How to Prep Lewis Acid Sites for Maximum Activity?
AUG 26, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lewis Acid Catalysis Background and Objectives
Lewis acid catalysis has emerged as a cornerstone of modern synthetic chemistry since the pioneering work of Gilbert N. Lewis in the early 20th century. These electron-pair acceptors facilitate numerous transformations by activating substrates through coordination with electron-rich sites. The evolution of Lewis acid catalysis has progressed from simple metal halides to sophisticated designer catalysts with precisely engineered active sites, enabling unprecedented control over reaction selectivity and efficiency.
The technological trajectory of Lewis acid catalysis has been marked by significant breakthroughs in understanding structure-activity relationships. Early applications primarily utilized aluminum, boron, and titanium-based Lewis acids in bulk quantities. However, recent decades have witnessed a paradigm shift toward atom-efficient catalysis with lower catalyst loadings and enhanced activity profiles, driven by environmental considerations and economic imperatives.
Current research focuses on maximizing the intrinsic activity of Lewis acid sites through rational design principles. This involves optimizing electronic properties, steric environments, and support interactions to enhance substrate binding while facilitating product release. The field has increasingly embraced interdisciplinary approaches, incorporating computational modeling, advanced spectroscopic techniques, and high-throughput experimentation to accelerate catalyst development.
The primary objective of Lewis acid site preparation research is to develop systematic methodologies that yield catalysts with maximum activity, selectivity, and stability. This entails precise control over the nature, density, strength, and accessibility of acid sites. Additionally, researchers aim to establish quantitative structure-activity relationships that enable predictive catalyst design rather than empirical optimization.
Industrial applications of Lewis acid catalysis span diverse sectors including petrochemicals, pharmaceuticals, fine chemicals, and materials science. The economic impact of improved Lewis acid catalysts is substantial, with potential for significant process intensification, waste reduction, and energy savings across these industries. Consequently, there is strong market pull for catalysts with enhanced performance metrics.
The environmental dimension of this research cannot be overstated. More active Lewis acid sites enable lower reaction temperatures, reduced catalyst loadings, and improved atom economy—all contributing to greener chemical processes. This aligns with global sustainability initiatives and regulatory trends toward more environmentally benign manufacturing practices.
Looking forward, the field is trending toward multifunctional catalytic systems that combine Lewis acidity with complementary functionalities such as Brønsted acidity, redox activity, or photosensitization. These integrated approaches promise to unlock novel reaction pathways and expand the synthetic utility of Lewis acid catalysis beyond its current boundaries.
The technological trajectory of Lewis acid catalysis has been marked by significant breakthroughs in understanding structure-activity relationships. Early applications primarily utilized aluminum, boron, and titanium-based Lewis acids in bulk quantities. However, recent decades have witnessed a paradigm shift toward atom-efficient catalysis with lower catalyst loadings and enhanced activity profiles, driven by environmental considerations and economic imperatives.
Current research focuses on maximizing the intrinsic activity of Lewis acid sites through rational design principles. This involves optimizing electronic properties, steric environments, and support interactions to enhance substrate binding while facilitating product release. The field has increasingly embraced interdisciplinary approaches, incorporating computational modeling, advanced spectroscopic techniques, and high-throughput experimentation to accelerate catalyst development.
The primary objective of Lewis acid site preparation research is to develop systematic methodologies that yield catalysts with maximum activity, selectivity, and stability. This entails precise control over the nature, density, strength, and accessibility of acid sites. Additionally, researchers aim to establish quantitative structure-activity relationships that enable predictive catalyst design rather than empirical optimization.
Industrial applications of Lewis acid catalysis span diverse sectors including petrochemicals, pharmaceuticals, fine chemicals, and materials science. The economic impact of improved Lewis acid catalysts is substantial, with potential for significant process intensification, waste reduction, and energy savings across these industries. Consequently, there is strong market pull for catalysts with enhanced performance metrics.
The environmental dimension of this research cannot be overstated. More active Lewis acid sites enable lower reaction temperatures, reduced catalyst loadings, and improved atom economy—all contributing to greener chemical processes. This aligns with global sustainability initiatives and regulatory trends toward more environmentally benign manufacturing practices.
Looking forward, the field is trending toward multifunctional catalytic systems that combine Lewis acidity with complementary functionalities such as Brønsted acidity, redox activity, or photosensitization. These integrated approaches promise to unlock novel reaction pathways and expand the synthetic utility of Lewis acid catalysis beyond its current boundaries.
Market Applications and Demand Analysis
Lewis acid sites play a crucial role across multiple industries, with the market demand for optimized Lewis acid catalysts showing significant growth. The global catalyst market, valued at approximately $35.5 billion in 2022, is projected to reach $47.9 billion by 2028, with Lewis acid catalysts representing a substantial segment of this market. This growth is driven by increasing demand for efficient chemical processes and sustainable manufacturing solutions.
The petroleum refining industry remains the largest consumer of Lewis acid catalysts, particularly in fluid catalytic cracking (FCC) and hydrocracking processes. These catalysts enable the conversion of heavy petroleum fractions into valuable lighter products such as gasoline and diesel. With global oil refining capacity exceeding 100 million barrels per day, the demand for highly active Lewis acid catalysts continues to expand, especially as refineries seek to process increasingly heavy and sour crude oil feedstocks.
Fine chemical and pharmaceutical manufacturing represents another significant market segment. Lewis acid catalysts facilitate numerous organic transformations including Friedel-Crafts reactions, Diels-Alder reactions, and various carbon-carbon bond formations. The pharmaceutical industry, with its emphasis on high-value, complex molecules, particularly values catalysts with enhanced selectivity and activity. Market analysis indicates that approximately 80% of pharmaceutical processes involve at least one catalytic step, with Lewis acid catalysis featuring prominently.
The polymer industry constitutes a rapidly growing application area, where Lewis acid catalysts are essential for polymerization reactions, particularly in the production of polyolefins. The global polyolefin market, exceeding $250 billion annually, relies heavily on advanced catalytic systems. Manufacturers are increasingly seeking catalysts with maximized Lewis acidity to achieve higher molecular weight control and stereoselectivity in polymer production.
Environmental applications represent an emerging market with substantial growth potential. Lewis acid sites in zeolites and metal-organic frameworks are being developed for applications in carbon capture, air purification, and wastewater treatment. The global environmental technology market is expanding at over 8% annually, with catalytic solutions for pollution abatement driving significant demand.
Market trends indicate growing customer preference for catalysts with longer lifespans, higher selectivity, and reduced environmental impact. This has created demand for novel preparation methods that can precisely control Lewis acid site density, strength, and accessibility. Additionally, regulatory pressures across industries are driving the development of non-toxic alternatives to traditional Lewis acid catalysts containing heavy metals, creating new market opportunities for innovative catalyst preparation technologies.
The petroleum refining industry remains the largest consumer of Lewis acid catalysts, particularly in fluid catalytic cracking (FCC) and hydrocracking processes. These catalysts enable the conversion of heavy petroleum fractions into valuable lighter products such as gasoline and diesel. With global oil refining capacity exceeding 100 million barrels per day, the demand for highly active Lewis acid catalysts continues to expand, especially as refineries seek to process increasingly heavy and sour crude oil feedstocks.
Fine chemical and pharmaceutical manufacturing represents another significant market segment. Lewis acid catalysts facilitate numerous organic transformations including Friedel-Crafts reactions, Diels-Alder reactions, and various carbon-carbon bond formations. The pharmaceutical industry, with its emphasis on high-value, complex molecules, particularly values catalysts with enhanced selectivity and activity. Market analysis indicates that approximately 80% of pharmaceutical processes involve at least one catalytic step, with Lewis acid catalysis featuring prominently.
The polymer industry constitutes a rapidly growing application area, where Lewis acid catalysts are essential for polymerization reactions, particularly in the production of polyolefins. The global polyolefin market, exceeding $250 billion annually, relies heavily on advanced catalytic systems. Manufacturers are increasingly seeking catalysts with maximized Lewis acidity to achieve higher molecular weight control and stereoselectivity in polymer production.
Environmental applications represent an emerging market with substantial growth potential. Lewis acid sites in zeolites and metal-organic frameworks are being developed for applications in carbon capture, air purification, and wastewater treatment. The global environmental technology market is expanding at over 8% annually, with catalytic solutions for pollution abatement driving significant demand.
Market trends indicate growing customer preference for catalysts with longer lifespans, higher selectivity, and reduced environmental impact. This has created demand for novel preparation methods that can precisely control Lewis acid site density, strength, and accessibility. Additionally, regulatory pressures across industries are driving the development of non-toxic alternatives to traditional Lewis acid catalysts containing heavy metals, creating new market opportunities for innovative catalyst preparation technologies.
Current State and Challenges in Lewis Acid Site Preparation
The preparation of Lewis acid sites with maximum catalytic activity remains a significant challenge in heterogeneous catalysis. Current methodologies span various approaches including impregnation, ion exchange, sol-gel synthesis, and atomic layer deposition, each with distinct advantages and limitations for creating optimal Lewis acid sites.
Globally, research efforts have intensified particularly in North America, Europe, and East Asia, with notable contributions from institutions in the United States, Germany, China, and Japan. Despite these advancements, several critical challenges persist in achieving maximum Lewis acid site activity.
One fundamental challenge involves precise control over the distribution and density of Lewis acid sites. Current preparation methods often result in heterogeneous site distribution, with varying strengths and accessibilities that compromise overall catalytic performance. The inability to selectively generate specific types of Lewis acid sites with uniform properties remains a significant limitation.
Site stability presents another major obstacle. Many Lewis acid sites suffer from deactivation through various mechanisms including poisoning, coking, sintering, and leaching during catalytic processes. This instability is particularly problematic in aqueous environments or high-temperature applications, where structural integrity is compromised.
Characterization limitations further complicate advancement in this field. Despite sophisticated analytical techniques like FTIR spectroscopy with probe molecules, solid-state NMR, and XPS, researchers still struggle to precisely quantify Lewis acid site concentration, strength distribution, and coordination environment under reaction conditions. This characterization gap hinders rational catalyst design.
The scalability of preparation methods represents a significant industrial challenge. Laboratory-scale techniques that produce highly active Lewis acid sites often face implementation barriers at commercial scale due to cost constraints, reproducibility issues, or complex processing requirements.
Additionally, the mechanistic understanding of how preparation parameters influence the final properties of Lewis acid sites remains incomplete. The complex interplay between precursor chemistry, support interactions, calcination conditions, and post-synthesis treatments creates a multidimensional parameter space that is difficult to navigate systematically.
Environmental and sustainability concerns also present challenges, as traditional preparation methods may involve toxic precursors, generate hazardous waste, or require energy-intensive processing steps. The development of greener preparation routes that maintain high Lewis acid site activity represents an emerging research direction.
Globally, research efforts have intensified particularly in North America, Europe, and East Asia, with notable contributions from institutions in the United States, Germany, China, and Japan. Despite these advancements, several critical challenges persist in achieving maximum Lewis acid site activity.
One fundamental challenge involves precise control over the distribution and density of Lewis acid sites. Current preparation methods often result in heterogeneous site distribution, with varying strengths and accessibilities that compromise overall catalytic performance. The inability to selectively generate specific types of Lewis acid sites with uniform properties remains a significant limitation.
Site stability presents another major obstacle. Many Lewis acid sites suffer from deactivation through various mechanisms including poisoning, coking, sintering, and leaching during catalytic processes. This instability is particularly problematic in aqueous environments or high-temperature applications, where structural integrity is compromised.
Characterization limitations further complicate advancement in this field. Despite sophisticated analytical techniques like FTIR spectroscopy with probe molecules, solid-state NMR, and XPS, researchers still struggle to precisely quantify Lewis acid site concentration, strength distribution, and coordination environment under reaction conditions. This characterization gap hinders rational catalyst design.
The scalability of preparation methods represents a significant industrial challenge. Laboratory-scale techniques that produce highly active Lewis acid sites often face implementation barriers at commercial scale due to cost constraints, reproducibility issues, or complex processing requirements.
Additionally, the mechanistic understanding of how preparation parameters influence the final properties of Lewis acid sites remains incomplete. The complex interplay between precursor chemistry, support interactions, calcination conditions, and post-synthesis treatments creates a multidimensional parameter space that is difficult to navigate systematically.
Environmental and sustainability concerns also present challenges, as traditional preparation methods may involve toxic precursors, generate hazardous waste, or require energy-intensive processing steps. The development of greener preparation routes that maintain high Lewis acid site activity represents an emerging research direction.
Current Methodologies for Lewis Acid Site Activation
01 Lewis acid site characterization and measurement techniques
Various techniques are employed to characterize and measure Lewis acid site activity in catalysts and materials. These methods include spectroscopic techniques such as infrared spectroscopy, temperature-programmed desorption, and probe molecule adsorption studies. These characterization techniques help determine the strength, density, and distribution of Lewis acid sites, which is crucial for understanding catalytic performance and optimizing catalyst formulations.- Lewis acid sites in zeolite catalysts: Lewis acid sites in zeolite catalysts play a crucial role in various catalytic reactions. The activity of these sites can be modified by controlling the framework composition, post-synthesis treatments, and metal incorporation. Zeolites with optimized Lewis acid site distribution and strength show enhanced catalytic performance in processes such as cracking, isomerization, and alkylation reactions. The balance between Brønsted and Lewis acidity is often critical for achieving desired selectivity and conversion rates.
- Metal oxide-based Lewis acid catalysts: Metal oxides such as alumina, silica-alumina, and various transition metal oxides exhibit Lewis acid properties that can be tuned for specific catalytic applications. The Lewis acid site activity in these materials depends on the metal coordination environment, surface hydroxyl groups, and structural defects. These catalysts are particularly effective for reactions requiring moderate to strong Lewis acidity, including dehydration, condensation, and certain oxidation processes. Surface modification techniques can be employed to enhance the Lewis acid site density and strength.
- Lewis acid sites in polymerization catalysts: Lewis acid sites are essential components in polymerization catalyst systems, particularly for olefin polymerization. The activity of these sites influences polymer properties such as molecular weight distribution, stereoselectivity, and branching. Metallocene catalysts and other single-site catalysts utilize Lewis acid co-catalysts to generate active cationic species. The strength and accessibility of Lewis acid sites can be modulated by ligand design, support materials, and activation methods to achieve desired polymer architectures.
- Characterization and measurement of Lewis acid site activity: Various analytical techniques are employed to characterize the activity, strength, and distribution of Lewis acid sites in catalytic materials. These include temperature-programmed desorption of probe molecules, infrared spectroscopy with adsorbed pyridine, solid-state NMR, and calorimetric measurements. Computational methods are also used to model Lewis acid site interactions with reactants. Quantitative assessment of Lewis acidity helps in understanding structure-activity relationships and in designing more efficient catalytic systems.
- Lewis acid sites in industrial processes and applications: Lewis acid sites are utilized in numerous industrial processes including petroleum refining, fine chemical synthesis, and environmental remediation. The activity of these sites is crucial for selective transformations such as Friedel-Crafts reactions, isomerizations, and certain oxidation processes. In biomass conversion, Lewis acid catalysts facilitate the transformation of carbohydrates to platform chemicals. The stability and regeneration of Lewis acid sites under industrial conditions are important considerations for commercial applications, with various strategies developed to prevent deactivation and extend catalyst lifetime.
02 Zeolite and molecular sieve Lewis acid catalysts
Zeolites and molecular sieves are important materials with Lewis acid sites that exhibit catalytic activity. These materials can be modified to enhance their Lewis acidity through various treatments including dealumination, metal ion exchange, and framework substitution. The controlled modification of zeolite structures allows for tuning the strength and accessibility of Lewis acid sites, making them valuable catalysts for various industrial processes including hydrocarbon transformations.Expand Specific Solutions03 Metal oxide-based Lewis acid catalysts
Metal oxides such as alumina, silica-alumina, titania, and zirconia exhibit Lewis acid properties that can be utilized in catalytic applications. The Lewis acid site activity in these materials can be enhanced through various methods including controlled synthesis conditions, doping with other metals, and surface modifications. These catalysts are widely used in organic transformations, petrochemical processes, and environmental applications due to their tunable acidity and stability.Expand Specific Solutions04 Lewis acid sites in polymerization catalysts
Lewis acid sites play a crucial role in polymerization catalysts, particularly in coordination polymerization systems. The activity of these sites influences polymer properties including molecular weight, stereochemistry, and chain architecture. Catalyst systems containing Lewis acidic components such as metallocene complexes, metal alkyls, and supported transition metals are widely used in industrial polymerization processes. The strength and accessibility of Lewis acid sites can be modified to control polymerization kinetics and polymer structure.Expand Specific Solutions05 Novel materials with enhanced Lewis acid site activity
Research has led to the development of novel materials with enhanced Lewis acid site activity, including metal-organic frameworks (MOFs), covalent organic frameworks (COFs), and composite materials. These materials feature well-defined structures with accessible Lewis acid sites that can be precisely engineered for specific catalytic applications. Innovations in synthesis methods allow for controlling the density, strength, and environment of Lewis acid sites, resulting in improved catalytic performance for various chemical transformations.Expand Specific Solutions
Key Industrial and Academic Players in Lewis Acid Catalysis
The field of Lewis acid site preparation for maximum activity is currently in a growth phase, with increasing market demand driven by catalytic applications in petrochemical, pharmaceutical, and fine chemical industries. The global market for advanced catalysts is estimated at $25-30 billion, with Lewis acid catalysts representing a significant segment. Technologically, this field shows varying maturity levels across different companies. Industry leaders like Sinopec, ExxonMobil Chemical, and IFP Energies Nouvelles have developed sophisticated approaches for Lewis acid site engineering, while academic institutions such as North Carolina State University and Zhejiang University are advancing fundamental understanding. Companies including Wanhua Chemical and Akzo Nobel are focusing on specialized applications, creating a competitive landscape that balances established industrial processes with emerging research breakthroughs in site-specific catalyst design.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has pioneered hierarchical porous materials with optimized Lewis acid sites for catalytic applications. Their approach focuses on creating multi-level pore structures that maximize accessibility to active sites while maintaining high site density. Sinopec's proprietary synthesis involves controlled hydrolysis of metal precursors (particularly Al, Ti, and Zr) followed by templated assembly to create materials with both micro and mesopores. Their catalysts undergo precise thermal activation protocols that remove coordinated water molecules without causing site agglomeration, resulting in coordinatively unsaturated metal centers with maximum Lewis acidity. The company has developed advanced surface modification techniques using organosilanes to create hydrophobic environments around Lewis acid sites, protecting them from deactivation in water-containing reaction media. This technology has shown particular success in biomass conversion processes, achieving yields approximately 25-30% higher than conventional catalysts.
Strengths: Excellent mass transport properties due to hierarchical structure; superior resistance to water deactivation; versatility across multiple reaction types. Weaknesses: Higher production costs compared to traditional catalysts; potential mechanical stability issues in certain reactor configurations; challenges in achieving uniform site distribution in large-scale production.
IFP Energies Nouvelles
Technical Solution: IFP Energies Nouvelles has developed innovative supported metal oxide catalysts with enhanced Lewis acidity for biomass valorization and petrochemical applications. Their approach centers on controlled deposition of metal oxides (particularly TiO2, ZrO2, and SnO2) on high-surface-area supports using advanced atomic layer deposition techniques. This creates highly dispersed, isolated Lewis acid sites with optimal coordination geometry. IFP's catalysts undergo proprietary activation procedures involving carefully controlled reduction-oxidation cycles that generate oxygen vacancies adjacent to metal centers, significantly enhancing Lewis acid strength. Their research has demonstrated that precise control of the metal-support interface is critical for maximizing activity, with their catalysts showing up to 3-fold increases in turnover frequency for model reactions compared to conventional systems. IFP has also pioneered the development of bifunctional catalysts combining Lewis acid sites with proximal basic sites, enabling cascade reactions in single-pot processes with significantly improved atom economy.
Strengths: Exceptional stability under hydrothermal conditions; precise control over site density and distribution; versatility for both gas and liquid phase reactions. Weaknesses: Higher production costs due to sophisticated synthesis methods; potential for metal leaching in certain reaction environments; challenges in scale-up while maintaining site uniformity.
Critical Patents and Literature on Lewis Acid Site Enhancement
Hydrotreating catalyst for hydrocarbon oil, method for producing the same, and method for hydrotreating hydrocarbon oil
PatentActiveUS11896959B2
Innovation
- A hydrotreating catalyst with an inorganic composite oxide carrier containing alumina, silicon, and phosphorus, supporting active metals like molybdenum and cobalt, with optimized Lewis and Brönsted acid amounts, and a specific surface area, to enhance desulfurization activity.
Process for chemical reaction of amino acids and amides yielding selective conversion products
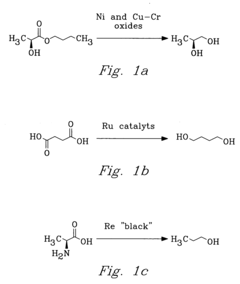
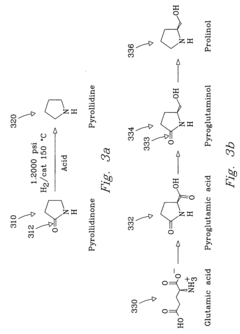
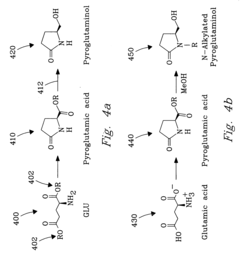
PatentInactiveUS20040225133A1
Innovation
- The process involves selecting starting materials such as L-glutamic acid and L-pyroglutamic acid, reacting them under controlled conditions with precious-metal catalysts at elevated temperatures and specific pH levels, and using a reduction catalyst to achieve high yields and selectivity in the formation of desirable products.
Characterization Techniques for Lewis Acid Site Analysis
The accurate characterization of Lewis acid sites is fundamental to understanding their activity and optimizing their preparation. Various analytical techniques have been developed to quantify, locate, and determine the strength of Lewis acid sites in catalytic materials.
Infrared (IR) spectroscopy with probe molecules such as pyridine, CO, and NH3 remains one of the most widely used techniques. The shift in vibrational frequencies of adsorbed probe molecules provides valuable information about the strength and nature of Lewis acid sites. For instance, the adsorption of pyridine on Lewis acid sites typically shows characteristic bands at 1450-1460 cm⁻¹, which can be distinguished from Brønsted acid sites.
Solid-state Nuclear Magnetic Resonance (NMR) spectroscopy offers atomic-level insights into the local environment of Lewis acid sites. ²⁷Al, ¹¹B, and ²⁹Si NMR are particularly useful for zeolites and metal-oxide catalysts, revealing coordination numbers and structural information critical for understanding Lewis acidity.
Temperature-programmed desorption (TPD) of basic probe molecules like NH₃ provides quantitative measurements of acid site density and strength distribution. The desorption temperature correlates with the strength of acid sites, allowing for comparative analysis between different catalyst preparations.
X-ray absorption spectroscopy (XAS), including XANES and EXAFS, enables the determination of oxidation states and coordination environments of metal centers that function as Lewis acid sites. This technique is especially valuable for supported metal catalysts where traditional methods may be less effective.
Electron paramagnetic resonance (EPR) spectroscopy is particularly useful for characterizing Lewis acid sites containing unpaired electrons, providing information about their electronic structure and coordination environment.
Advanced microscopy techniques such as high-resolution transmission electron microscopy (HRTEM) coupled with energy-dispersive X-ray spectroscopy (EDX) allow for spatial mapping of Lewis acid sites, critical for understanding structure-activity relationships in heterogeneous catalysts.
Computational methods, including density functional theory (DFT), have become increasingly important for interpreting experimental data and predicting Lewis acid site properties. These methods can calculate adsorption energies, structural changes upon adsorption, and activation barriers for catalytic reactions.
The integration of multiple characterization techniques is essential for comprehensive understanding, as each method provides complementary information. Modern approaches often combine spectroscopic methods with in-situ or operando measurements to observe Lewis acid sites under reaction conditions, bridging the gap between static characterization and dynamic catalytic performance.
Infrared (IR) spectroscopy with probe molecules such as pyridine, CO, and NH3 remains one of the most widely used techniques. The shift in vibrational frequencies of adsorbed probe molecules provides valuable information about the strength and nature of Lewis acid sites. For instance, the adsorption of pyridine on Lewis acid sites typically shows characteristic bands at 1450-1460 cm⁻¹, which can be distinguished from Brønsted acid sites.
Solid-state Nuclear Magnetic Resonance (NMR) spectroscopy offers atomic-level insights into the local environment of Lewis acid sites. ²⁷Al, ¹¹B, and ²⁹Si NMR are particularly useful for zeolites and metal-oxide catalysts, revealing coordination numbers and structural information critical for understanding Lewis acidity.
Temperature-programmed desorption (TPD) of basic probe molecules like NH₃ provides quantitative measurements of acid site density and strength distribution. The desorption temperature correlates with the strength of acid sites, allowing for comparative analysis between different catalyst preparations.
X-ray absorption spectroscopy (XAS), including XANES and EXAFS, enables the determination of oxidation states and coordination environments of metal centers that function as Lewis acid sites. This technique is especially valuable for supported metal catalysts where traditional methods may be less effective.
Electron paramagnetic resonance (EPR) spectroscopy is particularly useful for characterizing Lewis acid sites containing unpaired electrons, providing information about their electronic structure and coordination environment.
Advanced microscopy techniques such as high-resolution transmission electron microscopy (HRTEM) coupled with energy-dispersive X-ray spectroscopy (EDX) allow for spatial mapping of Lewis acid sites, critical for understanding structure-activity relationships in heterogeneous catalysts.
Computational methods, including density functional theory (DFT), have become increasingly important for interpreting experimental data and predicting Lewis acid site properties. These methods can calculate adsorption energies, structural changes upon adsorption, and activation barriers for catalytic reactions.
The integration of multiple characterization techniques is essential for comprehensive understanding, as each method provides complementary information. Modern approaches often combine spectroscopic methods with in-situ or operando measurements to observe Lewis acid sites under reaction conditions, bridging the gap between static characterization and dynamic catalytic performance.
Sustainability Aspects of Lewis Acid Catalyst Preparation
The sustainability of Lewis acid catalyst preparation has become increasingly critical as industries strive to meet environmental regulations while maintaining economic viability. Traditional preparation methods often involve hazardous chemicals, high energy consumption, and generate significant waste. Sustainable approaches focus on reducing environmental impact through green chemistry principles, including atom economy, waste reduction, and renewable feedstocks.
Water-based synthesis routes represent a significant advancement in sustainable Lewis acid catalyst preparation. By replacing organic solvents with water, these methods substantially reduce volatile organic compound (VOC) emissions and associated health risks. Recent developments in aqueous sol-gel techniques have enabled the synthesis of highly active Lewis acid catalysts with controlled site distribution and enhanced stability.
Energy-efficient preparation techniques have emerged as another crucial sustainability aspect. Microwave-assisted and mechanochemical synthesis methods significantly reduce energy requirements compared to conventional thermal treatments. Studies indicate that microwave-assisted preparation can reduce energy consumption by up to 85% while simultaneously decreasing reaction times from hours to minutes, resulting in catalysts with comparable or superior Lewis acid site activity.
Waste valorization approaches incorporate industrial by-products as precursors for Lewis acid catalyst synthesis. Red mud from aluminum production and fly ash from coal combustion contain valuable metal oxides that can be transformed into effective Lewis acid catalysts. This circular economy approach not only reduces waste disposal issues but also lowers the carbon footprint of catalyst production by minimizing the need for virgin raw materials.
Biobased templates and supports derived from agricultural waste provide renewable alternatives to petroleum-based materials traditionally used in catalyst preparation. Cellulose, lignin, and chitin-derived materials offer unique structural properties that can enhance Lewis acid site accessibility and stability. These renewable templates can be removed through low-temperature calcination processes, resulting in catalysts with high surface areas and well-distributed active sites.
Life cycle assessment (LCA) studies comparing conventional and sustainable preparation methods reveal significant environmental benefits. Comprehensive analyses show that green synthesis routes can reduce global warming potential by 40-60% and decrease freshwater ecotoxicity by up to 75%. However, these studies also highlight trade-offs, as some green methods may require specialized equipment or longer processing times, affecting economic feasibility.
Regulatory frameworks increasingly influence catalyst preparation strategies, with restrictions on hazardous substances driving innovation in sustainable synthesis. Forward-thinking companies are proactively developing preparation methods that anticipate future regulations, positioning themselves advantageously in an evolving regulatory landscape while ensuring their catalysts maintain maximum Lewis acid site activity.
Water-based synthesis routes represent a significant advancement in sustainable Lewis acid catalyst preparation. By replacing organic solvents with water, these methods substantially reduce volatile organic compound (VOC) emissions and associated health risks. Recent developments in aqueous sol-gel techniques have enabled the synthesis of highly active Lewis acid catalysts with controlled site distribution and enhanced stability.
Energy-efficient preparation techniques have emerged as another crucial sustainability aspect. Microwave-assisted and mechanochemical synthesis methods significantly reduce energy requirements compared to conventional thermal treatments. Studies indicate that microwave-assisted preparation can reduce energy consumption by up to 85% while simultaneously decreasing reaction times from hours to minutes, resulting in catalysts with comparable or superior Lewis acid site activity.
Waste valorization approaches incorporate industrial by-products as precursors for Lewis acid catalyst synthesis. Red mud from aluminum production and fly ash from coal combustion contain valuable metal oxides that can be transformed into effective Lewis acid catalysts. This circular economy approach not only reduces waste disposal issues but also lowers the carbon footprint of catalyst production by minimizing the need for virgin raw materials.
Biobased templates and supports derived from agricultural waste provide renewable alternatives to petroleum-based materials traditionally used in catalyst preparation. Cellulose, lignin, and chitin-derived materials offer unique structural properties that can enhance Lewis acid site accessibility and stability. These renewable templates can be removed through low-temperature calcination processes, resulting in catalysts with high surface areas and well-distributed active sites.
Life cycle assessment (LCA) studies comparing conventional and sustainable preparation methods reveal significant environmental benefits. Comprehensive analyses show that green synthesis routes can reduce global warming potential by 40-60% and decrease freshwater ecotoxicity by up to 75%. However, these studies also highlight trade-offs, as some green methods may require specialized equipment or longer processing times, affecting economic feasibility.
Regulatory frameworks increasingly influence catalyst preparation strategies, with restrictions on hazardous substances driving innovation in sustainable synthesis. Forward-thinking companies are proactively developing preparation methods that anticipate future regulations, positioning themselves advantageously in an evolving regulatory landscape while ensuring their catalysts maintain maximum Lewis acid site activity.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!