How to Optimize Hydrochloric Acid Neutralization Processes?
JUL 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HCl Neutralization Background and Objectives
Hydrochloric acid (HCl) neutralization is a fundamental process in various industrial applications, including wastewater treatment, chemical manufacturing, and environmental remediation. The optimization of this process has become increasingly crucial due to its widespread use and significant impact on operational efficiency, cost-effectiveness, and environmental sustainability.
The evolution of HCl neutralization techniques can be traced back to the early 20th century when basic chemical principles were applied in industrial settings. Over the decades, advancements in chemical engineering, process control, and materials science have contributed to the refinement of neutralization methods. The primary objective of these developments has been to achieve more efficient, safer, and environmentally friendly neutralization processes.
In recent years, the focus on optimizing HCl neutralization has intensified due to several factors. Stricter environmental regulations have necessitated more precise control over effluent pH levels and the reduction of chemical waste. Additionally, the increasing costs of raw materials and waste disposal have driven industries to seek more economical neutralization solutions. The advent of Industry 4.0 and smart manufacturing concepts has also opened new avenues for process optimization through data-driven approaches and automation.
The current technological landscape presents both challenges and opportunities for HCl neutralization optimization. Key areas of focus include improving reaction kinetics, enhancing mixing efficiency, developing more accurate pH monitoring and control systems, and exploring alternative neutralizing agents. Researchers and engineers are also investigating the potential of advanced materials, such as novel catalysts and ion-exchange resins, to improve the efficiency and selectivity of neutralization reactions.
Looking ahead, the technological goals for HCl neutralization optimization are multifaceted. They include developing real-time monitoring and control systems that can adapt to varying input conditions, minimizing the use of excess neutralizing agents, and reducing the overall environmental footprint of the process. There is also a growing interest in integrating neutralization processes with other treatment methods, such as membrane filtration or electrochemical systems, to create more comprehensive and efficient wastewater treatment solutions.
As industries continue to seek ways to improve their operational efficiency and environmental performance, the optimization of HCl neutralization processes remains a critical area of research and development. The ongoing efforts in this field are expected to yield innovative solutions that will not only enhance the effectiveness of neutralization processes but also contribute to broader sustainability goals across various industrial sectors.
The evolution of HCl neutralization techniques can be traced back to the early 20th century when basic chemical principles were applied in industrial settings. Over the decades, advancements in chemical engineering, process control, and materials science have contributed to the refinement of neutralization methods. The primary objective of these developments has been to achieve more efficient, safer, and environmentally friendly neutralization processes.
In recent years, the focus on optimizing HCl neutralization has intensified due to several factors. Stricter environmental regulations have necessitated more precise control over effluent pH levels and the reduction of chemical waste. Additionally, the increasing costs of raw materials and waste disposal have driven industries to seek more economical neutralization solutions. The advent of Industry 4.0 and smart manufacturing concepts has also opened new avenues for process optimization through data-driven approaches and automation.
The current technological landscape presents both challenges and opportunities for HCl neutralization optimization. Key areas of focus include improving reaction kinetics, enhancing mixing efficiency, developing more accurate pH monitoring and control systems, and exploring alternative neutralizing agents. Researchers and engineers are also investigating the potential of advanced materials, such as novel catalysts and ion-exchange resins, to improve the efficiency and selectivity of neutralization reactions.
Looking ahead, the technological goals for HCl neutralization optimization are multifaceted. They include developing real-time monitoring and control systems that can adapt to varying input conditions, minimizing the use of excess neutralizing agents, and reducing the overall environmental footprint of the process. There is also a growing interest in integrating neutralization processes with other treatment methods, such as membrane filtration or electrochemical systems, to create more comprehensive and efficient wastewater treatment solutions.
As industries continue to seek ways to improve their operational efficiency and environmental performance, the optimization of HCl neutralization processes remains a critical area of research and development. The ongoing efforts in this field are expected to yield innovative solutions that will not only enhance the effectiveness of neutralization processes but also contribute to broader sustainability goals across various industrial sectors.
Industrial Demand for Efficient Neutralization
The industrial demand for efficient hydrochloric acid neutralization processes has been steadily increasing across various sectors. Chemical manufacturing, water treatment, and metal processing industries are at the forefront of this demand, seeking more cost-effective and environmentally friendly solutions. The need for optimization stems from several factors, including rising operational costs, stringent environmental regulations, and the push for sustainable practices.
In the chemical manufacturing sector, hydrochloric acid is a common byproduct of many processes. Efficient neutralization is crucial for waste management and compliance with discharge regulations. Companies are looking for ways to reduce the volume of neutralizing agents used while maintaining effective pH control. This not only cuts down on material costs but also minimizes the environmental impact of the neutralization process.
The water treatment industry faces similar challenges, particularly in areas where industrial effluents contain high levels of hydrochloric acid. Municipal water treatment plants and industrial wastewater facilities are under pressure to handle increasing volumes of acidic waste while adhering to tighter environmental standards. There is a growing demand for neutralization technologies that can handle fluctuating acid concentrations efficiently and with minimal chemical usage.
Metal processing industries, including steel manufacturing and metal finishing, generate significant amounts of hydrochloric acid waste. These sectors are actively seeking optimized neutralization processes to reduce treatment costs and improve overall operational efficiency. The ability to recover and reuse neutralizing agents or to generate valuable byproducts from the neutralization process is becoming increasingly attractive to these industries.
The pharmaceutical industry is another significant contributor to the demand for efficient neutralization. With the production of various active pharmaceutical ingredients (APIs) often involving hydrochloric acid, there is a critical need for precise and efficient neutralization to ensure product quality and safety while minimizing waste.
Environmental concerns are driving innovation in neutralization technologies. Industries are looking for solutions that not only effectively neutralize hydrochloric acid but also reduce the carbon footprint of the process. This has led to increased interest in green chemistry approaches and the use of renewable or waste-derived neutralizing agents.
The global push towards circular economy models is influencing the demand for more efficient neutralization processes. Industries are exploring ways to integrate neutralization into broader waste management and resource recovery systems, aiming to transform what was once considered a costly treatment process into a potential source of value.
As automation and Industry 4.0 technologies continue to advance, there is a growing demand for smart neutralization systems that can adapt in real-time to changing acid concentrations and process conditions. This trend is driving the development of more sophisticated control systems and sensor technologies for optimized neutralization processes.
In the chemical manufacturing sector, hydrochloric acid is a common byproduct of many processes. Efficient neutralization is crucial for waste management and compliance with discharge regulations. Companies are looking for ways to reduce the volume of neutralizing agents used while maintaining effective pH control. This not only cuts down on material costs but also minimizes the environmental impact of the neutralization process.
The water treatment industry faces similar challenges, particularly in areas where industrial effluents contain high levels of hydrochloric acid. Municipal water treatment plants and industrial wastewater facilities are under pressure to handle increasing volumes of acidic waste while adhering to tighter environmental standards. There is a growing demand for neutralization technologies that can handle fluctuating acid concentrations efficiently and with minimal chemical usage.
Metal processing industries, including steel manufacturing and metal finishing, generate significant amounts of hydrochloric acid waste. These sectors are actively seeking optimized neutralization processes to reduce treatment costs and improve overall operational efficiency. The ability to recover and reuse neutralizing agents or to generate valuable byproducts from the neutralization process is becoming increasingly attractive to these industries.
The pharmaceutical industry is another significant contributor to the demand for efficient neutralization. With the production of various active pharmaceutical ingredients (APIs) often involving hydrochloric acid, there is a critical need for precise and efficient neutralization to ensure product quality and safety while minimizing waste.
Environmental concerns are driving innovation in neutralization technologies. Industries are looking for solutions that not only effectively neutralize hydrochloric acid but also reduce the carbon footprint of the process. This has led to increased interest in green chemistry approaches and the use of renewable or waste-derived neutralizing agents.
The global push towards circular economy models is influencing the demand for more efficient neutralization processes. Industries are exploring ways to integrate neutralization into broader waste management and resource recovery systems, aiming to transform what was once considered a costly treatment process into a potential source of value.
As automation and Industry 4.0 technologies continue to advance, there is a growing demand for smart neutralization systems that can adapt in real-time to changing acid concentrations and process conditions. This trend is driving the development of more sophisticated control systems and sensor technologies for optimized neutralization processes.
Current Challenges in HCl Neutralization
The optimization of hydrochloric acid (HCl) neutralization processes faces several significant challenges in the current industrial landscape. One of the primary issues is the control of reaction kinetics and heat generation. HCl neutralization is highly exothermic, and managing the heat release is crucial to prevent equipment damage and ensure process safety. The rapid reaction rate can lead to localized hot spots, potentially causing material degradation or unwanted side reactions.
Another challenge lies in the precise control of pH levels throughout the neutralization process. Achieving and maintaining the desired pH is critical for effective neutralization and subsequent waste treatment or product quality. However, the non-linear nature of the pH curve near the neutralization point makes it difficult to implement accurate control systems, often resulting in pH oscillations or overshooting.
The selection and efficiency of neutralizing agents pose additional challenges. While commonly used bases like sodium hydroxide (NaOH) or calcium hydroxide (Ca(OH)2) are effective, they may introduce unwanted ions into the system or create disposal issues for the resulting salt solutions. Finding cost-effective and environmentally friendly alternatives that do not compromise neutralization efficiency remains an ongoing challenge.
Corrosion management is another significant concern in HCl neutralization processes. The highly corrosive nature of HCl, especially at elevated temperatures, can lead to rapid deterioration of equipment and piping. This necessitates the use of expensive corrosion-resistant materials or frequent maintenance, both of which increase operational costs.
The handling and storage of HCl and neutralizing agents also present safety and logistical challenges. Proper containment, transfer systems, and personal protective equipment are essential to prevent accidents and ensure worker safety. Additionally, the transportation and on-site storage of large quantities of these chemicals require careful planning and adherence to strict regulations.
Waste management and environmental considerations further complicate HCl neutralization processes. The resulting salt solutions or sludges from neutralization often require additional treatment or disposal, which can be costly and environmentally impactful. Developing more sustainable neutralization methods that minimize waste generation or enable easier recycling of byproducts is an ongoing challenge for the industry.
Lastly, the optimization of HCl neutralization processes is hindered by the variability in feed composition and flow rates in many industrial applications. Fluctuations in acid concentration or impurities can significantly affect the neutralization process, requiring robust control systems and flexible process designs to maintain consistent performance under varying conditions.
Another challenge lies in the precise control of pH levels throughout the neutralization process. Achieving and maintaining the desired pH is critical for effective neutralization and subsequent waste treatment or product quality. However, the non-linear nature of the pH curve near the neutralization point makes it difficult to implement accurate control systems, often resulting in pH oscillations or overshooting.
The selection and efficiency of neutralizing agents pose additional challenges. While commonly used bases like sodium hydroxide (NaOH) or calcium hydroxide (Ca(OH)2) are effective, they may introduce unwanted ions into the system or create disposal issues for the resulting salt solutions. Finding cost-effective and environmentally friendly alternatives that do not compromise neutralization efficiency remains an ongoing challenge.
Corrosion management is another significant concern in HCl neutralization processes. The highly corrosive nature of HCl, especially at elevated temperatures, can lead to rapid deterioration of equipment and piping. This necessitates the use of expensive corrosion-resistant materials or frequent maintenance, both of which increase operational costs.
The handling and storage of HCl and neutralizing agents also present safety and logistical challenges. Proper containment, transfer systems, and personal protective equipment are essential to prevent accidents and ensure worker safety. Additionally, the transportation and on-site storage of large quantities of these chemicals require careful planning and adherence to strict regulations.
Waste management and environmental considerations further complicate HCl neutralization processes. The resulting salt solutions or sludges from neutralization often require additional treatment or disposal, which can be costly and environmentally impactful. Developing more sustainable neutralization methods that minimize waste generation or enable easier recycling of byproducts is an ongoing challenge for the industry.
Lastly, the optimization of HCl neutralization processes is hindered by the variability in feed composition and flow rates in many industrial applications. Fluctuations in acid concentration or impurities can significantly affect the neutralization process, requiring robust control systems and flexible process designs to maintain consistent performance under varying conditions.
Existing HCl Neutralization Methods
01 Continuous neutralization process
Optimization of hydrochloric acid neutralization can be achieved through continuous processes. This involves the use of specialized equipment and flow systems to ensure constant mixing and reaction of the acid with neutralizing agents. Continuous processes offer better control over reaction conditions, improved efficiency, and reduced waste compared to batch processes.- Optimization of neutralization reactors: Improving the design and operation of neutralization reactors can enhance the efficiency of hydrochloric acid neutralization processes. This includes optimizing reactor geometry, mixing mechanisms, and residence time to ensure complete neutralization while minimizing reagent consumption.
- Use of alternative neutralizing agents: Exploring and implementing alternative neutralizing agents can improve the efficiency and cost-effectiveness of hydrochloric acid neutralization. This may involve using industrial by-products, waste materials, or novel compounds that offer better neutralization performance or additional benefits.
- Process control and automation: Implementing advanced process control systems and automation technologies can optimize hydrochloric acid neutralization processes. This includes real-time monitoring of pH, temperature, and reagent dosing, as well as the use of predictive models and feedback control loops to maintain optimal operating conditions.
- Recovery and recycling of by-products: Developing methods to recover and recycle valuable by-products from the neutralization process can improve overall efficiency and reduce waste. This may include techniques for separating and purifying salts or other compounds formed during neutralization for reuse or sale.
- Energy efficiency improvements: Enhancing the energy efficiency of hydrochloric acid neutralization processes can lead to cost savings and reduced environmental impact. This involves optimizing heat recovery systems, implementing more efficient mixing and pumping technologies, and exploring low-energy neutralization methods.
02 Use of specific neutralizing agents
The choice of neutralizing agents plays a crucial role in optimizing hydrochloric acid neutralization. Various compounds such as calcium carbonate, sodium hydroxide, or magnesium hydroxide can be used. The selection depends on factors like reaction speed, cost-effectiveness, and the desired end product. Some agents may offer additional benefits like reduced sludge formation or easier handling.Expand Specific Solutions03 Temperature and pressure control
Optimizing the temperature and pressure conditions during the neutralization process can significantly improve efficiency and product quality. Careful control of these parameters can enhance reaction rates, reduce energy consumption, and prevent unwanted side reactions. Advanced monitoring and control systems are often employed to maintain optimal conditions throughout the process.Expand Specific Solutions04 Recycling and waste minimization
Optimization of hydrochloric acid neutralization processes often involves strategies for recycling and waste minimization. This can include recovering and reusing excess neutralizing agents, implementing closed-loop systems, or finding applications for byproducts. Such approaches not only improve process efficiency but also reduce environmental impact and operating costs.Expand Specific Solutions05 Advanced monitoring and control systems
The implementation of advanced monitoring and control systems can significantly optimize hydrochloric acid neutralization processes. These may include real-time pH monitoring, automated feed systems, and computer-controlled process management. Such systems allow for precise control of reaction conditions, rapid adjustments to process parameters, and improved overall efficiency and product consistency.Expand Specific Solutions
Key Players in Chemical Neutralization Industry
The hydrochloric acid neutralization process optimization market is in a mature stage, with established players and well-defined technologies. The global market size for this sector is estimated to be in the billions, driven by industrial applications across chemical, pharmaceutical, and water treatment industries. Technologically, the field is well-developed, with companies like Arkema France SA, Sumitomo Chemical Co., Ltd., and DuPont de Nemours, Inc. leading innovation. These firms are focusing on improving efficiency, reducing environmental impact, and developing advanced control systems. Emerging players such as Tianjin Jiuri New Materials Co., Ltd. and Aquaox, Inc. are introducing novel approaches, potentially disrupting the market with more sustainable and cost-effective solutions.
Arkema France SA
Technical Solution: Arkema has developed an innovative approach to optimize hydrochloric acid neutralization processes using advanced reactor designs and process control systems. Their method involves a multi-stage neutralization process with precise pH control at each stage[1]. The company utilizes a combination of inline pH sensors and automated dosing systems to maintain optimal neutralization conditions. Arkema's process also incorporates heat recovery systems to capture and reuse the exothermic heat generated during neutralization, improving overall energy efficiency[3]. Additionally, they have implemented a real-time monitoring system that uses predictive algorithms to anticipate pH fluctuations and adjust reagent addition rates accordingly, minimizing chemical waste and improving process stability[5].
Strengths: Precise pH control, energy efficiency through heat recovery, and predictive process control. Weaknesses: Potentially higher initial investment costs and complexity in system integration.
Sumitomo Chemical Co., Ltd.
Technical Solution: Sumitomo Chemical has developed a novel approach to hydrochloric acid neutralization that focuses on sustainability and resource recovery. Their process utilizes a proprietary adsorbent material that selectively captures chloride ions from the acidic solution[2]. This allows for the separation of chlorine compounds, which can be recycled back into the production process. The remaining solution undergoes a controlled neutralization process using a cascade of reaction vessels with optimized mixing and residence times[4]. Sumitomo's system also incorporates advanced process analytical technology (PAT) to monitor and control key parameters such as temperature, pH, and conductivity in real-time, ensuring consistent neutralization performance[6]. The company claims their method reduces alkali consumption by up to 15% compared to conventional neutralization processes.
Strengths: Resource recovery capabilities, reduced chemical consumption, and advanced process control. Weaknesses: Potentially higher operational complexity and specialized adsorbent material requirements.
Innovative Approaches in Acid Neutralization
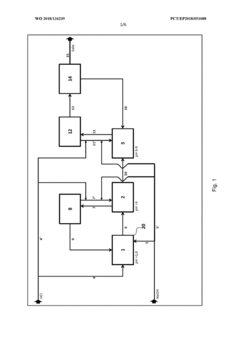
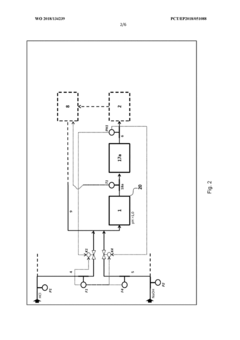
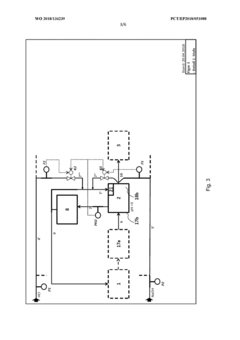
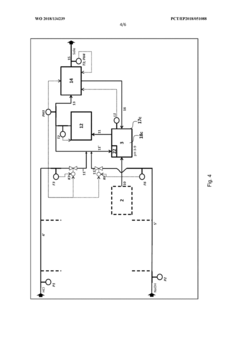
Method for flexibly controlling the use of hydrochloric acid from chemical production
PatentWO2018134239A1
Innovation
- A flexible control process for hydrochloric acid management involves neutralizing hydrochloric acid with concentrated alkali, specifically sodium hydroxide, in a multi-stage continuous process that adjusts pH values and compensates for flow and concentration variations, allowing for efficient handling and recycling of hydrochloric acid even when traditional acceptance points are unavailable.
Method and device for the continuous neutralization of hydrochloric acid
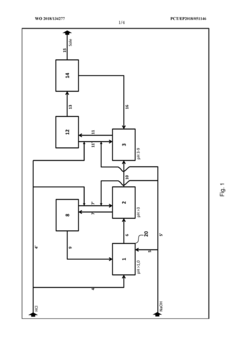
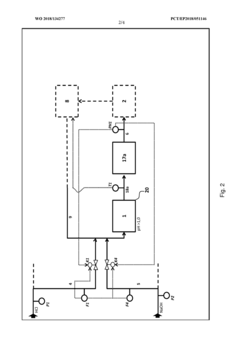
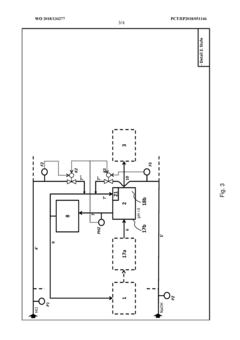
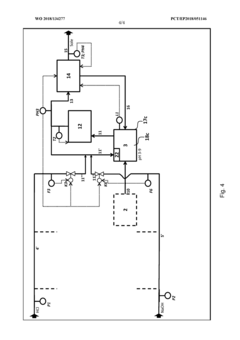
PatentWO2018134277A1
Innovation
- A three-stage neutralization process using cooled, recirculated partial streams of the reaction mixture, with static mixers and mixing nozzles for homogenization, and duplicated dosing valves for precise pH adjustment, allowing for continuous operation and heat dissipation through cooling water circuits.
Environmental Impact of Neutralization Processes
The environmental impact of hydrochloric acid neutralization processes is a critical consideration in industrial operations. These processes, while essential for maintaining pH balance and treating acidic waste streams, can have significant consequences for the surrounding ecosystem if not managed properly.
Neutralization reactions typically involve the combination of hydrochloric acid with alkaline substances, resulting in the formation of salt and water. However, this seemingly simple chemical process can lead to various environmental challenges. One of the primary concerns is the potential for the release of harmful byproducts into water bodies. Improperly treated effluents may contain residual chemicals, heavy metals, or other contaminants that can disrupt aquatic ecosystems and pose risks to marine life.
The disposal of neutralization byproducts, particularly the resulting salt solutions, presents another environmental challenge. These solutions often have high salinity levels, which can adversely affect soil quality and groundwater if not properly managed. In some cases, the accumulation of salts in soil can lead to reduced agricultural productivity and altered vegetation patterns in affected areas.
Air quality is also a concern in neutralization processes, particularly when volatile compounds are involved. The release of gaseous byproducts or fine particulate matter during the neutralization reaction can contribute to air pollution, potentially impacting both human health and the environment. Proper ventilation and emission control systems are crucial to mitigate these risks.
Energy consumption associated with neutralization processes is another environmental factor to consider. The production and transportation of neutralizing agents, as well as the operation of treatment facilities, contribute to the overall carbon footprint of these processes. Optimizing energy efficiency in neutralization operations is essential for reducing greenhouse gas emissions and aligning with sustainability goals.
Water usage is a significant environmental aspect of neutralization processes. Large volumes of water are often required for dilution, rinsing, and cooling purposes. In water-stressed regions, this high demand can strain local water resources and potentially impact ecosystems dependent on these water sources. Implementing water recycling and conservation measures can help mitigate this impact.
The long-term effects of neutralization processes on local ecosystems must also be considered. Continuous discharge of treated effluents, even when compliant with regulatory standards, can lead to gradual changes in the receiving environment. This may include alterations in pH levels, mineral content, and biological communities in water bodies over time.
To address these environmental concerns, industries are increasingly adopting more sustainable neutralization practices. These include the use of less harmful neutralizing agents, implementation of closed-loop systems to minimize waste discharge, and the development of more efficient treatment technologies. Additionally, there is a growing focus on waste minimization and resource recovery, aiming to extract valuable materials from waste streams and reduce the overall environmental footprint of neutralization processes.
Neutralization reactions typically involve the combination of hydrochloric acid with alkaline substances, resulting in the formation of salt and water. However, this seemingly simple chemical process can lead to various environmental challenges. One of the primary concerns is the potential for the release of harmful byproducts into water bodies. Improperly treated effluents may contain residual chemicals, heavy metals, or other contaminants that can disrupt aquatic ecosystems and pose risks to marine life.
The disposal of neutralization byproducts, particularly the resulting salt solutions, presents another environmental challenge. These solutions often have high salinity levels, which can adversely affect soil quality and groundwater if not properly managed. In some cases, the accumulation of salts in soil can lead to reduced agricultural productivity and altered vegetation patterns in affected areas.
Air quality is also a concern in neutralization processes, particularly when volatile compounds are involved. The release of gaseous byproducts or fine particulate matter during the neutralization reaction can contribute to air pollution, potentially impacting both human health and the environment. Proper ventilation and emission control systems are crucial to mitigate these risks.
Energy consumption associated with neutralization processes is another environmental factor to consider. The production and transportation of neutralizing agents, as well as the operation of treatment facilities, contribute to the overall carbon footprint of these processes. Optimizing energy efficiency in neutralization operations is essential for reducing greenhouse gas emissions and aligning with sustainability goals.
Water usage is a significant environmental aspect of neutralization processes. Large volumes of water are often required for dilution, rinsing, and cooling purposes. In water-stressed regions, this high demand can strain local water resources and potentially impact ecosystems dependent on these water sources. Implementing water recycling and conservation measures can help mitigate this impact.
The long-term effects of neutralization processes on local ecosystems must also be considered. Continuous discharge of treated effluents, even when compliant with regulatory standards, can lead to gradual changes in the receiving environment. This may include alterations in pH levels, mineral content, and biological communities in water bodies over time.
To address these environmental concerns, industries are increasingly adopting more sustainable neutralization practices. These include the use of less harmful neutralizing agents, implementation of closed-loop systems to minimize waste discharge, and the development of more efficient treatment technologies. Additionally, there is a growing focus on waste minimization and resource recovery, aiming to extract valuable materials from waste streams and reduce the overall environmental footprint of neutralization processes.
Safety Protocols in Acid Neutralization
Safety protocols in acid neutralization processes are paramount to ensure the well-being of personnel and the integrity of equipment. When optimizing hydrochloric acid neutralization, implementing robust safety measures is crucial. These protocols encompass a wide range of practices, from personal protective equipment (PPE) to emergency response procedures.
Proper PPE is the first line of defense against acid-related hazards. Workers must wear acid-resistant gloves, goggles, face shields, and protective clothing. Respiratory protection may be necessary depending on the concentration of acid fumes. Regular inspection and maintenance of PPE are essential to ensure its effectiveness.
Ventilation systems play a critical role in maintaining a safe working environment. Adequate local exhaust ventilation should be installed to remove acid vapors and mists. Regular monitoring of air quality and ventilation efficiency is necessary to prevent the accumulation of harmful fumes.
Proper storage and handling of hydrochloric acid are fundamental to safety. Acid should be stored in corrosion-resistant containers in well-ventilated areas, away from incompatible materials. Clear labeling and segregation of chemicals are essential to prevent accidental mixing.
Emergency response planning is crucial in acid neutralization processes. This includes the installation of easily accessible emergency showers and eyewash stations. Workers must be trained in their use and location. Spill containment and neutralization kits should be readily available, and personnel must be trained in proper spill response procedures.
Regular safety training and drills are essential components of a comprehensive safety protocol. Workers should be educated on the hazards of hydrochloric acid, proper handling techniques, and emergency procedures. Periodic refresher courses and practical exercises help maintain a high level of safety awareness.
Implementing a robust system for hazard communication is vital. This includes clear signage, material safety data sheets (MSDS) readily available, and regular safety briefings. A well-defined chain of communication for reporting incidents and near-misses should be established.
Continuous monitoring and control of the neutralization process are critical for safety. This includes regular pH checks, temperature monitoring, and control of reaction rates. Automated systems with alarms for detecting abnormal conditions can significantly enhance safety.
Regular maintenance and inspection of equipment used in acid neutralization are essential. This includes checking for corrosion, leaks, and proper functioning of control systems. A preventive maintenance schedule should be established and strictly followed.
By implementing these comprehensive safety protocols, organizations can significantly reduce the risks associated with hydrochloric acid neutralization processes, ensuring a safer working environment and more efficient operations.
Proper PPE is the first line of defense against acid-related hazards. Workers must wear acid-resistant gloves, goggles, face shields, and protective clothing. Respiratory protection may be necessary depending on the concentration of acid fumes. Regular inspection and maintenance of PPE are essential to ensure its effectiveness.
Ventilation systems play a critical role in maintaining a safe working environment. Adequate local exhaust ventilation should be installed to remove acid vapors and mists. Regular monitoring of air quality and ventilation efficiency is necessary to prevent the accumulation of harmful fumes.
Proper storage and handling of hydrochloric acid are fundamental to safety. Acid should be stored in corrosion-resistant containers in well-ventilated areas, away from incompatible materials. Clear labeling and segregation of chemicals are essential to prevent accidental mixing.
Emergency response planning is crucial in acid neutralization processes. This includes the installation of easily accessible emergency showers and eyewash stations. Workers must be trained in their use and location. Spill containment and neutralization kits should be readily available, and personnel must be trained in proper spill response procedures.
Regular safety training and drills are essential components of a comprehensive safety protocol. Workers should be educated on the hazards of hydrochloric acid, proper handling techniques, and emergency procedures. Periodic refresher courses and practical exercises help maintain a high level of safety awareness.
Implementing a robust system for hazard communication is vital. This includes clear signage, material safety data sheets (MSDS) readily available, and regular safety briefings. A well-defined chain of communication for reporting incidents and near-misses should be established.
Continuous monitoring and control of the neutralization process are critical for safety. This includes regular pH checks, temperature monitoring, and control of reaction rates. Automated systems with alarms for detecting abnormal conditions can significantly enhance safety.
Regular maintenance and inspection of equipment used in acid neutralization are essential. This includes checking for corrosion, leaks, and proper functioning of control systems. A preventive maintenance schedule should be established and strictly followed.
By implementing these comprehensive safety protocols, organizations can significantly reduce the risks associated with hydrochloric acid neutralization processes, ensuring a safer working environment and more efficient operations.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!