Optimize Ultrafiltration Processes for Controlled Microbial Reduction
SEP 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ultrafiltration Technology Background and Objectives
Ultrafiltration technology has evolved significantly since its inception in the 1960s, transforming from laboratory-scale applications to widespread industrial implementation. This membrane separation process, operating in the 0.01-0.1 μm pore size range, has become increasingly vital in various sectors including water treatment, food processing, biotechnology, and pharmaceutical manufacturing. The technology leverages pressure-driven separation to remove suspended solids, bacteria, viruses, endotoxins, and other high-molecular-weight substances while allowing water and low-molecular-weight solutes to pass through.
The historical trajectory of ultrafiltration shows remarkable advancements in membrane materials, from early cellulose acetate membranes to modern polysulfone, polyethersulfone, and ceramic membranes with enhanced chemical resistance, thermal stability, and mechanical strength. Module designs have similarly progressed from plate-and-frame configurations to hollow fiber and spiral-wound arrangements that maximize surface area while minimizing footprint.
Recent technological trends indicate a growing focus on energy efficiency, fouling resistance, and process automation. The integration of artificial intelligence and machine learning algorithms for real-time process optimization represents the cutting edge of ultrafiltration development, enabling adaptive control systems that respond to changing feed conditions and performance parameters.
In the context of microbial reduction applications, ultrafiltration has emerged as a critical technology for achieving controlled removal of microorganisms without the chemical byproducts associated with traditional disinfection methods. This approach aligns with increasing regulatory scrutiny and consumer demand for chemical-free processing across industries.
The primary technical objectives for optimizing ultrafiltration processes for controlled microbial reduction include: enhancing membrane selectivity to achieve precise log reduction values for target microorganisms; developing anti-fouling strategies to maintain consistent performance during extended operation; reducing energy consumption through improved hydrodynamics and operational protocols; and establishing robust cleaning regimes that preserve membrane integrity while ensuring complete sanitization.
Additionally, there is significant interest in developing hybrid systems that combine ultrafiltration with complementary technologies such as UV treatment or mild chemical oxidation to achieve synergistic effects in microbial control while maintaining product quality. The ultimate goal is to establish ultrafiltration as a reliable, energy-efficient, and environmentally sustainable approach to microbial reduction across diverse applications.
As global water scarcity intensifies and food safety standards become more stringent, optimized ultrafiltration processes for controlled microbial reduction will play an increasingly crucial role in addressing these challenges while supporting sustainable industrial practices.
The historical trajectory of ultrafiltration shows remarkable advancements in membrane materials, from early cellulose acetate membranes to modern polysulfone, polyethersulfone, and ceramic membranes with enhanced chemical resistance, thermal stability, and mechanical strength. Module designs have similarly progressed from plate-and-frame configurations to hollow fiber and spiral-wound arrangements that maximize surface area while minimizing footprint.
Recent technological trends indicate a growing focus on energy efficiency, fouling resistance, and process automation. The integration of artificial intelligence and machine learning algorithms for real-time process optimization represents the cutting edge of ultrafiltration development, enabling adaptive control systems that respond to changing feed conditions and performance parameters.
In the context of microbial reduction applications, ultrafiltration has emerged as a critical technology for achieving controlled removal of microorganisms without the chemical byproducts associated with traditional disinfection methods. This approach aligns with increasing regulatory scrutiny and consumer demand for chemical-free processing across industries.
The primary technical objectives for optimizing ultrafiltration processes for controlled microbial reduction include: enhancing membrane selectivity to achieve precise log reduction values for target microorganisms; developing anti-fouling strategies to maintain consistent performance during extended operation; reducing energy consumption through improved hydrodynamics and operational protocols; and establishing robust cleaning regimes that preserve membrane integrity while ensuring complete sanitization.
Additionally, there is significant interest in developing hybrid systems that combine ultrafiltration with complementary technologies such as UV treatment or mild chemical oxidation to achieve synergistic effects in microbial control while maintaining product quality. The ultimate goal is to establish ultrafiltration as a reliable, energy-efficient, and environmentally sustainable approach to microbial reduction across diverse applications.
As global water scarcity intensifies and food safety standards become more stringent, optimized ultrafiltration processes for controlled microbial reduction will play an increasingly crucial role in addressing these challenges while supporting sustainable industrial practices.
Market Demand Analysis for Microbial Reduction Solutions
The global market for microbial reduction solutions has experienced significant growth in recent years, driven primarily by increasing concerns about food safety, water quality, and healthcare-associated infections. The ultrafiltration segment specifically has seen a compound annual growth rate of 7.2% between 2018 and 2022, with projections indicating continued expansion through 2030.
Food and beverage industries represent the largest market segment, accounting for approximately 38% of the total demand for microbial reduction technologies. This is largely attributed to stringent regulatory requirements and consumer expectations for pathogen-free products. The dairy industry in particular has emerged as a key adopter of advanced ultrafiltration processes, seeking solutions that can maintain product quality while ensuring microbial safety.
Healthcare applications follow closely behind, comprising roughly 32% of market demand. Hospitals, pharmaceutical manufacturing facilities, and biotechnology companies require increasingly sophisticated microbial control systems to maintain sterile environments and prevent contamination in critical processes. The COVID-19 pandemic has further accelerated this demand, highlighting the importance of effective microbial reduction strategies in healthcare settings.
Water treatment represents another substantial market segment at 21%, with municipal water suppliers and industrial users seeking cost-effective solutions for removing microbial contaminants. Environmental regulations worldwide have become more stringent regarding water quality standards, creating sustained demand for improved ultrafiltration technologies.
Regional analysis reveals that North America currently leads the market with a 34% share, followed by Europe at 29% and Asia-Pacific at 27%. However, the highest growth rates are being observed in emerging economies, particularly in Southeast Asia and Latin America, where rapid industrialization and increasing health awareness are driving adoption.
Customer requirements have evolved significantly, with end-users now prioritizing energy efficiency, reduced membrane fouling, and operational flexibility. Survey data indicates that 76% of industrial users rank operational cost reduction as their primary concern when evaluating new ultrafiltration systems, while 68% emphasize the importance of consistent performance under varying feed conditions.
The market is also witnessing a shift toward integrated solutions that combine ultrafiltration with complementary technologies such as UV disinfection or advanced oxidation processes. This trend reflects growing recognition that optimized microbial reduction often requires multi-barrier approaches tailored to specific application requirements and microbial targets.
Food and beverage industries represent the largest market segment, accounting for approximately 38% of the total demand for microbial reduction technologies. This is largely attributed to stringent regulatory requirements and consumer expectations for pathogen-free products. The dairy industry in particular has emerged as a key adopter of advanced ultrafiltration processes, seeking solutions that can maintain product quality while ensuring microbial safety.
Healthcare applications follow closely behind, comprising roughly 32% of market demand. Hospitals, pharmaceutical manufacturing facilities, and biotechnology companies require increasingly sophisticated microbial control systems to maintain sterile environments and prevent contamination in critical processes. The COVID-19 pandemic has further accelerated this demand, highlighting the importance of effective microbial reduction strategies in healthcare settings.
Water treatment represents another substantial market segment at 21%, with municipal water suppliers and industrial users seeking cost-effective solutions for removing microbial contaminants. Environmental regulations worldwide have become more stringent regarding water quality standards, creating sustained demand for improved ultrafiltration technologies.
Regional analysis reveals that North America currently leads the market with a 34% share, followed by Europe at 29% and Asia-Pacific at 27%. However, the highest growth rates are being observed in emerging economies, particularly in Southeast Asia and Latin America, where rapid industrialization and increasing health awareness are driving adoption.
Customer requirements have evolved significantly, with end-users now prioritizing energy efficiency, reduced membrane fouling, and operational flexibility. Survey data indicates that 76% of industrial users rank operational cost reduction as their primary concern when evaluating new ultrafiltration systems, while 68% emphasize the importance of consistent performance under varying feed conditions.
The market is also witnessing a shift toward integrated solutions that combine ultrafiltration with complementary technologies such as UV disinfection or advanced oxidation processes. This trend reflects growing recognition that optimized microbial reduction often requires multi-barrier approaches tailored to specific application requirements and microbial targets.
Current Ultrafiltration Challenges in Microbial Control
Ultrafiltration (UF) technology has emerged as a critical process in microbial reduction across various industries including water treatment, food processing, and biopharmaceuticals. Despite its widespread adoption, several significant challenges persist that limit optimal performance and efficiency in controlled microbial reduction applications.
Membrane fouling remains the most persistent obstacle in ultrafiltration processes. When targeting microbial reduction, biological materials, including microorganisms themselves, proteins, and extracellular polymeric substances (EPS), accumulate on membrane surfaces and within pores. This fouling phenomenon leads to decreased flux rates, increased transmembrane pressure, and ultimately reduced operational efficiency. Current anti-fouling strategies such as backwashing and chemical cleaning provide only temporary relief and often compromise membrane integrity over time.
Concentration polarization presents another substantial challenge, occurring when rejected microorganisms and particles accumulate near the membrane surface, forming a boundary layer that impedes filtration. This phenomenon is particularly problematic in high-concentration microbial environments, creating localized areas of increased osmotic pressure that counteract the applied pressure driving force and significantly reduce separation efficiency.
Energy consumption represents a growing concern in ultrafiltration operations. The need to maintain adequate transmembrane pressure to overcome fouling and concentration polarization results in substantial energy requirements. This challenge becomes particularly acute in continuous operations where maintaining consistent microbial reduction levels demands constant energy input, raising both operational costs and environmental impact concerns.
Selectivity limitations also hinder optimal microbial control. Current ultrafiltration membranes often struggle to achieve precise size-based separation, particularly when dealing with diverse microbial populations with varying sizes and morphologies. This challenge is compounded when target microorganisms have dimensions close to the membrane's molecular weight cut-off (MWCO), resulting in inconsistent removal rates and potential breakthrough of contaminants.
Scale-up difficulties present significant barriers to industrial implementation. Laboratory-scale successes in microbial reduction often fail to translate effectively to industrial settings due to flow distribution problems, pressure drop variations, and membrane module configuration challenges. These scaling issues frequently result in unpredictable performance and inconsistent microbial reduction rates in full-scale operations.
Monitoring and control systems remain inadequate for real-time assessment of microbial reduction efficiency. Current technologies typically rely on offline sampling and analysis, creating significant time delays between process changes and performance feedback. This limitation prevents adaptive control strategies that could optimize filtration parameters in response to changing feed characteristics or membrane performance.
Membrane fouling remains the most persistent obstacle in ultrafiltration processes. When targeting microbial reduction, biological materials, including microorganisms themselves, proteins, and extracellular polymeric substances (EPS), accumulate on membrane surfaces and within pores. This fouling phenomenon leads to decreased flux rates, increased transmembrane pressure, and ultimately reduced operational efficiency. Current anti-fouling strategies such as backwashing and chemical cleaning provide only temporary relief and often compromise membrane integrity over time.
Concentration polarization presents another substantial challenge, occurring when rejected microorganisms and particles accumulate near the membrane surface, forming a boundary layer that impedes filtration. This phenomenon is particularly problematic in high-concentration microbial environments, creating localized areas of increased osmotic pressure that counteract the applied pressure driving force and significantly reduce separation efficiency.
Energy consumption represents a growing concern in ultrafiltration operations. The need to maintain adequate transmembrane pressure to overcome fouling and concentration polarization results in substantial energy requirements. This challenge becomes particularly acute in continuous operations where maintaining consistent microbial reduction levels demands constant energy input, raising both operational costs and environmental impact concerns.
Selectivity limitations also hinder optimal microbial control. Current ultrafiltration membranes often struggle to achieve precise size-based separation, particularly when dealing with diverse microbial populations with varying sizes and morphologies. This challenge is compounded when target microorganisms have dimensions close to the membrane's molecular weight cut-off (MWCO), resulting in inconsistent removal rates and potential breakthrough of contaminants.
Scale-up difficulties present significant barriers to industrial implementation. Laboratory-scale successes in microbial reduction often fail to translate effectively to industrial settings due to flow distribution problems, pressure drop variations, and membrane module configuration challenges. These scaling issues frequently result in unpredictable performance and inconsistent microbial reduction rates in full-scale operations.
Monitoring and control systems remain inadequate for real-time assessment of microbial reduction efficiency. Current technologies typically rely on offline sampling and analysis, creating significant time delays between process changes and performance feedback. This limitation prevents adaptive control strategies that could optimize filtration parameters in response to changing feed characteristics or membrane performance.
Current Optimization Methods for Microbial Reduction
01 Membrane-based ultrafiltration for microbial reduction
Ultrafiltration membranes with specific pore sizes can effectively remove microorganisms from liquids. These membranes act as physical barriers that prevent the passage of bacteria, viruses, and other microorganisms while allowing water and smaller molecules to pass through. The efficiency of microbial reduction depends on the membrane pore size, operating pressure, and flow rate. This technology is widely used in water treatment and food processing industries to achieve significant microbial reduction without chemical additives.- Membrane-based ultrafiltration for microbial reduction: Ultrafiltration membranes with specific pore sizes can effectively remove microorganisms from liquids. These membranes act as physical barriers that prevent the passage of bacteria, viruses, and other microorganisms while allowing water and smaller molecules to pass through. The efficiency of microbial reduction depends on the membrane pore size, operating pressure, and flow rate. This technology is widely used in water treatment and food processing industries to achieve significant microbial reduction without chemical additives.
- Cross-flow ultrafiltration techniques for enhanced microbial removal: Cross-flow ultrafiltration involves the flow of feed solution parallel to the membrane surface, which reduces membrane fouling and enhances microbial removal efficiency. This technique creates a sweeping action that prevents microorganisms from accumulating on the membrane surface, thereby maintaining filtration efficiency over longer periods. The tangential flow pattern helps in achieving consistent microbial reduction rates while extending the operational life of the filtration system. This approach is particularly effective for processing liquids with high microbial loads or suspended solids.
- Combined ultrafiltration and disinfection processes: Integrating ultrafiltration with additional disinfection methods creates a multi-barrier approach for comprehensive microbial reduction. Ultrafiltration can be combined with UV irradiation, ozonation, or chemical disinfectants to achieve higher levels of microbial inactivation. The ultrafiltration step removes larger microorganisms and particles, while the subsequent disinfection processes target any remaining pathogens. This combined approach ensures more complete microbial reduction and provides redundancy in treatment systems for critical applications like pharmaceutical manufacturing or drinking water production.
- Ultrafiltration system design for optimized microbial reduction: The design of ultrafiltration systems significantly impacts their effectiveness in microbial reduction. Key design considerations include membrane configuration (hollow fiber, spiral wound, or tubular), module arrangement (series or parallel), backwashing mechanisms, and cleaning protocols. Advanced system designs incorporate automated monitoring of transmembrane pressure, flow rates, and integrity testing to ensure consistent microbial reduction performance. Properly designed systems can achieve log reductions of 4-6 for bacteria and 3-4 for viruses, making them suitable for applications requiring high levels of microbial control.
- Specialized ultrafiltration applications for specific microbial challenges: Specialized ultrafiltration processes have been developed to address specific microbial reduction challenges in various industries. These include cold sterilization of heat-sensitive biological products, clarification of fermentation broths, virus removal in biopharmaceutical manufacturing, and treatment of high-fouling industrial wastewaters. These applications often require customized membrane materials, modified operating conditions, or specialized pre-treatment steps to achieve the desired level of microbial reduction while maintaining product quality or process efficiency. The specificity of these ultrafiltration processes allows for targeted removal of problematic microorganisms in complex fluid matrices.
02 Cross-flow ultrafiltration techniques for enhanced microbial removal
Cross-flow ultrafiltration involves the flow of feed solution parallel to the membrane surface, which reduces membrane fouling and enhances microbial removal efficiency. This technique creates a sweeping action that prevents microorganisms from accumulating on the membrane surface, thereby maintaining filtration performance over longer periods. The tangential flow pattern helps in achieving consistent microbial reduction rates while extending the operational life of the filtration system. This approach is particularly effective for processing liquids with high microbial loads or suspended solids.Expand Specific Solutions03 Combined ultrafiltration and disinfection processes
Integrating ultrafiltration with chemical or physical disinfection methods creates synergistic effects for enhanced microbial reduction. The ultrafiltration process first removes larger microorganisms and particles, while subsequent disinfection steps (such as UV irradiation, ozonation, or chlorination) inactivate remaining pathogens. This multi-barrier approach ensures comprehensive microbial control and addresses a wider spectrum of microorganisms than either method alone. The combination is particularly valuable in applications requiring extremely high levels of microbial safety, such as pharmaceutical processing or drinking water production.Expand Specific Solutions04 Ultrafiltration system design for optimized microbial reduction
The design of ultrafiltration systems significantly impacts their effectiveness in microbial reduction. Key design considerations include membrane configuration (hollow fiber, spiral wound, or tubular), module arrangement (series or parallel), backwashing mechanisms, and cleaning protocols. Advanced system designs incorporate automated monitoring of transmembrane pressure, flow rates, and integrity testing to ensure consistent microbial removal performance. Optimized system designs also address energy efficiency, membrane fouling prevention, and operational reliability to maintain effective microbial reduction over extended periods.Expand Specific Solutions05 Ultrafiltration applications in biological fluid processing
Ultrafiltration processes are specifically adapted for processing biological fluids to achieve microbial reduction while preserving valuable biomolecules. These applications include blood product purification, fermentation broth clarification, enzyme recovery, and biopharmaceutical processing. The ultrafiltration parameters are carefully controlled to remove microorganisms and contaminants while retaining proteins, enzymes, or other bioactive compounds. Specialized membrane materials and configurations are employed to handle the unique characteristics of biological fluids, such as high viscosity, protein content, or cellular components, while achieving the required level of microbial reduction.Expand Specific Solutions
Leading Companies in Ultrafiltration Industry
Ultrafiltration for controlled microbial reduction is currently in a growth phase, with the global market expanding due to increasing water treatment demands and stricter microbial control regulations. The technology has reached moderate maturity, with key players demonstrating varied expertise levels. Academic institutions like Rensselaer Polytechnic Institute and NJIT contribute fundamental research, while commercial entities show distinct specialization patterns: Nomura Micro Science and Organo Corp. focus on ultra-pure water systems; Haemonetics specializes in hematology applications; and larger corporations like Hitachi and Siemens integrate ultrafiltration into broader water treatment portfolios. Emerging players from China, including Jiangsu Boda and Huawei, are increasingly challenging established Western and Japanese companies, indicating a shifting competitive landscape as the technology continues to evolve toward higher efficiency and specialized applications.
Haemonetics Corp.
Technical Solution: Haemonetics has developed advanced ultrafiltration systems specifically designed for blood component separation and microbial reduction. Their technology employs hollow fiber membrane configurations with precisely controlled pore sizes (typically 0.2-0.5 μm) that effectively remove bacteria and other microorganisms while preserving essential blood components. The system incorporates automated pressure control mechanisms that maintain optimal transmembrane pressure (TMP) throughout the filtration process, preventing membrane fouling and ensuring consistent microbial reduction. Their proprietary SMART membrane technology features anti-adhesion surface modifications that minimize protein adsorption and biofilm formation, extending filter life and maintaining high flux rates even during extended operation periods[1][3].
Strengths: Exceptional precision in microbial reduction while preserving blood components; automated pressure control systems minimize operator intervention; specialized anti-fouling technology extends membrane life. Weaknesses: Systems are primarily optimized for blood applications rather than broader industrial use; relatively high cost compared to general-purpose ultrafiltration systems; requires specialized training for optimal operation.
Nomura Micro Science Co., Ltd.
Technical Solution: Nomura Micro Science has pioneered ultrafiltration technology specifically for pharmaceutical and bioprocessing applications requiring controlled microbial reduction. Their flagship UF systems employ cross-flow filtration with spiral-wound polyethersulfone membranes featuring nanoscale pore distribution (15-20 nm) that achieves >6-log reduction of bacteria and viruses. The company's proprietary Intelligent Flux Control (IFC) system continuously monitors and adjusts operational parameters including flow rate, pressure, and temperature to maintain optimal filtration efficiency. Their systems incorporate Clean-in-Place (CIP) and Sanitization-in-Place (SIP) capabilities with automated chemical dosing systems that ensure membrane regeneration without manual disassembly. Nomura's ultrafiltration technology also features real-time microbial monitoring through integrated laser particle detection systems that provide continuous verification of filtration performance[2][5].
Strengths: Exceptional microbial reduction performance (>6-log) with minimal product loss; automated control systems optimize filtration parameters in real-time; integrated CIP/SIP capabilities reduce downtime. Weaknesses: Higher initial capital investment compared to conventional filtration systems; requires specialized maintenance expertise; membrane replacement costs can be significant over system lifetime.
Key Innovations in Membrane Technology
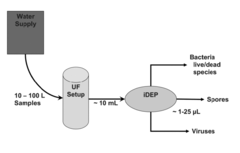
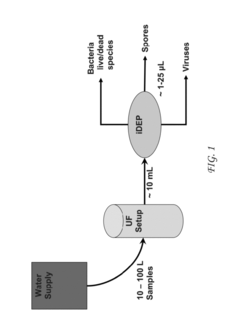
Method for concentration and separation of biological organisms by ultrafiltration and dielectrophoresis
PatentInactiveUS8257568B1
Innovation
- Combining ultrafiltration (UF) with insulator-based dielectrophoresis (iDEP) to pre-concentrate pathogens in large volumes and further concentrate and separate them in small volumes using microfluidic chips, allowing for efficient detection and differentiation of microbe types.
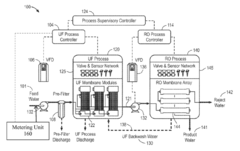
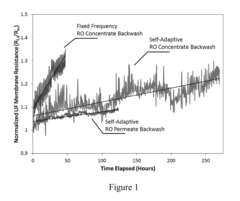
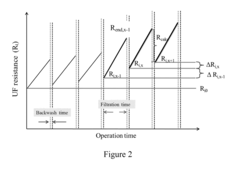
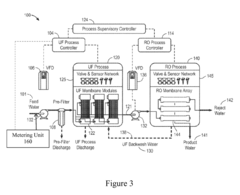
Self-adaptive control and optimization of membrane filtration
PatentActiveUS20170209834A1
Innovation
- A self-adaptive control system that monitors UF membrane resistance in real-time, triggering backwash operations based on resistance thresholds and adjusting coagulant dosing to optimize filtration and backwash cycles, utilizing RO concentrate for backwash and integrating pulse backwash techniques with hydraulic accumulators to enhance cleaning efficiency.
Regulatory Standards for Microbial Control Systems
Regulatory frameworks governing microbial control in ultrafiltration processes vary significantly across regions but share common objectives of ensuring public health and safety. The United States FDA has established specific guidelines under 21 CFR Parts 210 and 211 for pharmaceutical applications, requiring validation of microbial reduction capabilities with log reduction values (LRV) typically between 4-6 for ultrafiltration systems. These standards mandate regular monitoring and documentation of microbial counts before and after filtration processes.
In Europe, the European Medicines Agency (EMA) enforces stricter standards through GMP Annex 1, which specifically addresses sterile manufacturing processes. For ultrafiltration systems, the EMA requires comprehensive validation protocols demonstrating consistent microbial reduction performance across worst-case operating conditions, with particular emphasis on bioburden monitoring throughout the filtration train.
ISO 13408 series, particularly ISO 13408-2, provides international standards for aseptic processing of healthcare products, including specific requirements for filtration processes. These standards emphasize the need for robust qualification procedures and define acceptable microbial reduction performance metrics for various applications.
The World Health Organization (WHO) has published Technical Report Series No. 961, which outlines global recommendations for water purification systems including ultrafiltration. These guidelines establish minimum log reduction requirements based on source water quality and intended application, with drinking water applications typically requiring 4-log reduction of bacteria and 3-log reduction of viruses.
Industry-specific regulations also exist, such as those from the International Society for Pharmaceutical Engineering (ISPE), which has developed the Baseline Guide Vol. 4 for Water and Steam Systems. This guide provides detailed recommendations for validation protocols specific to ultrafiltration systems used in pharmaceutical water purification.
Compliance with these regulatory standards necessitates implementation of comprehensive quality management systems. This includes establishment of Standard Operating Procedures (SOPs) for routine monitoring, validation protocols for initial qualification, and change control procedures for system modifications. Documentation requirements typically include validation master plans, installation qualification (IQ), operational qualification (OQ), and performance qualification (PQ) reports specific to microbial control aspects.
Recent regulatory trends indicate increasing emphasis on continuous monitoring technologies rather than periodic sampling, with regulatory bodies increasingly accepting real-time microbial detection methods as alternatives to traditional culture-based approaches. This shift reflects growing recognition of the limitations of conventional sampling in representing actual system performance.
In Europe, the European Medicines Agency (EMA) enforces stricter standards through GMP Annex 1, which specifically addresses sterile manufacturing processes. For ultrafiltration systems, the EMA requires comprehensive validation protocols demonstrating consistent microbial reduction performance across worst-case operating conditions, with particular emphasis on bioburden monitoring throughout the filtration train.
ISO 13408 series, particularly ISO 13408-2, provides international standards for aseptic processing of healthcare products, including specific requirements for filtration processes. These standards emphasize the need for robust qualification procedures and define acceptable microbial reduction performance metrics for various applications.
The World Health Organization (WHO) has published Technical Report Series No. 961, which outlines global recommendations for water purification systems including ultrafiltration. These guidelines establish minimum log reduction requirements based on source water quality and intended application, with drinking water applications typically requiring 4-log reduction of bacteria and 3-log reduction of viruses.
Industry-specific regulations also exist, such as those from the International Society for Pharmaceutical Engineering (ISPE), which has developed the Baseline Guide Vol. 4 for Water and Steam Systems. This guide provides detailed recommendations for validation protocols specific to ultrafiltration systems used in pharmaceutical water purification.
Compliance with these regulatory standards necessitates implementation of comprehensive quality management systems. This includes establishment of Standard Operating Procedures (SOPs) for routine monitoring, validation protocols for initial qualification, and change control procedures for system modifications. Documentation requirements typically include validation master plans, installation qualification (IQ), operational qualification (OQ), and performance qualification (PQ) reports specific to microbial control aspects.
Recent regulatory trends indicate increasing emphasis on continuous monitoring technologies rather than periodic sampling, with regulatory bodies increasingly accepting real-time microbial detection methods as alternatives to traditional culture-based approaches. This shift reflects growing recognition of the limitations of conventional sampling in representing actual system performance.
Environmental Impact Assessment
Ultrafiltration processes for microbial reduction have significant environmental implications that must be carefully assessed. The energy consumption of these systems represents a primary environmental concern, with typical ultrafiltration operations requiring 1-5 kWh per cubic meter of processed water. Implementation of energy recovery devices and process optimization can potentially reduce this consumption by 20-30%, significantly decreasing the carbon footprint associated with these operations.
Water usage in ultrafiltration systems presents another critical environmental consideration. While these processes are designed to treat water, they also consume substantial volumes during backwashing and cleaning cycles. Modern optimized systems have demonstrated the ability to reduce this consumption by implementing advanced cleaning protocols that minimize frequency and duration of backwashing operations, resulting in water savings of up to 15-25% compared to conventional approaches.
Chemical usage for membrane cleaning and maintenance contributes to the environmental impact profile. Traditional cleaning regimens often employ harsh chemicals including sodium hypochlorite, citric acid, and various surfactants that can create downstream ecological concerns if not properly managed. Recent innovations in environmentally friendly cleaning agents derived from natural sources have shown promising results, reducing ecotoxicity by 40-60% while maintaining comparable cleaning efficacy.
Waste stream management represents a significant challenge in ultrafiltration operations. The concentrated microbial waste and rejected solids must undergo appropriate treatment before discharge. Advanced waste handling protocols incorporating thermal treatment, additional filtration stages, or biological processing can reduce environmental hazards associated with these concentrated streams. Studies indicate that properly managed ultrafiltration waste can achieve 95-99% reduction in pathogenic load before environmental release.
The life cycle assessment of ultrafiltration membranes reveals additional environmental considerations. With typical operational lifespans of 3-7 years, membrane replacement contributes to material consumption and waste generation. Emerging membrane technologies utilizing biodegradable polymers and recyclable components offer promising alternatives, potentially reducing end-of-life environmental impact by 30-50% compared to conventional membrane materials.
When implemented with appropriate environmental controls, ultrafiltration processes for microbial reduction can deliver net positive environmental outcomes by preventing waterborne disease transmission and reducing the need for chemical disinfection methods that may produce harmful byproducts. Comprehensive environmental management systems that address energy efficiency, water conservation, chemical usage, and waste handling are essential for maximizing these benefits while minimizing ecological footprint.
Water usage in ultrafiltration systems presents another critical environmental consideration. While these processes are designed to treat water, they also consume substantial volumes during backwashing and cleaning cycles. Modern optimized systems have demonstrated the ability to reduce this consumption by implementing advanced cleaning protocols that minimize frequency and duration of backwashing operations, resulting in water savings of up to 15-25% compared to conventional approaches.
Chemical usage for membrane cleaning and maintenance contributes to the environmental impact profile. Traditional cleaning regimens often employ harsh chemicals including sodium hypochlorite, citric acid, and various surfactants that can create downstream ecological concerns if not properly managed. Recent innovations in environmentally friendly cleaning agents derived from natural sources have shown promising results, reducing ecotoxicity by 40-60% while maintaining comparable cleaning efficacy.
Waste stream management represents a significant challenge in ultrafiltration operations. The concentrated microbial waste and rejected solids must undergo appropriate treatment before discharge. Advanced waste handling protocols incorporating thermal treatment, additional filtration stages, or biological processing can reduce environmental hazards associated with these concentrated streams. Studies indicate that properly managed ultrafiltration waste can achieve 95-99% reduction in pathogenic load before environmental release.
The life cycle assessment of ultrafiltration membranes reveals additional environmental considerations. With typical operational lifespans of 3-7 years, membrane replacement contributes to material consumption and waste generation. Emerging membrane technologies utilizing biodegradable polymers and recyclable components offer promising alternatives, potentially reducing end-of-life environmental impact by 30-50% compared to conventional membrane materials.
When implemented with appropriate environmental controls, ultrafiltration processes for microbial reduction can deliver net positive environmental outcomes by preventing waterborne disease transmission and reducing the need for chemical disinfection methods that may produce harmful byproducts. Comprehensive environmental management systems that address energy efficiency, water conservation, chemical usage, and waste handling are essential for maximizing these benefits while minimizing ecological footprint.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!