emerging concern (CECs) such as pharmaceuticals, personal care products, and industrial chemicals.
Geographically, North America dominates the market with a 34% share, followed by Europe (28%) and Asia-Pacific (25%). However, the Asia-Pacific region is expected to witness the highest growth rate at 14.2% annually, driven by rapid industrialization, urbanization, and increasing regulatory pressure in countries like China and India.
Customer willingness to pay for advanced ultrafiltration solutions varies significantly by sector. Municipal utilities demonstrate price sensitivity but are increasingly mandated by regulations to adopt advanced technologies. Industrial end-users, particularly in pharmaceuticals and electronics manufacturing, show greater willingness to invest in premium solutions that offer higher rejection rates for specific contaminants critical to their operations.
Market research indicates that customers prioritize four key factors when selecting advanced contaminant rejection systems: rejection efficiency (cited by 87% of surveyed customers), operational costs (82%), system lifespan (76%), and ease of integration with existing infrastructure (71%). Solutions that can demonstrate superior performance in rejecting emerging contaminants while maintaining reasonable operational costs are positioned to capture premium market segments.
Competition in this space is intensifying, with over 200 companies globally offering various ultrafiltration solutions. Market consolidation is evident, with the top five players controlling approximately 43% of the market share. Technological differentiation, particularly in membrane materials and module designs that enhance rejection rates for emerging contaminants, represents the primary competitive advantage in this evolving marketplace.
Current Limitations in Emerging Contaminant Removal
Despite significant advancements in ultrafiltration (UF) technology, several critical limitations persist in effectively removing emerging contaminants from water sources. The primary challenge lies in the molecular size discrepancy between UF membrane pore sizes (typically 0.01-0.1 μm) and many emerging contaminants such as pharmaceuticals, personal care products, and endocrine disrupting compounds, which often have molecular weights below the rejection threshold of conventional UF membranes. This fundamental size mismatch results in poor rejection rates, particularly for low molecular weight compounds below 300 Da.
Membrane fouling represents another significant limitation, as organic and inorganic substances accumulate on membrane surfaces and within pores during filtration processes. This fouling phenomenon not only reduces water flux and operational efficiency but can also create unpredictable rejection behaviors for emerging contaminants. Studies have demonstrated that while initial fouling may temporarily improve rejection rates through pore constriction, extended fouling ultimately compromises membrane integrity and separation performance.
The variability in emerging contaminant properties presents additional challenges. These compounds exhibit diverse physicochemical characteristics including varying hydrophobicity, charge states, and molecular structures. Current UF membrane designs struggle to simultaneously address this wide spectrum of properties, resulting in inconsistent removal efficiencies across different contaminant classes. For instance, hydrophilic compounds typically demonstrate lower rejection rates compared to hydrophobic compounds of similar molecular weight.
Operational parameters significantly impact rejection performance but remain difficult to optimize across diverse water matrices. Factors such as pH, temperature, transmembrane pressure, and cross-flow velocity can dramatically alter rejection mechanisms and efficiencies. The complex interplay between these parameters creates substantial challenges in maintaining consistent removal rates across varying environmental conditions and water compositions.
The absence of standardized testing protocols for emerging contaminants further complicates technology assessment and comparison. Unlike conventional pollutants with established monitoring frameworks, emerging contaminants lack unified testing methodologies, making it difficult to reliably evaluate and compare different UF technologies and their actual field performance.
Energy consumption remains a persistent concern, particularly when attempting to enhance rejection rates through increased pressure or additional treatment steps. Current UF systems face an inherent trade-off between improved contaminant removal and energy efficiency, limiting their sustainable implementation in resource-constrained settings or large-scale applications.
Membrane fouling represents another significant limitation, as organic and inorganic substances accumulate on membrane surfaces and within pores during filtration processes. This fouling phenomenon not only reduces water flux and operational efficiency but can also create unpredictable rejection behaviors for emerging contaminants. Studies have demonstrated that while initial fouling may temporarily improve rejection rates through pore constriction, extended fouling ultimately compromises membrane integrity and separation performance.
The variability in emerging contaminant properties presents additional challenges. These compounds exhibit diverse physicochemical characteristics including varying hydrophobicity, charge states, and molecular structures. Current UF membrane designs struggle to simultaneously address this wide spectrum of properties, resulting in inconsistent removal efficiencies across different contaminant classes. For instance, hydrophilic compounds typically demonstrate lower rejection rates compared to hydrophobic compounds of similar molecular weight.
Operational parameters significantly impact rejection performance but remain difficult to optimize across diverse water matrices. Factors such as pH, temperature, transmembrane pressure, and cross-flow velocity can dramatically alter rejection mechanisms and efficiencies. The complex interplay between these parameters creates substantial challenges in maintaining consistent removal rates across varying environmental conditions and water compositions.
The absence of standardized testing protocols for emerging contaminants further complicates technology assessment and comparison. Unlike conventional pollutants with established monitoring frameworks, emerging contaminants lack unified testing methodologies, making it difficult to reliably evaluate and compare different UF technologies and their actual field performance.
Energy consumption remains a persistent concern, particularly when attempting to enhance rejection rates through increased pressure or additional treatment steps. Current UF systems face an inherent trade-off between improved contaminant removal and energy efficiency, limiting their sustainable implementation in resource-constrained settings or large-scale applications.
Current Ultrafiltration Solutions for Emerging Contaminants
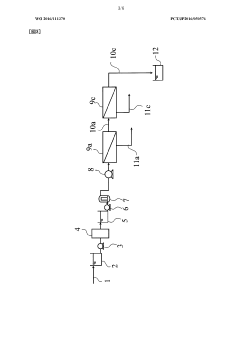
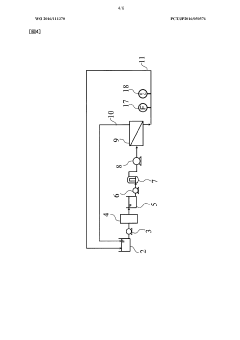
01 Membrane material composition affecting rejection rates
The composition of ultrafiltration membranes significantly impacts rejection rates. Different polymeric materials such as polysulfone, polyethersulfone, and cellulose derivatives exhibit varying rejection characteristics for different solutes. Membrane materials can be modified with additives or surface treatments to enhance selectivity and improve rejection of specific contaminants while maintaining permeability. The chemical structure and properties of the membrane material determine its interaction with various solutes and consequently affect the rejection efficiency.- Membrane material selection for optimizing rejection rates: The choice of membrane material significantly impacts ultrafiltration rejection rates. Different polymeric materials such as polysulfone, polyethersulfone, and cellulose derivatives offer varying rejection capabilities for different solutes. Advanced composite membranes incorporating nanomaterials can enhance selectivity and rejection performance. The chemical composition and surface properties of the membrane material determine its interaction with specific contaminants, affecting overall filtration efficiency.
- Pore size control and distribution techniques: Precise control of membrane pore size and distribution is crucial for achieving targeted rejection rates in ultrafiltration systems. Manufacturing techniques that enable uniform pore formation improve consistency in rejection performance. Methods such as phase inversion, track-etching, and stretching processes can be optimized to create specific pore architectures. Narrow pore size distribution reduces variability in rejection rates and enhances separation efficiency for specific molecular weight cutoffs.
- Operating parameter optimization for enhanced rejection: Operating parameters significantly influence ultrafiltration rejection rates. Factors such as transmembrane pressure, cross-flow velocity, temperature, and pH can be optimized to enhance rejection performance. Higher pressures may increase flux but can lead to membrane compaction and reduced rejection efficiency. Optimizing cross-flow velocity helps minimize concentration polarization and fouling, maintaining consistent rejection rates over time. Temperature affects solution viscosity and diffusion rates, thereby impacting rejection behavior.
- Fouling mitigation strategies to maintain rejection performance: Membrane fouling significantly impacts ultrafiltration rejection rates over time. Various pretreatment methods, cleaning protocols, and surface modifications can be implemented to mitigate fouling and maintain consistent rejection performance. Feed water pretreatment reduces foulant load, while optimized backwashing and chemical cleaning regimes restore membrane performance. Surface modifications that increase hydrophilicity or incorporate antimicrobial properties help maintain stable rejection rates during extended operation.
- Advanced monitoring and control systems for rejection rate stability: Real-time monitoring and control systems enable maintenance of target rejection rates in ultrafiltration processes. Sensors that track parameters such as pressure differential, turbidity, and conductivity provide data for automated adjustments to operating conditions. Machine learning algorithms can predict rejection rate changes and optimize system performance. Integrated control systems that respond to variations in feed water quality help maintain consistent rejection rates despite fluctuating input conditions.
02 Pore size distribution and control methods
Ultrafiltration rejection rates are directly related to the membrane pore size distribution. Precise control of pore size during membrane fabrication enables targeted rejection of specific molecular weight compounds. Manufacturing techniques such as phase inversion, stretching, and track-etching can be employed to achieve desired pore characteristics. Narrow pore size distribution results in more consistent rejection rates, while broader distributions may lead to variable performance. Advanced techniques for measuring and characterizing pore size distribution help in predicting and optimizing rejection performance.Expand Specific Solutions03 Operating conditions influencing rejection efficiency
Operating parameters significantly affect ultrafiltration rejection rates. Factors such as transmembrane pressure, cross-flow velocity, temperature, and pH can be optimized to enhance rejection performance. Higher pressures may initially improve rejection but can lead to membrane compaction or fouling over time. Temperature affects both solution viscosity and diffusion rates, thereby influencing rejection behavior. The pH of the feed solution impacts the charge characteristics of both the membrane and solutes, affecting electrostatic interactions and rejection mechanisms. Proper control of these operating conditions is essential for maintaining consistent rejection rates.Expand Specific Solutions04 Fouling effects on rejection performance
Membrane fouling significantly impacts ultrafiltration rejection rates over time. Organic, inorganic, and biological foulants can accumulate on the membrane surface or within pores, altering the effective pore size and surface properties. This fouling phenomenon typically leads to changes in rejection behavior, often increasing rejection of some solutes while potentially creating preferential flow paths that reduce rejection of others. Various pretreatment strategies, membrane surface modifications, and cleaning protocols can be implemented to mitigate fouling effects and maintain consistent rejection performance throughout the membrane's operational life.Expand Specific Solutions05 Novel membrane configurations for enhanced rejection
Innovative membrane configurations and module designs can significantly improve ultrafiltration rejection rates. Multilayer composite membranes with tailored properties in each layer can provide enhanced selectivity while maintaining high flux. Hollow fiber, spiral wound, and tubular configurations offer different advantages in terms of rejection performance and fouling resistance. Dynamic or secondary membranes formed during filtration can further enhance rejection capabilities. Recent advances include stimuli-responsive membranes that can adjust their rejection properties in response to environmental changes, and hybrid systems combining ultrafiltration with other separation techniques to achieve superior rejection performance.Expand Specific Solutions
Leading Companies in Ultrafiltration Industry
The ultrafiltration market for emerging contaminant rejection is currently in a growth phase, with increasing regulatory pressure driving adoption. The global market size is expanding rapidly, expected to reach significant value as water scarcity and contamination concerns intensify worldwide. Technologically, ultrafiltration for emerging contaminants shows varying maturity levels across applications. Leading companies like Toray Industries, Kurita Water Industries, and EMD Millipore have developed advanced membrane technologies with improved selectivity and fouling resistance. Academic institutions including Sichuan University and Tongji University are contributing breakthrough research in membrane modification techniques. Meanwhile, Siemens AG and LivaNova are integrating ultrafiltration into comprehensive water treatment systems, while specialized players like Norgen Biotek focus on niche applications requiring high rejection rates.
Kurita Water Industries Ltd.
Technical Solution: Kurita Water Industries has developed the KURARAY™ UF system specifically designed to enhance rejection of emerging contaminants through their patented "Dual-Phase Filtration" technology. This innovative approach combines traditional ultrafiltration with in-situ formation of metal hydroxide microparticles that serve as adsorption sites for organic micropollutants. Their membrane modules utilize modified polyethersulfone (PES) with hydrophilic additives that reduce organic fouling while maintaining high permeability (>300 LMH/bar). Kurita's system incorporates a proprietary pre-treatment stage where specific metal salts are dosed and partially precipitated, creating nanoscale adsorption sites that bind to contaminants before they reach the membrane surface. The company has implemented an advanced control system that continuously adjusts chemical dosing based on real-time water quality parameters, optimizing both rejection performance and operational efficiency. Field implementations have demonstrated sustained removal rates of 92-97% for pharmaceuticals, personal care products, and endocrine disrupting compounds across varying influent concentrations. The technology maintains stable performance even during significant fluctuations in feed water quality, making it particularly suitable for municipal applications.
Strengths: Exceptional adaptability to varying water quality conditions through dynamic chemical dosing; combines physical filtration with chemical adsorption for comprehensive contaminant removal; lower membrane fouling rates extend operational cycles. Weaknesses: Requires continuous chemical consumption increasing operational costs; more complex control systems demand higher operator expertise; generates chemical sludge requiring additional handling and disposal.
Toray Industries, Inc.
Technical Solution: Toray has developed advanced ultrafiltration (UF) membranes with modified surface chemistry specifically targeting emerging contaminants. Their proprietary technology incorporates hydrophilic nanoparticles into polyvinylidene fluoride (PVDF) membrane matrices, creating enhanced rejection capabilities for micropollutants including pharmaceuticals and personal care products. The company's ROMEMBRA™ UF series utilizes a unique multi-layer composite structure with controlled pore size distribution (average 0.01-0.03 μm) and increased surface charge density to improve electrostatic repulsion of charged contaminants. Toray has implemented surface grafting techniques with functional polymers containing amide and carboxyl groups to increase hydrogen bonding with polar contaminants, achieving up to 95% rejection rates for compounds previously difficult to remove with conventional UF. Their membranes maintain high flux rates (>120 LMH) while operating at lower transmembrane pressures compared to competitors.
Strengths: Superior rejection of polar and charged contaminants through advanced surface chemistry modifications; maintains high flux rates while achieving improved rejection; established manufacturing infrastructure for commercial-scale production. Weaknesses: Higher production costs compared to conventional UF membranes; potential for increased fouling due to surface modifications; requires more frequent cleaning cycles in high-contaminant environments.
Key Innovations in Membrane Material Science
Method for producing concentrated liquid of stock solution
PatentWO2024143357A1
Innovation
- The method involves using an ultrafiltration membrane with a molecular weight cutoff ratio between 0.05 and 0.30 relative to the weight average molecular weight of the solute, and filtering at a transmembrane pressure differential equal to or higher than the critical pressure, to improve the rejection rate and prevent component leakage.
Water treatment method
PatentWO2016111370A1
Innovation
- A water treatment method involving the use of a rejection improving liquid with a specific agent, such as polyethylene glycol, is supplied to the semipermeable membrane unit, with real-time monitoring of supply pressure and permeate flow rate to adjust the treatment process, ensuring accurate and efficient improvement of the rejection rate without significant permeation flux reduction.
Regulatory Framework for Water Purification Technologies
The regulatory landscape governing water purification technologies has become increasingly stringent in response to growing concerns about emerging contaminants. At the international level, organizations such as the World Health Organization (WHO) have established guidelines for drinking water quality that address a wide range of contaminants, including emerging pollutants like pharmaceuticals, personal care products, and industrial chemicals. These guidelines serve as a reference point for national regulatory bodies developing their own standards.
In the United States, the Environmental Protection Agency (EPA) regulates drinking water quality through the Safe Drinking Water Act (SDWA), which has been amended to address emerging contaminants. The EPA's Contaminant Candidate List (CCL) identifies unregulated contaminants that may require future regulation, with several emerging contaminants appearing on recent lists. Additionally, the EPA's Unregulated Contaminant Monitoring Rule (UCMR) requires water utilities to monitor for selected unregulated contaminants, providing data that informs regulatory decisions.
The European Union has implemented the Water Framework Directive (WFD) and the Drinking Water Directive, which establish comprehensive frameworks for water protection and quality standards. The EU has also developed a "watch list" mechanism to monitor substances of emerging concern in aquatic environments, with data collected informing potential regulatory actions.
Regulatory approaches to ultrafiltration technologies specifically focus on performance validation and operational requirements. Standards organizations such as NSF International and the American Water Works Association (AWWA) have developed certification protocols for membrane filtration systems, including ultrafiltration. These protocols typically specify minimum rejection rates for various contaminants and establish testing methodologies to verify performance claims.
Recent regulatory trends indicate a shift toward more comprehensive approaches to water quality management. This includes the adoption of risk-based frameworks that consider the entire water treatment process rather than focusing solely on end-product testing. Such approaches recognize that multiple barriers, including ultrafiltration, may be necessary to effectively address emerging contaminants.
Compliance requirements for ultrafiltration systems are becoming more sophisticated, with many jurisdictions now requiring continuous monitoring of membrane integrity and performance. Parameters such as turbidity, pressure differential, and particle counts are commonly used as surrogate measures to ensure that ultrafiltration systems maintain their rejection capabilities for emerging contaminants throughout their operational life.
Future regulatory developments are likely to include more specific requirements for the rejection of emerging contaminants by ultrafiltration systems, as analytical methods improve and more data becomes available on the occurrence and health effects of these substances. Water treatment facilities implementing ultrafiltration technologies must therefore remain vigilant about evolving regulatory requirements and be prepared to adapt their treatment processes accordingly.
In the United States, the Environmental Protection Agency (EPA) regulates drinking water quality through the Safe Drinking Water Act (SDWA), which has been amended to address emerging contaminants. The EPA's Contaminant Candidate List (CCL) identifies unregulated contaminants that may require future regulation, with several emerging contaminants appearing on recent lists. Additionally, the EPA's Unregulated Contaminant Monitoring Rule (UCMR) requires water utilities to monitor for selected unregulated contaminants, providing data that informs regulatory decisions.
The European Union has implemented the Water Framework Directive (WFD) and the Drinking Water Directive, which establish comprehensive frameworks for water protection and quality standards. The EU has also developed a "watch list" mechanism to monitor substances of emerging concern in aquatic environments, with data collected informing potential regulatory actions.
Regulatory approaches to ultrafiltration technologies specifically focus on performance validation and operational requirements. Standards organizations such as NSF International and the American Water Works Association (AWWA) have developed certification protocols for membrane filtration systems, including ultrafiltration. These protocols typically specify minimum rejection rates for various contaminants and establish testing methodologies to verify performance claims.
Recent regulatory trends indicate a shift toward more comprehensive approaches to water quality management. This includes the adoption of risk-based frameworks that consider the entire water treatment process rather than focusing solely on end-product testing. Such approaches recognize that multiple barriers, including ultrafiltration, may be necessary to effectively address emerging contaminants.
Compliance requirements for ultrafiltration systems are becoming more sophisticated, with many jurisdictions now requiring continuous monitoring of membrane integrity and performance. Parameters such as turbidity, pressure differential, and particle counts are commonly used as surrogate measures to ensure that ultrafiltration systems maintain their rejection capabilities for emerging contaminants throughout their operational life.
Future regulatory developments are likely to include more specific requirements for the rejection of emerging contaminants by ultrafiltration systems, as analytical methods improve and more data becomes available on the occurrence and health effects of these substances. Water treatment facilities implementing ultrafiltration technologies must therefore remain vigilant about evolving regulatory requirements and be prepared to adapt their treatment processes accordingly.
Environmental Impact Assessment of Ultrafiltration Systems
Ultrafiltration systems, while offering significant benefits for water treatment, also present various environmental implications that must be carefully assessed. The environmental footprint of ultrafiltration technology extends across multiple dimensions, from energy consumption to waste generation and chemical usage.
Energy consumption represents one of the most significant environmental considerations for ultrafiltration systems. These systems require substantial electrical power for maintaining transmembrane pressure and operating auxiliary equipment. The carbon footprint associated with this energy usage varies considerably depending on the energy source, with renewable energy-powered systems demonstrating markedly lower environmental impact compared to those relying on fossil fuels.
Membrane manufacturing and disposal contribute to the life-cycle environmental impact of ultrafiltration systems. Most ultrafiltration membranes are produced from synthetic polymers derived from petrochemical sources, involving energy-intensive manufacturing processes. The disposal of spent membranes presents additional environmental challenges, as these materials typically have limited biodegradability and may persist in landfills for extended periods.
Chemical usage in membrane cleaning and maintenance processes represents another environmental consideration. Cleaning agents such as sodium hypochlorite, citric acid, and various surfactants can potentially impact aquatic ecosystems if discharged without proper treatment. Advanced ultrafiltration systems increasingly incorporate chemical recovery and neutralization processes to mitigate these effects.
Water conservation benefits represent a positive environmental aspect of ultrafiltration technology. By enabling water reuse and reducing discharge volumes, these systems can significantly decrease overall water withdrawal from natural sources. This benefit becomes particularly valuable in water-stressed regions where conservation is critical for ecosystem preservation.
The rejection of emerging contaminants by ultrafiltration systems directly impacts environmental quality. Higher rejection rates prevent potentially harmful substances from entering natural water bodies, protecting aquatic ecosystems and reducing bioaccumulation risks in the food chain. As rejection efficiency improves, the environmental protection value of these systems increases proportionally.
Land use requirements for ultrafiltration facilities must also be considered in environmental impact assessments. Modern ultrafiltration systems typically have smaller footprints compared to conventional treatment technologies, potentially reducing habitat disruption and preserving natural spaces. This advantage becomes particularly significant in densely populated areas where land availability is limited.
Noise pollution and visual impacts, while often overlooked, contribute to the overall environmental assessment of ultrafiltration installations. These factors can affect local wildlife and human communities, necessitating appropriate mitigation measures such as sound insulation and thoughtful facility design that harmonizes with surrounding landscapes.
Energy consumption represents one of the most significant environmental considerations for ultrafiltration systems. These systems require substantial electrical power for maintaining transmembrane pressure and operating auxiliary equipment. The carbon footprint associated with this energy usage varies considerably depending on the energy source, with renewable energy-powered systems demonstrating markedly lower environmental impact compared to those relying on fossil fuels.
Membrane manufacturing and disposal contribute to the life-cycle environmental impact of ultrafiltration systems. Most ultrafiltration membranes are produced from synthetic polymers derived from petrochemical sources, involving energy-intensive manufacturing processes. The disposal of spent membranes presents additional environmental challenges, as these materials typically have limited biodegradability and may persist in landfills for extended periods.
Chemical usage in membrane cleaning and maintenance processes represents another environmental consideration. Cleaning agents such as sodium hypochlorite, citric acid, and various surfactants can potentially impact aquatic ecosystems if discharged without proper treatment. Advanced ultrafiltration systems increasingly incorporate chemical recovery and neutralization processes to mitigate these effects.
Water conservation benefits represent a positive environmental aspect of ultrafiltration technology. By enabling water reuse and reducing discharge volumes, these systems can significantly decrease overall water withdrawal from natural sources. This benefit becomes particularly valuable in water-stressed regions where conservation is critical for ecosystem preservation.
The rejection of emerging contaminants by ultrafiltration systems directly impacts environmental quality. Higher rejection rates prevent potentially harmful substances from entering natural water bodies, protecting aquatic ecosystems and reducing bioaccumulation risks in the food chain. As rejection efficiency improves, the environmental protection value of these systems increases proportionally.
Land use requirements for ultrafiltration facilities must also be considered in environmental impact assessments. Modern ultrafiltration systems typically have smaller footprints compared to conventional treatment technologies, potentially reducing habitat disruption and preserving natural spaces. This advantage becomes particularly significant in densely populated areas where land availability is limited.
Noise pollution and visual impacts, while often overlooked, contribute to the overall environmental assessment of ultrafiltration installations. These factors can affect local wildlife and human communities, necessitating appropriate mitigation measures such as sound insulation and thoughtful facility design that harmonizes with surrounding landscapes.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!