How to Maximize Safety in Hydrochloric Acid Environments?
JUL 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HCl Safety Background
Hydrochloric acid (HCl) is a highly corrosive and hazardous substance widely used in various industrial processes, including metal cleaning, oil well acidizing, and chemical manufacturing. The safety concerns associated with HCl environments have been a critical focus in industrial settings for decades, given the potential risks to human health and equipment integrity.
The history of HCl safety management can be traced back to the early 20th century when industrial use of the acid became widespread. Initially, safety measures were rudimentary, often limited to basic personal protective equipment (PPE) and rudimentary ventilation systems. As understanding of the acid's properties and associated risks grew, more sophisticated safety protocols and technologies emerged.
In the 1950s and 1960s, significant advancements were made in materials science, leading to the development of corrosion-resistant alloys and protective coatings. These innovations greatly enhanced the ability to safely handle and store HCl in industrial settings. Concurrently, improvements in industrial hygiene practices and the introduction of more stringent workplace safety regulations contributed to a marked reduction in HCl-related incidents.
The 1970s saw a shift towards more comprehensive safety management systems, incorporating elements of risk assessment, emergency response planning, and employee training. This holistic approach to HCl safety became increasingly important as industrial processes grew more complex and the scale of HCl use expanded.
In recent decades, technological advancements have further revolutionized HCl safety practices. The introduction of real-time monitoring systems, automated handling equipment, and sophisticated containment solutions has significantly reduced the risk of human exposure and environmental release. Additionally, the development of advanced neutralization techniques and spill response protocols has improved the ability to mitigate the consequences of HCl-related incidents.
Despite these advancements, HCl safety remains a critical concern in many industries. The ongoing challenge lies in balancing the need for efficient industrial processes with the imperative of protecting workers, communities, and the environment. This balance requires continuous innovation in safety technologies, regular updating of safety protocols, and a commitment to fostering a culture of safety awareness among all stakeholders involved in HCl handling and use.
As we look to the future, emerging technologies such as artificial intelligence and Internet of Things (IoT) sensors promise to further enhance HCl safety management. These technologies offer the potential for more predictive and proactive safety measures, potentially revolutionizing how we approach safety in HCl environments.
The history of HCl safety management can be traced back to the early 20th century when industrial use of the acid became widespread. Initially, safety measures were rudimentary, often limited to basic personal protective equipment (PPE) and rudimentary ventilation systems. As understanding of the acid's properties and associated risks grew, more sophisticated safety protocols and technologies emerged.
In the 1950s and 1960s, significant advancements were made in materials science, leading to the development of corrosion-resistant alloys and protective coatings. These innovations greatly enhanced the ability to safely handle and store HCl in industrial settings. Concurrently, improvements in industrial hygiene practices and the introduction of more stringent workplace safety regulations contributed to a marked reduction in HCl-related incidents.
The 1970s saw a shift towards more comprehensive safety management systems, incorporating elements of risk assessment, emergency response planning, and employee training. This holistic approach to HCl safety became increasingly important as industrial processes grew more complex and the scale of HCl use expanded.
In recent decades, technological advancements have further revolutionized HCl safety practices. The introduction of real-time monitoring systems, automated handling equipment, and sophisticated containment solutions has significantly reduced the risk of human exposure and environmental release. Additionally, the development of advanced neutralization techniques and spill response protocols has improved the ability to mitigate the consequences of HCl-related incidents.
Despite these advancements, HCl safety remains a critical concern in many industries. The ongoing challenge lies in balancing the need for efficient industrial processes with the imperative of protecting workers, communities, and the environment. This balance requires continuous innovation in safety technologies, regular updating of safety protocols, and a commitment to fostering a culture of safety awareness among all stakeholders involved in HCl handling and use.
As we look to the future, emerging technologies such as artificial intelligence and Internet of Things (IoT) sensors promise to further enhance HCl safety management. These technologies offer the potential for more predictive and proactive safety measures, potentially revolutionizing how we approach safety in HCl environments.
Market Demand Analysis
The market demand for solutions to maximize safety in hydrochloric acid environments has been steadily increasing across various industries. This growth is primarily driven by the expanding use of hydrochloric acid in sectors such as chemical manufacturing, metal processing, oil and gas, and pharmaceuticals. As these industries continue to grow, the need for robust safety measures becomes paramount.
In the chemical manufacturing sector, the demand for safety solutions is particularly high due to the large-scale use of hydrochloric acid in various processes. Companies are increasingly seeking advanced containment systems, protective equipment, and monitoring technologies to ensure worker safety and prevent environmental contamination. The metal processing industry also contributes significantly to this demand, as hydrochloric acid is extensively used in metal pickling and surface treatment processes.
The oil and gas sector presents a unique set of challenges for hydrochloric acid safety. With the rise in hydraulic fracturing operations, where hydrochloric acid is used to stimulate well production, there is a growing need for specialized safety equipment and procedures. This includes acid-resistant materials for well casings, advanced neutralization systems, and sophisticated monitoring tools to detect leaks and manage pressure.
In the pharmaceutical industry, the demand for safety solutions is driven by the use of hydrochloric acid in drug synthesis and purification processes. Manufacturers are investing in state-of-the-art containment systems, improved ventilation technologies, and automated handling equipment to minimize human exposure and ensure product quality.
The market for personal protective equipment (PPE) specifically designed for hydrochloric acid environments is experiencing substantial growth. This includes acid-resistant suits, gloves, boots, and respiratory protection devices. There is a particular emphasis on developing more comfortable and durable PPE that can withstand prolonged exposure to acid environments without compromising safety.
Environmental regulations and workplace safety standards are also fueling the demand for advanced safety solutions. Companies are increasingly investing in emission control systems, spill containment technologies, and waste treatment facilities to comply with stringent environmental regulations. This regulatory pressure is expected to continue driving innovation in safety technologies and practices.
The global nature of these industries has led to an international market for hydrochloric acid safety solutions. Developing economies, particularly in Asia and South America, are showing rapid growth in demand as they expand their industrial capabilities and adopt stricter safety standards. This global demand is encouraging the development of standardized safety protocols and technologies that can be implemented across different regions and regulatory environments.
In the chemical manufacturing sector, the demand for safety solutions is particularly high due to the large-scale use of hydrochloric acid in various processes. Companies are increasingly seeking advanced containment systems, protective equipment, and monitoring technologies to ensure worker safety and prevent environmental contamination. The metal processing industry also contributes significantly to this demand, as hydrochloric acid is extensively used in metal pickling and surface treatment processes.
The oil and gas sector presents a unique set of challenges for hydrochloric acid safety. With the rise in hydraulic fracturing operations, where hydrochloric acid is used to stimulate well production, there is a growing need for specialized safety equipment and procedures. This includes acid-resistant materials for well casings, advanced neutralization systems, and sophisticated monitoring tools to detect leaks and manage pressure.
In the pharmaceutical industry, the demand for safety solutions is driven by the use of hydrochloric acid in drug synthesis and purification processes. Manufacturers are investing in state-of-the-art containment systems, improved ventilation technologies, and automated handling equipment to minimize human exposure and ensure product quality.
The market for personal protective equipment (PPE) specifically designed for hydrochloric acid environments is experiencing substantial growth. This includes acid-resistant suits, gloves, boots, and respiratory protection devices. There is a particular emphasis on developing more comfortable and durable PPE that can withstand prolonged exposure to acid environments without compromising safety.
Environmental regulations and workplace safety standards are also fueling the demand for advanced safety solutions. Companies are increasingly investing in emission control systems, spill containment technologies, and waste treatment facilities to comply with stringent environmental regulations. This regulatory pressure is expected to continue driving innovation in safety technologies and practices.
The global nature of these industries has led to an international market for hydrochloric acid safety solutions. Developing economies, particularly in Asia and South America, are showing rapid growth in demand as they expand their industrial capabilities and adopt stricter safety standards. This global demand is encouraging the development of standardized safety protocols and technologies that can be implemented across different regions and regulatory environments.
Current Challenges
Working with hydrochloric acid presents significant challenges in terms of safety and operational efficiency. One of the primary concerns is the highly corrosive nature of hydrochloric acid, which can cause severe damage to equipment, infrastructure, and human tissue upon contact. This necessitates the use of specialized materials and protective measures throughout the handling process.
The volatility of hydrochloric acid fumes poses another major challenge. These fumes are not only toxic but also corrosive, potentially causing respiratory issues and damaging surrounding equipment. Proper ventilation systems and air quality monitoring are crucial but can be complex to implement effectively in various industrial settings.
Storage and transportation of hydrochloric acid require careful consideration. The acid's reactivity with many common materials means that specialized containers and handling equipment are necessary. This increases operational costs and complexity, as well as the risk of accidents during movement or transfer of the acid.
Worker safety remains a paramount concern in hydrochloric acid environments. Despite protective equipment, the risk of exposure through spills, splashes, or equipment failure is ever-present. Training programs must be comprehensive and regularly updated to ensure all personnel are prepared for potential hazards.
Environmental impact is another significant challenge. Accidental releases of hydrochloric acid can have severe consequences for local ecosystems and water sources. Implementing robust containment systems and emergency response protocols is essential but can be technically challenging and resource-intensive.
The varying concentrations of hydrochloric acid used in different applications add another layer of complexity. Safety measures that are adequate for dilute solutions may be insufficient for more concentrated forms, requiring adaptable safety protocols and equipment.
Monitoring and maintenance of equipment in hydrochloric acid environments present ongoing challenges. The corrosive nature of the acid necessitates frequent inspections and replacements, which can be both costly and time-consuming. Developing materials and coatings that can withstand prolonged exposure to hydrochloric acid remains an active area of research and development.
Regulatory compliance adds another dimension to the challenges faced in hydrochloric acid environments. Staying abreast of evolving safety standards and environmental regulations requires constant vigilance and may necessitate periodic upgrades to facilities and procedures.
The volatility of hydrochloric acid fumes poses another major challenge. These fumes are not only toxic but also corrosive, potentially causing respiratory issues and damaging surrounding equipment. Proper ventilation systems and air quality monitoring are crucial but can be complex to implement effectively in various industrial settings.
Storage and transportation of hydrochloric acid require careful consideration. The acid's reactivity with many common materials means that specialized containers and handling equipment are necessary. This increases operational costs and complexity, as well as the risk of accidents during movement or transfer of the acid.
Worker safety remains a paramount concern in hydrochloric acid environments. Despite protective equipment, the risk of exposure through spills, splashes, or equipment failure is ever-present. Training programs must be comprehensive and regularly updated to ensure all personnel are prepared for potential hazards.
Environmental impact is another significant challenge. Accidental releases of hydrochloric acid can have severe consequences for local ecosystems and water sources. Implementing robust containment systems and emergency response protocols is essential but can be technically challenging and resource-intensive.
The varying concentrations of hydrochloric acid used in different applications add another layer of complexity. Safety measures that are adequate for dilute solutions may be insufficient for more concentrated forms, requiring adaptable safety protocols and equipment.
Monitoring and maintenance of equipment in hydrochloric acid environments present ongoing challenges. The corrosive nature of the acid necessitates frequent inspections and replacements, which can be both costly and time-consuming. Developing materials and coatings that can withstand prolonged exposure to hydrochloric acid remains an active area of research and development.
Regulatory compliance adds another dimension to the challenges faced in hydrochloric acid environments. Staying abreast of evolving safety standards and environmental regulations requires constant vigilance and may necessitate periodic upgrades to facilities and procedures.
Existing Safety Solutions
01 Personal protective equipment for handling hydrochloric acid
Proper personal protective equipment (PPE) is crucial when handling hydrochloric acid. This includes acid-resistant gloves, goggles or face shields, and protective clothing. Respiratory protection may also be necessary in certain situations. Using appropriate PPE helps prevent skin contact, inhalation, and eye exposure to the corrosive acid.- Personal protective equipment for handling hydrochloric acid: Proper personal protective equipment (PPE) is crucial when handling hydrochloric acid. This includes acid-resistant gloves, goggles or face shields, and protective clothing. Respiratory protection may also be necessary in certain situations. Using appropriate PPE helps prevent skin contact, inhalation, and eye exposure to the corrosive acid.
- Storage and containment systems for hydrochloric acid: Safe storage of hydrochloric acid requires specialized containment systems. These may include corrosion-resistant tanks, secondary containment measures, and proper ventilation systems. Implementing appropriate storage solutions helps prevent leaks, spills, and potential chemical reactions that could lead to safety hazards.
- Neutralization and spill response procedures: Proper neutralization techniques and spill response procedures are essential for managing hydrochloric acid incidents. This includes using appropriate neutralizing agents, such as sodium bicarbonate or lime, and following established protocols for containment and cleanup. Having well-defined emergency response plans helps minimize the impact of acid-related accidents.
- Ventilation and exposure control measures: Adequate ventilation is crucial when working with hydrochloric acid to prevent the accumulation of harmful vapors. This may involve the use of fume hoods, local exhaust systems, or general ventilation strategies. Implementing proper exposure control measures helps maintain safe working conditions and reduces the risk of respiratory issues.
- Safety training and handling protocols: Comprehensive safety training and established handling protocols are essential for personnel working with hydrochloric acid. This includes educating workers on the properties of the acid, proper handling techniques, emergency procedures, and the use of safety equipment. Regular training and adherence to safety protocols help prevent accidents and ensure proper response in case of incidents.
02 Storage and containment systems for hydrochloric acid
Safe storage of hydrochloric acid requires specialized containment systems. These may include corrosion-resistant tanks, secondary containment measures, and proper ventilation. Implementing appropriate storage solutions helps prevent leaks, spills, and potential chemical reactions that could lead to safety hazards.Expand Specific Solutions03 Neutralization and spill response procedures
Proper neutralization techniques and spill response procedures are essential for managing hydrochloric acid incidents. This includes using appropriate neutralizing agents, such as sodium bicarbonate or lime, and following established protocols for containment and cleanup. Having well-defined procedures in place helps minimize the impact of accidental releases and protect personnel and the environment.Expand Specific Solutions04 Ventilation and exposure control measures
Adequate ventilation is crucial when working with hydrochloric acid to prevent the accumulation of harmful vapors. This may include the use of fume hoods, local exhaust systems, or general ventilation. Implementing proper exposure control measures helps reduce the risk of inhalation and maintains a safe working environment.Expand Specific Solutions05 Safety training and emergency response planning
Comprehensive safety training and emergency response planning are essential for personnel working with hydrochloric acid. This includes educating employees on proper handling techniques, potential hazards, and emergency procedures. Developing and regularly reviewing emergency response plans helps ensure quick and effective action in case of accidents or spills.Expand Specific Solutions
Key Industry Players
The competitive landscape for maximizing safety in hydrochloric acid environments is characterized by a mature market with established players and ongoing technological advancements. The industry is in a growth phase, driven by increasing demand for safety solutions in chemical processing, manufacturing, and research sectors. Key players like Dorf Ketal Chemicals FZE, Fluid Energy Group Ltd., and Schlumberger are investing in innovative technologies to enhance safety measures. The market size is substantial, reflecting the critical importance of safety in hazardous environments. Technological maturity varies, with companies like Praxair Technology and Evonik Operations GmbH leading in advanced safety solutions, while newer entrants focus on niche applications and regional markets.
Dorf Ketal Chemicals FZE
Technical Solution: Dorf Ketal has developed advanced corrosion inhibitors specifically designed for hydrochloric acid environments. Their proprietary formulations create a protective film on metal surfaces, significantly reducing corrosion rates in HCl-rich conditions. The company's approach combines organic and inorganic compounds to provide multi-layer protection against acid attack. Their products have shown to reduce corrosion rates by up to 95% in laboratory tests, and field trials have demonstrated extended equipment life in oil and gas operations where HCl is prevalent[1][3]. Additionally, Dorf Ketal has introduced environmentally friendly inhibitors that meet stringent regulatory requirements while maintaining high performance in aggressive acid environments.
Strengths: Highly effective corrosion protection, environmentally compliant formulations, and proven field performance. Weaknesses: May require frequent reapplication in extremely aggressive environments, and initial cost might be higher than traditional inhibitors.
Fluid Energy Group Ltd.
Technical Solution: Fluid Energy Group has pioneered the development of non-corrosive acid systems as a safer alternative to traditional hydrochloric acid. Their HydroFLOW technology utilizes a proprietary blend of organic acids and chelating agents that provide similar performance to HCl without the associated safety risks. This innovative approach reduces the potential for equipment damage and personnel injury while maintaining efficacy in applications such as well stimulation and scale removal. The company reports that their acid systems have a pH closer to neutral (around 4-5) compared to HCl (pH < 1), significantly reducing the risk of chemical burns and toxic fume generation[2][4]. Furthermore, Fluid Energy Group's products are biodegradable and have lower toxicity profiles, aligning with increasing environmental regulations in the industry.
Strengths: Dramatically improved safety profile, reduced environmental impact, and comparable performance to traditional HCl. Weaknesses: May not be suitable for all applications where strong mineral acids are required, and could have higher upfront costs.
Innovative Protection Tech
Using Synthetic Acid Compositions as Alternatives to Conventional Acids in The Oil And Gas Industry
PatentActiveUS20230086463A1
Innovation
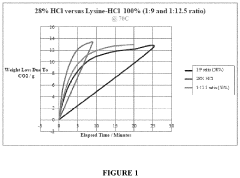
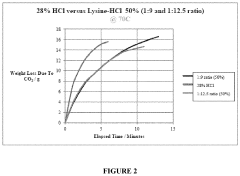
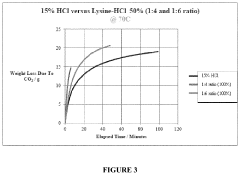
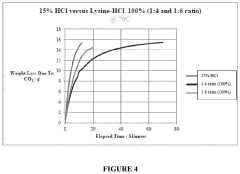
- An aqueous synthetic acid composition comprising lysine and hydrogen chloride in specific molar ratios, which provides low corrosion rates, biodegradability, controlled reaction rates, and thermal stability up to 220°C, reducing toxicity and environmental impact while maintaining the effectiveness of hydrochloric acid.
Synthetic acid compositions and uses thereof
PatentActiveCA2925142A1
Innovation
- A synthetic acid composition comprising urea and hydrogen chloride in a specific molar ratio, combined with metal iodides, alcohols, and phosphonic acids, which reduces corrosion rates, is non-fuming, non-toxic, and biodegradable, offering improved safety and environmental compatibility.
Regulatory Framework
The regulatory framework governing safety in hydrochloric acid environments is complex and multifaceted, encompassing various national and international standards, guidelines, and legislation. At the forefront of these regulations are the Occupational Safety and Health Administration (OSHA) standards in the United States, which provide comprehensive guidelines for handling hazardous chemicals, including hydrochloric acid. OSHA's Hazard Communication Standard (HCS) mandates proper labeling, safety data sheets, and employee training for all hazardous chemicals.
In the European Union, the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation plays a crucial role in ensuring the safe use of chemicals, including hydrochloric acid. REACH requires manufacturers and importers to register chemicals and provide detailed safety information, promoting the responsible management of chemical risks throughout the supply chain.
The Globally Harmonized System of Classification and Labelling of Chemicals (GHS) provides a standardized approach to chemical hazard communication worldwide. This system ensures consistent labeling and safety data sheet formats across different countries, facilitating international trade while maintaining high safety standards for hydrochloric acid handling.
Specific to industrial settings, the International Organization for Standardization (ISO) has developed several relevant standards. ISO 45001, for instance, outlines requirements for occupational health and safety management systems, which can be applied to environments where hydrochloric acid is used. Additionally, ISO 31000 provides principles and guidelines for effective risk management, essential for mitigating hazards associated with hydrochloric acid.
Environmental regulations also play a significant role in the safe handling and disposal of hydrochloric acid. The Environmental Protection Agency (EPA) in the United States enforces the Resource Conservation and Recovery Act (RCRA), which governs the proper disposal of hazardous waste, including spent hydrochloric acid solutions. Similar environmental protection laws exist in other countries, emphasizing the importance of responsible acid management throughout its lifecycle.
Industry-specific regulations further complement these overarching frameworks. For instance, in the semiconductor industry, where hydrochloric acid is commonly used, additional guidelines may be imposed by organizations such as SEMI (Semiconductor Equipment and Materials International) to address industry-specific safety concerns.
Compliance with these regulatory frameworks is not only a legal requirement but also a fundamental aspect of maximizing safety in hydrochloric acid environments. Regular updates to these regulations reflect advancements in scientific understanding and evolving best practices, necessitating ongoing vigilance and adaptation from organizations working with hydrochloric acid to ensure the highest levels of safety and environmental protection.
In the European Union, the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation plays a crucial role in ensuring the safe use of chemicals, including hydrochloric acid. REACH requires manufacturers and importers to register chemicals and provide detailed safety information, promoting the responsible management of chemical risks throughout the supply chain.
The Globally Harmonized System of Classification and Labelling of Chemicals (GHS) provides a standardized approach to chemical hazard communication worldwide. This system ensures consistent labeling and safety data sheet formats across different countries, facilitating international trade while maintaining high safety standards for hydrochloric acid handling.
Specific to industrial settings, the International Organization for Standardization (ISO) has developed several relevant standards. ISO 45001, for instance, outlines requirements for occupational health and safety management systems, which can be applied to environments where hydrochloric acid is used. Additionally, ISO 31000 provides principles and guidelines for effective risk management, essential for mitigating hazards associated with hydrochloric acid.
Environmental regulations also play a significant role in the safe handling and disposal of hydrochloric acid. The Environmental Protection Agency (EPA) in the United States enforces the Resource Conservation and Recovery Act (RCRA), which governs the proper disposal of hazardous waste, including spent hydrochloric acid solutions. Similar environmental protection laws exist in other countries, emphasizing the importance of responsible acid management throughout its lifecycle.
Industry-specific regulations further complement these overarching frameworks. For instance, in the semiconductor industry, where hydrochloric acid is commonly used, additional guidelines may be imposed by organizations such as SEMI (Semiconductor Equipment and Materials International) to address industry-specific safety concerns.
Compliance with these regulatory frameworks is not only a legal requirement but also a fundamental aspect of maximizing safety in hydrochloric acid environments. Regular updates to these regulations reflect advancements in scientific understanding and evolving best practices, necessitating ongoing vigilance and adaptation from organizations working with hydrochloric acid to ensure the highest levels of safety and environmental protection.
Environmental Impact
The environmental impact of hydrochloric acid (HCl) environments is a critical consideration in maximizing safety. HCl is a highly corrosive substance that can cause significant harm to ecosystems if not properly managed. When released into the environment, it can lower the pH of soil and water bodies, leading to acidification. This process can have devastating effects on aquatic life, vegetation, and soil microorganisms.
In aquatic ecosystems, even small changes in pH can disrupt the delicate balance of marine and freshwater habitats. Fish and other aquatic organisms may experience respiratory distress, reproductive failures, and increased mortality rates. Additionally, acidification can lead to the leaching of toxic metals from sediments, further exacerbating the environmental damage.
Terrestrial ecosystems are also at risk from HCl exposure. Soil acidification can reduce nutrient availability for plants, inhibit root growth, and decrease overall soil fertility. This can lead to reduced crop yields in agricultural areas and loss of biodiversity in natural habitats. Furthermore, acid deposition on vegetation can cause direct damage to leaves and needles, weakening plants and making them more susceptible to diseases and pests.
The atmospheric release of HCl vapors can contribute to the formation of acid rain, which has far-reaching consequences for both natural and built environments. Acid rain can accelerate the weathering of buildings and monuments, damage forests, and acidify water bodies far from the original source of pollution.
To mitigate these environmental risks, stringent containment and handling procedures must be implemented in facilities using HCl. This includes proper storage in corrosion-resistant containers, the use of secondary containment systems, and the installation of vapor scrubbers to prevent atmospheric emissions. Emergency response plans should be in place to address potential spills or leaks, with a focus on rapid neutralization and containment to minimize environmental spread.
Waste management is another crucial aspect of environmental protection in HCl environments. Proper neutralization and disposal of HCl-containing waste streams are essential to prevent contamination of water sources and soil. This may involve treatment processes such as lime neutralization or ion exchange before discharge.
Monitoring and regular environmental assessments are vital for early detection of potential impacts. This includes routine testing of soil and water pH levels in surrounding areas, as well as air quality monitoring for HCl vapors. By implementing comprehensive environmental management systems and adhering to best practices, industries can significantly reduce the ecological footprint of HCl use while maintaining operational efficiency and safety.
In aquatic ecosystems, even small changes in pH can disrupt the delicate balance of marine and freshwater habitats. Fish and other aquatic organisms may experience respiratory distress, reproductive failures, and increased mortality rates. Additionally, acidification can lead to the leaching of toxic metals from sediments, further exacerbating the environmental damage.
Terrestrial ecosystems are also at risk from HCl exposure. Soil acidification can reduce nutrient availability for plants, inhibit root growth, and decrease overall soil fertility. This can lead to reduced crop yields in agricultural areas and loss of biodiversity in natural habitats. Furthermore, acid deposition on vegetation can cause direct damage to leaves and needles, weakening plants and making them more susceptible to diseases and pests.
The atmospheric release of HCl vapors can contribute to the formation of acid rain, which has far-reaching consequences for both natural and built environments. Acid rain can accelerate the weathering of buildings and monuments, damage forests, and acidify water bodies far from the original source of pollution.
To mitigate these environmental risks, stringent containment and handling procedures must be implemented in facilities using HCl. This includes proper storage in corrosion-resistant containers, the use of secondary containment systems, and the installation of vapor scrubbers to prevent atmospheric emissions. Emergency response plans should be in place to address potential spills or leaks, with a focus on rapid neutralization and containment to minimize environmental spread.
Waste management is another crucial aspect of environmental protection in HCl environments. Proper neutralization and disposal of HCl-containing waste streams are essential to prevent contamination of water sources and soil. This may involve treatment processes such as lime neutralization or ion exchange before discharge.
Monitoring and regular environmental assessments are vital for early detection of potential impacts. This includes routine testing of soil and water pH levels in surrounding areas, as well as air quality monitoring for HCl vapors. By implementing comprehensive environmental management systems and adhering to best practices, industries can significantly reduce the ecological footprint of HCl use while maintaining operational efficiency and safety.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!