How to Stabilize Hydrochloric Acid Storage Conditions?
JUL 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HCl Storage Background and Objectives
Hydrochloric acid (HCl) is a crucial chemical compound widely used in various industries, including chemical manufacturing, metal processing, and water treatment. The storage and handling of HCl present significant challenges due to its corrosive nature and potential safety hazards. As industries continue to rely on this versatile acid, the need for stable and secure storage conditions has become increasingly important.
The evolution of HCl storage technologies has been driven by the growing demand for safer and more efficient handling methods. Initially, glass or ceramic containers were used, but these proved fragile and unsuitable for large-scale industrial applications. The introduction of rubber-lined steel tanks in the mid-20th century marked a significant improvement, offering better durability and chemical resistance. However, these solutions still faced issues with long-term stability and potential leaks.
Recent technological advancements have focused on developing advanced materials and storage systems that can withstand the corrosive properties of HCl while maintaining its purity and concentration. These innovations aim to address key challenges such as material degradation, vapor emissions, and temperature fluctuations that can affect the stability of stored HCl.
The primary objective of stabilizing HCl storage conditions is to ensure the safety of personnel and the environment while maintaining the quality and integrity of the acid. This involves developing storage solutions that can effectively contain HCl, prevent contamination, and minimize the risk of leaks or spills. Additionally, there is a growing emphasis on creating storage systems that can adapt to varying environmental conditions and withstand long-term exposure to HCl without degradation.
Another critical goal is to optimize the storage capacity and efficiency of HCl facilities. As industrial demand for HCl continues to grow, there is a need for storage solutions that can accommodate larger volumes while minimizing the footprint and operational costs. This includes exploring innovative tank designs, automated monitoring systems, and advanced materials that can enhance storage capacity without compromising safety or stability.
Furthermore, the development of stable HCl storage conditions aims to comply with increasingly stringent environmental regulations and industry standards. This involves implementing technologies and practices that reduce emissions, improve containment, and facilitate safe handling and transportation of HCl. The ultimate goal is to create a sustainable and responsible approach to HCl storage that aligns with global efforts to minimize the environmental impact of industrial processes.
The evolution of HCl storage technologies has been driven by the growing demand for safer and more efficient handling methods. Initially, glass or ceramic containers were used, but these proved fragile and unsuitable for large-scale industrial applications. The introduction of rubber-lined steel tanks in the mid-20th century marked a significant improvement, offering better durability and chemical resistance. However, these solutions still faced issues with long-term stability and potential leaks.
Recent technological advancements have focused on developing advanced materials and storage systems that can withstand the corrosive properties of HCl while maintaining its purity and concentration. These innovations aim to address key challenges such as material degradation, vapor emissions, and temperature fluctuations that can affect the stability of stored HCl.
The primary objective of stabilizing HCl storage conditions is to ensure the safety of personnel and the environment while maintaining the quality and integrity of the acid. This involves developing storage solutions that can effectively contain HCl, prevent contamination, and minimize the risk of leaks or spills. Additionally, there is a growing emphasis on creating storage systems that can adapt to varying environmental conditions and withstand long-term exposure to HCl without degradation.
Another critical goal is to optimize the storage capacity and efficiency of HCl facilities. As industrial demand for HCl continues to grow, there is a need for storage solutions that can accommodate larger volumes while minimizing the footprint and operational costs. This includes exploring innovative tank designs, automated monitoring systems, and advanced materials that can enhance storage capacity without compromising safety or stability.
Furthermore, the development of stable HCl storage conditions aims to comply with increasingly stringent environmental regulations and industry standards. This involves implementing technologies and practices that reduce emissions, improve containment, and facilitate safe handling and transportation of HCl. The ultimate goal is to create a sustainable and responsible approach to HCl storage that aligns with global efforts to minimize the environmental impact of industrial processes.
Market Analysis for HCl Storage Solutions
The global market for hydrochloric acid (HCl) storage solutions is experiencing steady growth, driven by increasing demand from various industries such as chemical manufacturing, steel production, and water treatment. The need for safe and efficient storage of HCl has become paramount, leading to a surge in innovative storage solutions and technologies.
The market size for HCl storage solutions is projected to expand significantly over the next five years, with a compound annual growth rate (CAGR) expected to exceed the overall chemical storage market average. This growth is attributed to the rising industrial applications of HCl and stringent safety regulations imposed by governments worldwide.
Key market drivers include the expansion of the chemical industry in emerging economies, particularly in Asia-Pacific and Latin America. These regions are witnessing rapid industrialization and urbanization, leading to increased demand for HCl in various applications. Additionally, the growing focus on environmental protection and worker safety has spurred investments in advanced storage technologies that minimize the risk of leaks and spills.
The market is segmented based on storage type, with bulk storage tanks, intermediate bulk containers (IBCs), and specialized corrosion-resistant containers being the primary categories. Bulk storage tanks dominate the market share due to their cost-effectiveness and suitability for large-scale industrial applications. However, IBCs are gaining traction in the market owing to their versatility and ease of transportation.
Geographically, North America and Europe currently hold significant market shares due to their well-established chemical industries and stringent safety regulations. However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years, driven by rapid industrialization in countries like China and India.
The competitive landscape of the HCl storage solutions market is characterized by the presence of both global players and regional manufacturers. Key market players are focusing on product innovations, such as advanced corrosion-resistant materials and smart monitoring systems, to gain a competitive edge. Strategic partnerships and collaborations between storage solution providers and end-users are becoming increasingly common to develop tailored solutions for specific industry needs.
Challenges in the market include the high initial investment required for advanced storage systems and the stringent regulatory environment surrounding hazardous chemical storage. However, these challenges also present opportunities for innovation in cost-effective, compliant storage solutions.
Looking ahead, the market is expected to witness a shift towards more sustainable and environmentally friendly storage solutions. This trend is driven by increasing environmental concerns and the push for circular economy practices in the chemical industry. As a result, there is growing interest in recyclable and reusable storage containers, as well as in technologies that enable the safe recovery and reuse of HCl.
The market size for HCl storage solutions is projected to expand significantly over the next five years, with a compound annual growth rate (CAGR) expected to exceed the overall chemical storage market average. This growth is attributed to the rising industrial applications of HCl and stringent safety regulations imposed by governments worldwide.
Key market drivers include the expansion of the chemical industry in emerging economies, particularly in Asia-Pacific and Latin America. These regions are witnessing rapid industrialization and urbanization, leading to increased demand for HCl in various applications. Additionally, the growing focus on environmental protection and worker safety has spurred investments in advanced storage technologies that minimize the risk of leaks and spills.
The market is segmented based on storage type, with bulk storage tanks, intermediate bulk containers (IBCs), and specialized corrosion-resistant containers being the primary categories. Bulk storage tanks dominate the market share due to their cost-effectiveness and suitability for large-scale industrial applications. However, IBCs are gaining traction in the market owing to their versatility and ease of transportation.
Geographically, North America and Europe currently hold significant market shares due to their well-established chemical industries and stringent safety regulations. However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years, driven by rapid industrialization in countries like China and India.
The competitive landscape of the HCl storage solutions market is characterized by the presence of both global players and regional manufacturers. Key market players are focusing on product innovations, such as advanced corrosion-resistant materials and smart monitoring systems, to gain a competitive edge. Strategic partnerships and collaborations between storage solution providers and end-users are becoming increasingly common to develop tailored solutions for specific industry needs.
Challenges in the market include the high initial investment required for advanced storage systems and the stringent regulatory environment surrounding hazardous chemical storage. However, these challenges also present opportunities for innovation in cost-effective, compliant storage solutions.
Looking ahead, the market is expected to witness a shift towards more sustainable and environmentally friendly storage solutions. This trend is driven by increasing environmental concerns and the push for circular economy practices in the chemical industry. As a result, there is growing interest in recyclable and reusable storage containers, as well as in technologies that enable the safe recovery and reuse of HCl.
Current Challenges in HCl Storage
The storage of hydrochloric acid (HCl) presents several significant challenges due to its highly corrosive nature and potential for environmental hazards. One of the primary issues is the acid's tendency to release hydrogen chloride gas, which can lead to pressure build-up in storage containers. This gas release is particularly problematic in warmer climates or during temperature fluctuations, necessitating robust pressure relief systems and careful monitoring of storage conditions.
Corrosion of storage materials is another major concern. HCl rapidly attacks most metals, including carbon steel and many stainless steel alloys. This corrosion not only compromises the integrity of storage tanks but also contaminates the acid with metal ions, potentially rendering it unsuitable for certain applications. The selection of appropriate materials for tanks, piping, and valves is crucial, with options like rubber-lined steel, fiberglass-reinforced plastic (FRP), or high-grade alloys like Hastelloy being necessary, albeit at higher costs.
Moisture absorption is a significant challenge in HCl storage. The acid is hygroscopic, meaning it readily absorbs moisture from the air. This property can lead to concentration changes and potential overheating due to the exothermic nature of HCl dilution. Maintaining a sealed storage environment and implementing proper venting systems are essential to mitigate these risks.
Safety concerns extend beyond the immediate storage area. HCl vapors are highly toxic and can cause severe respiratory issues. Ensuring proper ventilation, implementing robust leak detection systems, and establishing comprehensive emergency response protocols are critical challenges in HCl storage facilities. The potential for accidental releases also necessitates careful consideration of storage location, with proximity to populated areas or sensitive ecosystems being a key factor.
Long-term storage stability is another challenge, particularly for high-purity HCl used in semiconductor manufacturing and other precision industries. Maintaining the acid's purity over extended periods requires specialized handling and storage techniques to prevent contamination from storage materials or external sources.
Regulatory compliance adds another layer of complexity to HCl storage. Stringent environmental and safety regulations govern the storage and handling of hazardous materials like HCl. Meeting these requirements, which can vary significantly across different regions, demands careful planning, documentation, and ongoing monitoring to ensure compliance and avoid potential legal and financial repercussions.
Corrosion of storage materials is another major concern. HCl rapidly attacks most metals, including carbon steel and many stainless steel alloys. This corrosion not only compromises the integrity of storage tanks but also contaminates the acid with metal ions, potentially rendering it unsuitable for certain applications. The selection of appropriate materials for tanks, piping, and valves is crucial, with options like rubber-lined steel, fiberglass-reinforced plastic (FRP), or high-grade alloys like Hastelloy being necessary, albeit at higher costs.
Moisture absorption is a significant challenge in HCl storage. The acid is hygroscopic, meaning it readily absorbs moisture from the air. This property can lead to concentration changes and potential overheating due to the exothermic nature of HCl dilution. Maintaining a sealed storage environment and implementing proper venting systems are essential to mitigate these risks.
Safety concerns extend beyond the immediate storage area. HCl vapors are highly toxic and can cause severe respiratory issues. Ensuring proper ventilation, implementing robust leak detection systems, and establishing comprehensive emergency response protocols are critical challenges in HCl storage facilities. The potential for accidental releases also necessitates careful consideration of storage location, with proximity to populated areas or sensitive ecosystems being a key factor.
Long-term storage stability is another challenge, particularly for high-purity HCl used in semiconductor manufacturing and other precision industries. Maintaining the acid's purity over extended periods requires specialized handling and storage techniques to prevent contamination from storage materials or external sources.
Regulatory compliance adds another layer of complexity to HCl storage. Stringent environmental and safety regulations govern the storage and handling of hazardous materials like HCl. Meeting these requirements, which can vary significantly across different regions, demands careful planning, documentation, and ongoing monitoring to ensure compliance and avoid potential legal and financial repercussions.
Existing HCl Stabilization Methods
01 Corrosion-resistant storage containers
Utilizing corrosion-resistant materials such as high-density polyethylene (HDPE) or specialized alloys for storage containers can significantly improve the storage stability of hydrochloric acid. These materials prevent chemical reactions between the acid and container, maintaining the acid's purity and concentration over time.- Corrosion-resistant storage containers: Utilizing corrosion-resistant materials such as high-density polyethylene (HDPE), polypropylene, or specially coated metals for storage containers can significantly improve the storage stability of hydrochloric acid. These materials prevent chemical reactions between the acid and container, maintaining the acid's purity and concentration over time.
- Temperature control systems: Implementing temperature control systems in storage facilities helps maintain the stability of hydrochloric acid. Keeping the acid at a consistent, optimal temperature range prevents degradation and reduces the risk of pressure build-up in containers, thereby enhancing long-term storage stability.
- Pressure relief mechanisms: Incorporating pressure relief valves or venting systems in storage containers and tanks is crucial for maintaining hydrochloric acid stability. These mechanisms prevent pressure build-up due to gas evolution, reducing the risk of container rupture and ensuring safe, long-term storage.
- Moisture control and sealing: Effective moisture control and proper sealing of storage containers are essential for maintaining hydrochloric acid stability. Utilizing desiccants, implementing airtight seals, and employing moisture-resistant packaging materials help prevent water absorption and maintain the acid's concentration during storage.
- Stabilizing additives: Incorporating specific stabilizing additives into hydrochloric acid formulations can enhance its storage stability. These additives may include antioxidants, corrosion inhibitors, or pH stabilizers that help maintain the acid's chemical properties and prevent degradation over extended storage periods.
02 Temperature control systems
Implementing temperature control systems in storage facilities helps maintain optimal conditions for hydrochloric acid storage. Proper temperature regulation prevents degradation and ensures the acid's stability, reducing the risk of pressure build-up or chemical changes that could affect its quality or safety.Expand Specific Solutions03 Ventilation and pressure relief mechanisms
Incorporating adequate ventilation systems and pressure relief mechanisms in storage tanks and facilities is crucial for maintaining hydrochloric acid stability. These features prevent the accumulation of potentially harmful vapors and manage internal pressure, ensuring safe and stable long-term storage.Expand Specific Solutions04 Moisture control and sealing techniques
Employing effective moisture control methods and advanced sealing techniques helps preserve the concentration and purity of stored hydrochloric acid. This includes using desiccants, implementing proper sealing mechanisms, and maintaining a dry environment to prevent water absorption and subsequent dilution or contamination of the acid.Expand Specific Solutions05 Monitoring and quality control systems
Implementing sophisticated monitoring and quality control systems enables real-time tracking of hydrochloric acid storage conditions. These systems can include sensors for measuring concentration, temperature, and pressure, allowing for prompt detection of any changes that might affect the acid's stability and facilitating timely corrective actions.Expand Specific Solutions
Key Players in HCl Storage Industry
The stabilization of hydrochloric acid storage conditions is a critical issue in the chemical industry, currently in a mature phase with established players and technologies. The global market for hydrochloric acid is substantial, estimated to reach $1.5 billion by 2025, driven by diverse industrial applications. Technologically, the field is well-developed, with companies like China Petroleum & Chemical Corp., Tokuyama Corp., and AGC, Inc. leading innovations in storage and handling solutions. These firms have implemented advanced corrosion-resistant materials, temperature control systems, and safety protocols to enhance stability and longevity of stored hydrochloric acid, reflecting the industry's focus on efficiency and safety in chemical storage practices.
Tokuyama Corp.
Technical Solution: Tokuyama Corp. has developed an innovative approach to stabilize hydrochloric acid storage conditions. Their method involves using a specially designed storage tank with a multi-layer lining system. The innermost layer is made of high-density polyethylene (HDPE) which is resistant to hydrochloric acid corrosion. This is followed by a layer of fiberglass-reinforced plastic (FRP) for added structural integrity. The outermost layer is a protective coating that helps prevent external damage and UV degradation. Additionally, Tokuyama Corp. implements a sophisticated temperature control system that maintains the acid at optimal temperatures, typically between 15-25°C, to prevent degradation and minimize vapor pressure[1]. They also utilize an advanced venting system that helps regulate internal pressure and prevents the buildup of potentially hazardous gases[3].
Strengths: Highly effective corrosion resistance, excellent temperature control, and improved safety through pressure regulation. Weaknesses: Higher initial cost compared to traditional storage methods, and potential complexity in maintenance and repairs.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed a comprehensive approach to stabilizing hydrochloric acid storage conditions. Their method involves using large-scale, rubber-lined steel tanks equipped with advanced monitoring systems. These tanks are designed with a double-wall construction, providing an extra layer of protection against leaks. The inner lining is made of a specialized rubber compound that is highly resistant to hydrochloric acid corrosion. Sinopec's system includes real-time pH and concentration monitoring, allowing for immediate detection of any changes in the acid's composition[2]. They also employ a closed-loop cooling system that maintains the acid at a constant temperature, typically around 20°C, to prevent thermal degradation and reduce vapor emissions. Furthermore, Sinopec has implemented an automated nitrogen blanketing system that displaces oxygen in the tank's headspace, reducing the risk of contamination and minimizing corrosion in the vapor space[4].
Strengths: Large-scale storage capability, advanced monitoring systems, and effective contamination prevention. Weaknesses: High capital investment required, and potential difficulties in retrofitting existing storage facilities.
Innovative HCl Storage Technologies
Storage stable solution comprising hypochlorous acid and/or hypochlorite
PatentPendingUS20250031702A1
Innovation
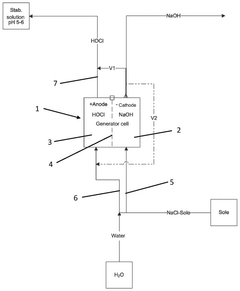
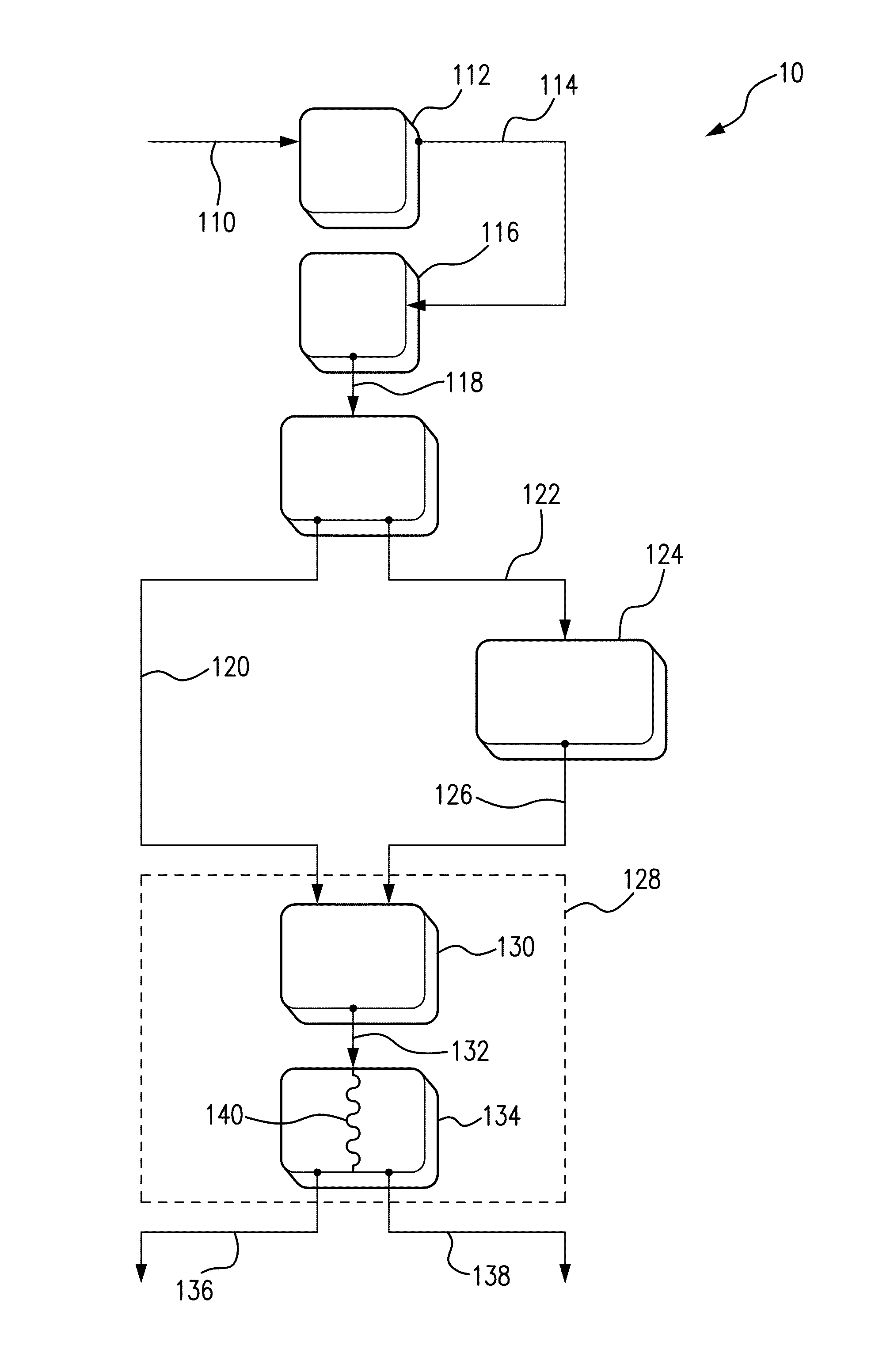
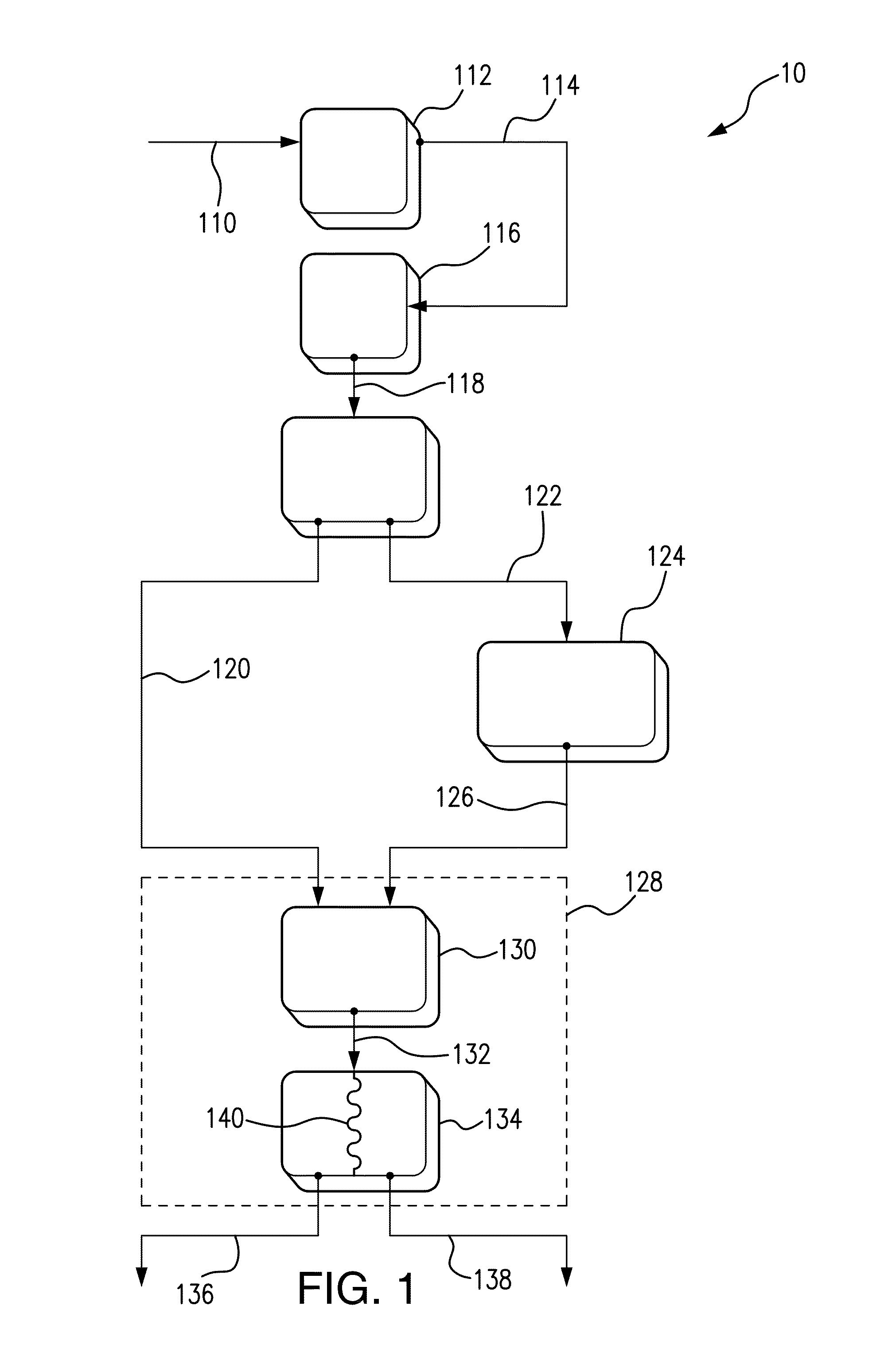
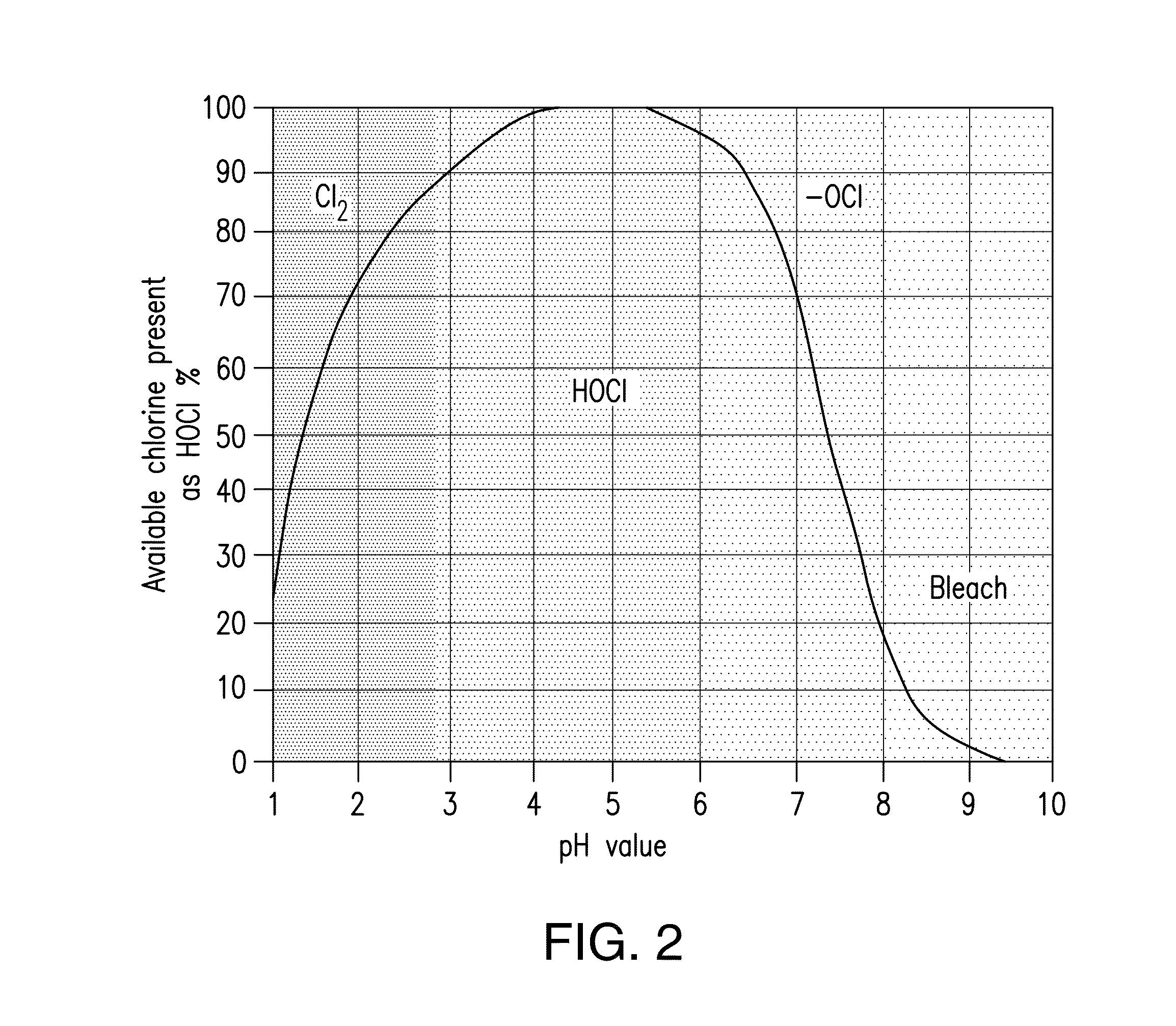
- A process involving electrolysis of a NaCl solution with specific conditions, including a pH adjustment to 5 to 6, is used to produce a storage-stable aqueous solution comprising hypochlorous acid and/or hypochlorite. This is achieved by introducing the aqueous NaCl solution into an electrolysis cell with separate compartments and applying direct current to produce and stabilize the solution.
Method for Producing Shelf Stable Hypochlorous Acid Solutions
PatentInactiveUS20150119245A1
Innovation
- A method involving the production of hypochlorous acid using high purity water from a combination of softening and reverse osmosis steps, followed by electrolysis with a bipolar membrane, and storage in opaque PET bottles or Nylon/PE bags, which significantly extends the shelf life to 24 months.
Safety Regulations for HCl Storage
The storage of hydrochloric acid (HCl) is subject to stringent safety regulations due to its corrosive and hazardous nature. These regulations are designed to protect workers, the environment, and the integrity of storage facilities. In the United States, the Occupational Safety and Health Administration (OSHA) and the Environmental Protection Agency (EPA) provide comprehensive guidelines for HCl storage.
OSHA standards require that HCl storage areas be equipped with proper ventilation systems to prevent the accumulation of toxic fumes. The ventilation system must be corrosion-resistant and capable of maintaining airborne concentrations of HCl below permissible exposure limits. Additionally, storage facilities must have emergency eyewash stations and safety showers within the immediate work area for quick decontamination in case of accidental exposure.
Containment is a critical aspect of HCl storage regulations. Storage tanks and containers must be constructed of materials resistant to HCl corrosion, such as high-density polyethylene (HDPE) or fiberglass-reinforced plastic (FRP). Secondary containment systems, such as dikes or berms, are mandatory to prevent environmental contamination in case of leaks or spills. These systems must have a capacity of at least 110% of the largest container or 10% of the total volume stored, whichever is greater.
Temperature control is another key regulatory requirement for HCl storage. Facilities must maintain temperatures within a safe range to prevent pressure build-up and potential container failure. Typically, storage temperatures should be kept below 30°C (86°F) to ensure stability. Monitoring systems must be in place to alert personnel of any temperature fluctuations outside the acceptable range.
Personal protective equipment (PPE) regulations for HCl storage areas are stringent. Workers must be provided with and trained in the use of appropriate PPE, including chemical-resistant gloves, goggles, face shields, and protective clothing. Respiratory protection may also be required depending on the concentration and potential for exposure.
Labeling and signage regulations are crucial for HCl storage safety. All containers must be clearly labeled with the chemical name, concentration, hazard warnings, and appropriate safety precautions. Storage areas must display prominent hazard communication signs, including NFPA 704 diamond placards indicating the specific hazards associated with HCl.
Emergency response planning is a regulatory requirement for facilities storing HCl. This includes developing and maintaining a comprehensive emergency action plan, conducting regular drills, and ensuring that all personnel are trained in emergency procedures. The plan must address potential scenarios such as spills, leaks, fires, and worker exposure.
Regular inspections and maintenance of storage facilities are mandated by safety regulations. This includes routine checks of containment systems, valves, piping, and monitoring equipment. Any deficiencies must be promptly addressed to maintain compliance and ensure safe storage conditions.
OSHA standards require that HCl storage areas be equipped with proper ventilation systems to prevent the accumulation of toxic fumes. The ventilation system must be corrosion-resistant and capable of maintaining airborne concentrations of HCl below permissible exposure limits. Additionally, storage facilities must have emergency eyewash stations and safety showers within the immediate work area for quick decontamination in case of accidental exposure.
Containment is a critical aspect of HCl storage regulations. Storage tanks and containers must be constructed of materials resistant to HCl corrosion, such as high-density polyethylene (HDPE) or fiberglass-reinforced plastic (FRP). Secondary containment systems, such as dikes or berms, are mandatory to prevent environmental contamination in case of leaks or spills. These systems must have a capacity of at least 110% of the largest container or 10% of the total volume stored, whichever is greater.
Temperature control is another key regulatory requirement for HCl storage. Facilities must maintain temperatures within a safe range to prevent pressure build-up and potential container failure. Typically, storage temperatures should be kept below 30°C (86°F) to ensure stability. Monitoring systems must be in place to alert personnel of any temperature fluctuations outside the acceptable range.
Personal protective equipment (PPE) regulations for HCl storage areas are stringent. Workers must be provided with and trained in the use of appropriate PPE, including chemical-resistant gloves, goggles, face shields, and protective clothing. Respiratory protection may also be required depending on the concentration and potential for exposure.
Labeling and signage regulations are crucial for HCl storage safety. All containers must be clearly labeled with the chemical name, concentration, hazard warnings, and appropriate safety precautions. Storage areas must display prominent hazard communication signs, including NFPA 704 diamond placards indicating the specific hazards associated with HCl.
Emergency response planning is a regulatory requirement for facilities storing HCl. This includes developing and maintaining a comprehensive emergency action plan, conducting regular drills, and ensuring that all personnel are trained in emergency procedures. The plan must address potential scenarios such as spills, leaks, fires, and worker exposure.
Regular inspections and maintenance of storage facilities are mandated by safety regulations. This includes routine checks of containment systems, valves, piping, and monitoring equipment. Any deficiencies must be promptly addressed to maintain compliance and ensure safe storage conditions.
Environmental Impact of HCl Storage
The storage of hydrochloric acid (HCl) poses significant environmental risks that require careful consideration and management. Accidental releases or improper handling of HCl can lead to severe consequences for both the immediate surroundings and broader ecosystems. When exposed to air, HCl readily forms a corrosive mist that can cause damage to vegetation, soil, and water bodies in the vicinity of storage facilities.
One of the primary environmental concerns is the potential for soil contamination. HCl spills can dramatically alter soil pH, leading to increased soil acidity. This change in soil chemistry can have far-reaching effects on plant life, microbial communities, and soil-dwelling organisms. The acidification of soil can result in reduced fertility, impaired nutrient uptake by plants, and the mobilization of toxic metals, further exacerbating environmental damage.
Water pollution is another critical issue associated with HCl storage. Accidental discharges into water systems can cause rapid pH changes, potentially leading to fish kills and disruption of aquatic ecosystems. The acidification of water bodies can also impact the solubility of various chemicals and metals, potentially increasing the bioavailability of harmful substances to aquatic life.
Air quality is also at risk from HCl storage. Vapor emissions from storage tanks or during transfer operations can contribute to the formation of acid rain, which has wide-ranging environmental impacts. Acid rain can damage forests, crops, and aquatic ecosystems far from the original source of pollution. Additionally, HCl emissions can react with other atmospheric pollutants, potentially exacerbating air quality issues in urban and industrial areas.
The long-term environmental effects of HCl storage must also be considered. Chronic low-level releases can lead to gradual changes in local ecosystems, potentially altering biodiversity and ecological balance over time. This can result in the loss of sensitive species and the dominance of more acid-tolerant organisms, fundamentally changing the composition of affected ecosystems.
To mitigate these environmental risks, stringent storage and handling protocols are essential. This includes the use of corrosion-resistant materials for storage tanks, implementation of secondary containment systems, and regular inspections to prevent leaks and spills. Proper ventilation systems and scrubbers can help reduce air emissions, while emergency response plans should be in place to quickly address any accidental releases.
Furthermore, the location of HCl storage facilities should be carefully chosen to minimize potential environmental impact. This includes considering factors such as proximity to water bodies, sensitive ecosystems, and populated areas. By implementing comprehensive environmental management strategies, the risks associated with HCl storage can be significantly reduced, helping to protect both human health and the environment.
One of the primary environmental concerns is the potential for soil contamination. HCl spills can dramatically alter soil pH, leading to increased soil acidity. This change in soil chemistry can have far-reaching effects on plant life, microbial communities, and soil-dwelling organisms. The acidification of soil can result in reduced fertility, impaired nutrient uptake by plants, and the mobilization of toxic metals, further exacerbating environmental damage.
Water pollution is another critical issue associated with HCl storage. Accidental discharges into water systems can cause rapid pH changes, potentially leading to fish kills and disruption of aquatic ecosystems. The acidification of water bodies can also impact the solubility of various chemicals and metals, potentially increasing the bioavailability of harmful substances to aquatic life.
Air quality is also at risk from HCl storage. Vapor emissions from storage tanks or during transfer operations can contribute to the formation of acid rain, which has wide-ranging environmental impacts. Acid rain can damage forests, crops, and aquatic ecosystems far from the original source of pollution. Additionally, HCl emissions can react with other atmospheric pollutants, potentially exacerbating air quality issues in urban and industrial areas.
The long-term environmental effects of HCl storage must also be considered. Chronic low-level releases can lead to gradual changes in local ecosystems, potentially altering biodiversity and ecological balance over time. This can result in the loss of sensitive species and the dominance of more acid-tolerant organisms, fundamentally changing the composition of affected ecosystems.
To mitigate these environmental risks, stringent storage and handling protocols are essential. This includes the use of corrosion-resistant materials for storage tanks, implementation of secondary containment systems, and regular inspections to prevent leaks and spills. Proper ventilation systems and scrubbers can help reduce air emissions, while emergency response plans should be in place to quickly address any accidental releases.
Furthermore, the location of HCl storage facilities should be carefully chosen to minimize potential environmental impact. This includes considering factors such as proximity to water bodies, sensitive ecosystems, and populated areas. By implementing comprehensive environmental management strategies, the risks associated with HCl storage can be significantly reduced, helping to protect both human health and the environment.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!