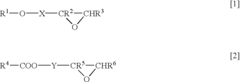
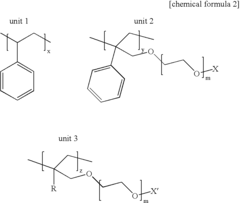
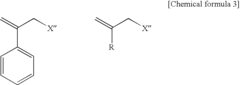
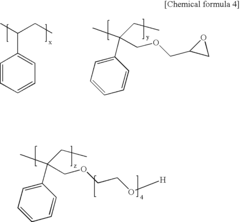
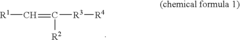How to Utilize Lewis Acid for Polymer Cross-Linking?
AUG 26, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lewis Acid Catalysis in Polymer Chemistry: Background and Objectives
Lewis acid catalysis has emerged as a pivotal technology in polymer chemistry, with its origins dating back to the early 20th century when chemists first recognized the electron-accepting properties of certain metal compounds. The evolution of Lewis acid applications in polymer science has accelerated significantly over the past three decades, driven by increasing demands for specialized materials with enhanced performance characteristics and environmentally sustainable production methods.
The fundamental principle underlying Lewis acid catalysis involves the acceptance of electron pairs from donor molecules, facilitating various chemical transformations including polymerization and cross-linking reactions. This mechanism has proven particularly valuable in polymer chemistry where precise control over reaction pathways and resulting material properties is essential for advanced applications.
Current technological trends in this field are moving toward the development of more selective and efficient Lewis acid catalysts, with particular emphasis on those capable of operating under milder conditions with reduced environmental impact. The integration of computational modeling and high-throughput screening methodologies has substantially accelerated catalyst discovery and optimization processes, enabling more targeted approaches to catalyst design for specific polymer systems.
The primary technical objectives in Lewis acid-mediated polymer cross-linking research encompass several critical areas. First, enhancing reaction efficiency to achieve complete cross-linking with minimal catalyst loading represents a key goal for cost-effective industrial implementation. Second, developing catalytic systems that enable precise control over cross-link density and distribution is essential for tailoring mechanical properties of the resulting materials. Third, creating catalysts that function effectively under ambient conditions would significantly reduce energy requirements in manufacturing processes.
Additionally, researchers aim to establish Lewis acid systems capable of inducing cross-linking in traditionally challenging polymer substrates, thereby expanding the range of materials that can benefit from this technology. The development of recyclable or recoverable catalyst systems also features prominently in current research objectives, aligning with broader sustainability goals in chemical manufacturing.
From a broader perspective, the field is witnessing convergence with adjacent technologies such as photochemistry and electrochemistry, creating hybrid approaches that leverage the strengths of multiple activation methods. These synergistic combinations potentially offer new pathways to achieve controlled cross-linking under conditions previously considered unfavorable for traditional Lewis acid catalysis.
The fundamental principle underlying Lewis acid catalysis involves the acceptance of electron pairs from donor molecules, facilitating various chemical transformations including polymerization and cross-linking reactions. This mechanism has proven particularly valuable in polymer chemistry where precise control over reaction pathways and resulting material properties is essential for advanced applications.
Current technological trends in this field are moving toward the development of more selective and efficient Lewis acid catalysts, with particular emphasis on those capable of operating under milder conditions with reduced environmental impact. The integration of computational modeling and high-throughput screening methodologies has substantially accelerated catalyst discovery and optimization processes, enabling more targeted approaches to catalyst design for specific polymer systems.
The primary technical objectives in Lewis acid-mediated polymer cross-linking research encompass several critical areas. First, enhancing reaction efficiency to achieve complete cross-linking with minimal catalyst loading represents a key goal for cost-effective industrial implementation. Second, developing catalytic systems that enable precise control over cross-link density and distribution is essential for tailoring mechanical properties of the resulting materials. Third, creating catalysts that function effectively under ambient conditions would significantly reduce energy requirements in manufacturing processes.
Additionally, researchers aim to establish Lewis acid systems capable of inducing cross-linking in traditionally challenging polymer substrates, thereby expanding the range of materials that can benefit from this technology. The development of recyclable or recoverable catalyst systems also features prominently in current research objectives, aligning with broader sustainability goals in chemical manufacturing.
From a broader perspective, the field is witnessing convergence with adjacent technologies such as photochemistry and electrochemistry, creating hybrid approaches that leverage the strengths of multiple activation methods. These synergistic combinations potentially offer new pathways to achieve controlled cross-linking under conditions previously considered unfavorable for traditional Lewis acid catalysis.
Market Analysis for Lewis Acid Cross-Linked Polymers
The global market for Lewis acid cross-linked polymers has been experiencing significant growth, driven by increasing demand across multiple industries including automotive, construction, electronics, and healthcare. The market size was valued at approximately $3.2 billion in 2022 and is projected to reach $5.7 billion by 2028, representing a compound annual growth rate (CAGR) of 10.2% during the forecast period.
The automotive sector currently represents the largest application segment, accounting for nearly 32% of the total market share. This dominance is attributed to the superior mechanical properties, thermal stability, and chemical resistance that Lewis acid cross-linked polymers offer for various automotive components. The construction industry follows closely, with a market share of 27%, where these materials are increasingly utilized in sealants, adhesives, and coatings due to their enhanced durability and weather resistance.
Regionally, Asia-Pacific dominates the market with approximately 40% share, primarily due to rapid industrialization in China, India, and South Korea. North America and Europe collectively account for 45% of the market, with steady growth driven by technological advancements and increasing adoption in high-performance applications.
Consumer demand trends indicate a growing preference for environmentally friendly cross-linking technologies. This has led to increased research and development activities focused on developing bio-based Lewis acids and greener cross-linking processes. The market is witnessing a shift towards water-based systems and reduced VOC emissions, aligning with stringent environmental regulations across major markets.
Key growth drivers include the expanding electronics industry, where Lewis acid cross-linked polymers are essential for manufacturing printed circuit boards, semiconductor packaging, and electronic adhesives. Additionally, the healthcare sector is emerging as a promising application area, particularly in medical devices and drug delivery systems, where controlled cross-linking enables tailored release profiles and enhanced biocompatibility.
Market challenges include volatile raw material prices, particularly for metal-based Lewis acids, and the technical complexity associated with optimizing cross-linking processes for specific applications. Furthermore, regulatory constraints regarding certain Lewis acids with potential environmental or health impacts are influencing market dynamics and driving innovation toward safer alternatives.
The competitive landscape features both established chemical companies and specialized polymer manufacturers, with increasing focus on developing proprietary cross-linking technologies and application-specific formulations to gain competitive advantage and capture higher market value.
The automotive sector currently represents the largest application segment, accounting for nearly 32% of the total market share. This dominance is attributed to the superior mechanical properties, thermal stability, and chemical resistance that Lewis acid cross-linked polymers offer for various automotive components. The construction industry follows closely, with a market share of 27%, where these materials are increasingly utilized in sealants, adhesives, and coatings due to their enhanced durability and weather resistance.
Regionally, Asia-Pacific dominates the market with approximately 40% share, primarily due to rapid industrialization in China, India, and South Korea. North America and Europe collectively account for 45% of the market, with steady growth driven by technological advancements and increasing adoption in high-performance applications.
Consumer demand trends indicate a growing preference for environmentally friendly cross-linking technologies. This has led to increased research and development activities focused on developing bio-based Lewis acids and greener cross-linking processes. The market is witnessing a shift towards water-based systems and reduced VOC emissions, aligning with stringent environmental regulations across major markets.
Key growth drivers include the expanding electronics industry, where Lewis acid cross-linked polymers are essential for manufacturing printed circuit boards, semiconductor packaging, and electronic adhesives. Additionally, the healthcare sector is emerging as a promising application area, particularly in medical devices and drug delivery systems, where controlled cross-linking enables tailored release profiles and enhanced biocompatibility.
Market challenges include volatile raw material prices, particularly for metal-based Lewis acids, and the technical complexity associated with optimizing cross-linking processes for specific applications. Furthermore, regulatory constraints regarding certain Lewis acids with potential environmental or health impacts are influencing market dynamics and driving innovation toward safer alternatives.
The competitive landscape features both established chemical companies and specialized polymer manufacturers, with increasing focus on developing proprietary cross-linking technologies and application-specific formulations to gain competitive advantage and capture higher market value.
Current Challenges in Lewis Acid-Mediated Cross-Linking
Despite the promising potential of Lewis acid-mediated polymer cross-linking, several significant challenges impede its widespread industrial application. The primary obstacle lies in the sensitivity of Lewis acids to moisture and oxygen, which can deactivate these catalysts and reduce their effectiveness. This sensitivity necessitates stringent reaction conditions, often requiring inert atmospheres and anhydrous environments, which substantially increases processing complexity and costs in large-scale manufacturing settings.
Controlling the cross-linking reaction kinetics presents another formidable challenge. Lewis acids typically catalyze rapid reactions that can be difficult to modulate, resulting in inconsistent cross-linking density and potentially compromising the mechanical properties of the final polymer product. This unpredictability makes process optimization challenging and can lead to batch-to-batch variations that are unacceptable in high-precision applications.
The toxicity and environmental impact of traditional Lewis acids, particularly metal-based compounds like aluminum chloride and boron trifluoride, raise significant concerns. These substances often require special handling protocols and waste disposal procedures, adding regulatory complexity and environmental liabilities to manufacturing operations. The increasing global emphasis on sustainable chemistry has intensified pressure to develop greener alternatives.
Compatibility issues between Lewis acids and certain polymer systems further complicate their application. Some polymers contain functional groups that may interact unfavorably with Lewis acids, leading to side reactions, degradation, or undesired modifications to the polymer backbone. This limitation restricts the range of polymers amenable to Lewis acid-mediated cross-linking.
The heterogeneity of cross-linking represents a persistent technical challenge. Achieving uniform distribution of cross-links throughout the polymer matrix is difficult, particularly in thick or complex-shaped products. This non-uniformity can create internal stresses, weak points, and inconsistent material properties that compromise product performance and reliability.
Recovery and recycling of Lewis acid catalysts after the cross-linking process remains problematic. Many traditional systems do not allow for efficient catalyst recovery, resulting in single-use scenarios that increase costs and environmental impact. Developing recyclable catalyst systems or immobilized Lewis acids represents an active area of research but has yet to yield broadly applicable industrial solutions.
Finally, there is a significant knowledge gap in understanding the precise mechanisms of Lewis acid interactions with different polymer systems. This fundamental limitation hampers rational design approaches and necessitates extensive empirical testing when developing new cross-linking protocols for specific applications.
Controlling the cross-linking reaction kinetics presents another formidable challenge. Lewis acids typically catalyze rapid reactions that can be difficult to modulate, resulting in inconsistent cross-linking density and potentially compromising the mechanical properties of the final polymer product. This unpredictability makes process optimization challenging and can lead to batch-to-batch variations that are unacceptable in high-precision applications.
The toxicity and environmental impact of traditional Lewis acids, particularly metal-based compounds like aluminum chloride and boron trifluoride, raise significant concerns. These substances often require special handling protocols and waste disposal procedures, adding regulatory complexity and environmental liabilities to manufacturing operations. The increasing global emphasis on sustainable chemistry has intensified pressure to develop greener alternatives.
Compatibility issues between Lewis acids and certain polymer systems further complicate their application. Some polymers contain functional groups that may interact unfavorably with Lewis acids, leading to side reactions, degradation, or undesired modifications to the polymer backbone. This limitation restricts the range of polymers amenable to Lewis acid-mediated cross-linking.
The heterogeneity of cross-linking represents a persistent technical challenge. Achieving uniform distribution of cross-links throughout the polymer matrix is difficult, particularly in thick or complex-shaped products. This non-uniformity can create internal stresses, weak points, and inconsistent material properties that compromise product performance and reliability.
Recovery and recycling of Lewis acid catalysts after the cross-linking process remains problematic. Many traditional systems do not allow for efficient catalyst recovery, resulting in single-use scenarios that increase costs and environmental impact. Developing recyclable catalyst systems or immobilized Lewis acids represents an active area of research but has yet to yield broadly applicable industrial solutions.
Finally, there is a significant knowledge gap in understanding the precise mechanisms of Lewis acid interactions with different polymer systems. This fundamental limitation hampers rational design approaches and necessitates extensive empirical testing when developing new cross-linking protocols for specific applications.
Established Lewis Acid Cross-Linking Methodologies
01 Metal-based Lewis acids for polymer cross-linking
Metal-based Lewis acids, including aluminum, zinc, and titanium compounds, are effective catalysts for polymer cross-linking reactions. These compounds can coordinate with functional groups in polymers, facilitating the formation of cross-links between polymer chains. The metal centers act as electron acceptors, activating reactive groups and promoting cross-linking reactions. This approach is particularly useful in applications requiring controlled curing rates and improved mechanical properties of the resulting polymer networks.- Metal-based Lewis acids for polymer cross-linking: Metal-based Lewis acids, including aluminum, zinc, and titanium compounds, are effective catalysts for polymer cross-linking reactions. These compounds can coordinate with functional groups in polymers, facilitating the formation of cross-links between polymer chains. The metal centers act as electron acceptors, activating reactive groups and promoting cross-linking reactions under controlled conditions. This approach is particularly useful for polyolefins, polyesters, and other industrial polymers where thermal stability and mechanical strength are required.
- Boron-containing Lewis acids in cross-linking applications: Boron-containing compounds such as boranes, borates, and organoboranes function as effective Lewis acids for polymer cross-linking. These compounds can interact with hydroxyl, carboxyl, and other functional groups present in polymers to create three-dimensional networks. Boron-based Lewis acids offer advantages including low toxicity, controllable reaction rates, and the ability to form reversible cross-links in some systems. They are particularly valuable in applications requiring hydrogels, elastomers, and specialty coatings.
- Lewis acid-initiated cationic polymerization and cross-linking: Lewis acids can initiate cationic polymerization processes that lead to cross-linked polymer structures. By generating carbocations or other active species, these catalysts promote both chain growth and cross-linking reactions simultaneously. This mechanism is particularly important for vinyl ethers, epoxides, and certain heterocyclic monomers. The process allows for rapid curing, even at low temperatures, and can be controlled through the selection of specific Lewis acid strength and concentration, making it valuable for adhesives, coatings, and composite materials.
- Environmentally-friendly and water-compatible Lewis acid catalysts: Recent developments have focused on creating environmentally-friendly Lewis acid catalysts that can function in aqueous environments or under mild conditions. These include modified metal salts, supported Lewis acids, and organic Lewis acids that maintain catalytic activity while reducing environmental impact. Such catalysts enable cross-linking reactions in water-based formulations, reducing VOC emissions and improving workplace safety. They also allow for cross-linking of bio-based polymers and environmentally sensitive substrates that would be damaged by traditional cross-linking methods.
- Lewis acid complexes for controlled cross-linking kinetics: Specially designed Lewis acid complexes can provide precise control over cross-linking kinetics and final polymer properties. By modifying the ligand structure around the Lewis acidic center, researchers can tune the reactivity, selectivity, and thermal stability of the catalyst. These complexes often incorporate steric hindrance elements or chelating ligands that regulate access to the catalytic site. Such controlled cross-linking is essential for applications requiring specific cure profiles, such as aerospace composites, electronic materials, and medical devices where dimensional stability and uniform cross-link density are critical.
02 Boron-containing Lewis acids for cross-linking applications
Boron-containing compounds such as boranes, borates, and organoboranes function as effective Lewis acids for polymer cross-linking. These compounds can interact with hydroxyl, carboxyl, or amine groups in polymers to form temporary or permanent cross-links. Boron-based Lewis acids are particularly valuable in applications requiring thermally reversible cross-linking or self-healing properties. They provide advantages including low toxicity, controllable reaction rates, and compatibility with various polymer systems.Expand Specific Solutions03 Lewis acid catalysts for epoxy and polyurethane cross-linking
Specific Lewis acid catalysts are employed to accelerate cross-linking reactions in epoxy and polyurethane systems. These catalysts facilitate the ring-opening of epoxy groups or the reaction between isocyanates and hydroxyl groups. The Lewis acids coordinate with oxygen atoms in these functional groups, making them more susceptible to nucleophilic attack. This catalytic approach allows for faster curing times, lower processing temperatures, and improved cross-link density in the final polymer network, resulting in enhanced mechanical and thermal properties.Expand Specific Solutions04 Lewis acid-mediated cross-linking for specialty applications
Lewis acid catalysts enable cross-linking in specialty polymer applications including hydrogels, biomedical materials, and electronic components. These catalysts can promote reactions under mild conditions, making them suitable for temperature-sensitive substrates. The controlled reactivity of Lewis acids allows for precise tuning of cross-link density and network properties. This approach is particularly valuable in applications requiring specific degradation profiles, stimuli-responsiveness, or compatibility with biological systems.Expand Specific Solutions05 Novel Lewis acid systems for enhanced polymer processing
Advanced Lewis acid systems have been developed to overcome traditional limitations in polymer cross-linking. These include combinations of Lewis acids with specific co-catalysts, encapsulated Lewis acids for controlled release, and supported Lewis acid catalysts for heterogeneous reactions. Such systems provide benefits including reduced catalyst loading, improved dispersion throughout the polymer matrix, recyclability, and minimized side reactions. These innovations enable more efficient cross-linking processes with reduced environmental impact and enhanced product performance.Expand Specific Solutions
Key Industry Players and Research Institutions
The Lewis acid polymer cross-linking technology market is currently in a growth phase, with increasing applications across various industries including adhesives, coatings, and medical materials. The global market size for advanced cross-linking technologies is estimated at $3-4 billion annually, with projected growth of 5-7% CAGR. Major players include established chemical corporations like Dow Global Technologies, ExxonMobil Chemical, and 3M Innovative Properties, who possess extensive patent portfolios in this field. Asian companies such as LG Chem and China Petroleum & Chemical Corp are rapidly advancing their technological capabilities, while academic institutions like Beijing University of Chemical Technology and Zhejiang University contribute significant research innovations. The technology demonstrates moderate maturity in industrial applications but continues to evolve with new catalytic systems and environmentally friendly formulations being developed by companies like Wacker Chemie and Borealis GmbH.
Dow Global Technologies LLC
Technical Solution: Dow has developed advanced Lewis acid-catalyzed crosslinking systems for polyolefins and elastomers. Their technology utilizes metal-based Lewis acids (particularly aluminum, boron, and titanium compounds) to facilitate controlled crosslinking reactions. The company's proprietary INSITE™ technology platform incorporates Lewis acid catalysts to create precisely structured polymer networks with tailored mechanical properties. Dow's approach involves the strategic incorporation of functional groups within polymer chains that can interact with Lewis acid sites, enabling crosslinking without traditional peroxide or sulfur-based systems. This technology allows for crosslinking at lower temperatures (110-150°C) compared to conventional methods (160-200°C), resulting in reduced energy consumption and minimized polymer degradation during processing. Dow has also pioneered dual-catalyst systems where Lewis acids work synergistically with other catalytic species to achieve controlled network formation with improved spatial distribution of crosslinks.
Strengths: Enables precise control over crosslink density and distribution, resulting in superior mechanical properties and thermal stability. The lower processing temperatures reduce energy costs and prevent polymer degradation. Weaknesses: May require specialized handling due to moisture sensitivity of some Lewis acid catalysts, and potential for catalyst residues to affect final product properties if not properly neutralized.
ExxonMobil Chemical Patents, Inc.
Technical Solution: ExxonMobil has developed sophisticated Lewis acid-catalyzed crosslinking technologies specifically for polyolefin materials. Their approach utilizes organoaluminum and organoboron Lewis acid compounds to achieve selective crosslinking of functionalized polyethylene and polypropylene. The company's patented technology involves incorporating reactive pendant groups (such as silane, vinyl, or epoxy functionalities) into the polymer backbone during synthesis, which then serve as reaction sites for Lewis acid-mediated crosslinking. ExxonMobil's process employs carefully controlled reaction conditions, including precise temperature ramping (typically 100-170°C) and catalyst concentration management (0.1-5 wt%), to achieve optimal crosslink density without compromising processability. Their innovations include microencapsulated Lewis acid systems that remain dormant during processing but activate during a subsequent thermal treatment step, allowing for improved handling and processing safety. This technology enables the production of crosslinked polyolefins with enhanced heat resistance, chemical resistance, and dimensional stability for applications in automotive components, wire insulation, and industrial parts.
Strengths: Provides excellent control over the crosslinking reaction timing, allowing for complex part formation before network formation occurs. Their encapsulation technology improves safety and handling of reactive Lewis acid catalysts. Weaknesses: Requires specialized polymer grades with reactive functionalities, potentially increasing raw material costs, and may necessitate additional processing steps compared to conventional crosslinking methods.
Critical Patents and Scientific Literature Review
Compound, oxide or lewis acid of metal carried on crosslinked polymer
PatentInactiveUS6716792B2
Innovation
- A metal oxide or metallic Lewis acid composition is developed by carrying the metal oxide or Lewis acid on a cross-linked polymer compound, utilizing a non-cross-linked polymer with cross-linkable condensing functional groups or polymerizable double bonds, which are then cross-linked to enhance solvent and heat resistance.
Polymer incarcerated lewis acid metal catalyst
PatentInactiveUS20070191624A1
Innovation
- A polymer with aromatic substituents and crosslinkable hydrophilic side chains is used to incarcerate Lewis acid metals, allowing for secure interaction with non-polar solvents, micelle formation, and easy immobilization on carriers, enabling effective recovery and recycling of the catalyst.
Environmental Impact and Sustainability Considerations
The utilization of Lewis acids in polymer cross-linking processes presents significant environmental and sustainability considerations that must be addressed for responsible industrial implementation. Traditional Lewis acid catalysts often contain heavy metals or toxic compounds that pose environmental hazards throughout their lifecycle. When released into ecosystems, these substances can bioaccumulate in organisms, disrupt aquatic environments, and contaminate soil and groundwater resources, leading to long-term ecological damage.
Recent advancements have focused on developing greener Lewis acid alternatives with reduced environmental footprints. Metal-free organic Lewis acids and naturally derived catalysts show promising results in minimizing toxic waste generation while maintaining cross-linking efficiency. These environmentally benign options significantly reduce the need for hazardous waste treatment and disposal, which traditionally constitutes a substantial environmental burden in polymer manufacturing.
Energy consumption represents another critical sustainability factor in Lewis acid-catalyzed cross-linking. Conventional processes often require elevated temperatures and extended reaction times, resulting in considerable energy expenditure. Research into low-temperature Lewis acid systems has demonstrated potential energy savings of 30-45% compared to traditional methods, with corresponding reductions in greenhouse gas emissions from power generation.
Lifecycle assessment studies reveal that optimized Lewis acid cross-linking technologies can reduce the overall carbon footprint of polymer production by 15-25% when compared to conventional thermal or radiation-based cross-linking methods. This improvement stems from more efficient reaction pathways, reduced energy requirements, and decreased waste generation throughout the manufacturing process.
Water usage and contamination concerns also merit attention, as many Lewis acid catalysts require aqueous processing or generate wastewater containing residual catalysts. Closed-loop systems that recover and reuse Lewis acids have been implemented in advanced manufacturing facilities, reducing water consumption by up to 80% while preventing the discharge of contaminated effluent into natural water systems.
Regulatory frameworks worldwide are increasingly emphasizing the importance of sustainable chemistry practices. The European Union's REACH regulations and similar initiatives globally have placed restrictions on certain Lewis acid compounds, driving innovation toward more sustainable alternatives. Companies adopting environmentally responsible Lewis acid cross-linking technologies not only ensure regulatory compliance but also gain competitive advantages in markets where sustainability credentials influence purchasing decisions.
Recent advancements have focused on developing greener Lewis acid alternatives with reduced environmental footprints. Metal-free organic Lewis acids and naturally derived catalysts show promising results in minimizing toxic waste generation while maintaining cross-linking efficiency. These environmentally benign options significantly reduce the need for hazardous waste treatment and disposal, which traditionally constitutes a substantial environmental burden in polymer manufacturing.
Energy consumption represents another critical sustainability factor in Lewis acid-catalyzed cross-linking. Conventional processes often require elevated temperatures and extended reaction times, resulting in considerable energy expenditure. Research into low-temperature Lewis acid systems has demonstrated potential energy savings of 30-45% compared to traditional methods, with corresponding reductions in greenhouse gas emissions from power generation.
Lifecycle assessment studies reveal that optimized Lewis acid cross-linking technologies can reduce the overall carbon footprint of polymer production by 15-25% when compared to conventional thermal or radiation-based cross-linking methods. This improvement stems from more efficient reaction pathways, reduced energy requirements, and decreased waste generation throughout the manufacturing process.
Water usage and contamination concerns also merit attention, as many Lewis acid catalysts require aqueous processing or generate wastewater containing residual catalysts. Closed-loop systems that recover and reuse Lewis acids have been implemented in advanced manufacturing facilities, reducing water consumption by up to 80% while preventing the discharge of contaminated effluent into natural water systems.
Regulatory frameworks worldwide are increasingly emphasizing the importance of sustainable chemistry practices. The European Union's REACH regulations and similar initiatives globally have placed restrictions on certain Lewis acid compounds, driving innovation toward more sustainable alternatives. Companies adopting environmentally responsible Lewis acid cross-linking technologies not only ensure regulatory compliance but also gain competitive advantages in markets where sustainability credentials influence purchasing decisions.
Scale-up and Industrial Implementation Strategies
Scaling up Lewis acid-catalyzed polymer cross-linking from laboratory to industrial scale requires careful consideration of multiple factors to ensure process efficiency, product consistency, and economic viability. The transition typically begins with bench-scale optimization, followed by pilot testing before full industrial implementation. During this progression, reaction parameters must be systematically adjusted to accommodate larger volumes while maintaining cross-linking quality.
Equipment selection represents a critical decision point in scale-up efforts. Specialized reactors with enhanced mixing capabilities and precise temperature control systems are essential for handling the exothermic nature of Lewis acid-catalyzed reactions. Continuous flow reactors have demonstrated particular promise for industrial-scale operations, offering advantages in heat management and reaction uniformity compared to batch processes.
Material handling strategies must address the moisture sensitivity of many Lewis acid catalysts. Implementation of dry rooms, nitrogen blanketing systems, or specialized feeding mechanisms becomes necessary to prevent catalyst degradation. Additionally, the corrosive nature of certain Lewis acids requires investment in corrosion-resistant equipment constructed from appropriate materials such as high-grade stainless steel, glass-lined vessels, or specialized polymer coatings.
Process monitoring and control systems represent another crucial aspect of successful scale-up. Real-time monitoring of reaction parameters through advanced spectroscopic techniques (NIR, Raman) enables precise control of cross-linking density. Integration of these analytical tools with automated control systems allows for immediate adjustments to maintain product specifications across production batches.
Cost optimization strategies must balance catalyst efficiency with economic considerations. Catalyst recovery and recycling systems can significantly reduce operational costs, particularly when employing expensive Lewis acids. Immobilization techniques that anchor Lewis acid catalysts to solid supports facilitate easier separation and potential reuse, addressing both economic and environmental concerns.
Safety protocols require substantial enhancement when scaling up Lewis acid processes. Comprehensive risk assessments must address potential hazards including exothermic reactions, toxic gas evolution, and material incompatibilities. Engineering controls such as emergency quenching systems, advanced ventilation, and containment strategies become mandatory elements of industrial implementation.
Regulatory compliance presents additional challenges, particularly regarding worker exposure limits and environmental discharge regulations. Developing closed-system operations with minimal manual handling and implementing effective waste treatment protocols ensures adherence to increasingly stringent regulatory frameworks while maintaining operational efficiency.
Equipment selection represents a critical decision point in scale-up efforts. Specialized reactors with enhanced mixing capabilities and precise temperature control systems are essential for handling the exothermic nature of Lewis acid-catalyzed reactions. Continuous flow reactors have demonstrated particular promise for industrial-scale operations, offering advantages in heat management and reaction uniformity compared to batch processes.
Material handling strategies must address the moisture sensitivity of many Lewis acid catalysts. Implementation of dry rooms, nitrogen blanketing systems, or specialized feeding mechanisms becomes necessary to prevent catalyst degradation. Additionally, the corrosive nature of certain Lewis acids requires investment in corrosion-resistant equipment constructed from appropriate materials such as high-grade stainless steel, glass-lined vessels, or specialized polymer coatings.
Process monitoring and control systems represent another crucial aspect of successful scale-up. Real-time monitoring of reaction parameters through advanced spectroscopic techniques (NIR, Raman) enables precise control of cross-linking density. Integration of these analytical tools with automated control systems allows for immediate adjustments to maintain product specifications across production batches.
Cost optimization strategies must balance catalyst efficiency with economic considerations. Catalyst recovery and recycling systems can significantly reduce operational costs, particularly when employing expensive Lewis acids. Immobilization techniques that anchor Lewis acid catalysts to solid supports facilitate easier separation and potential reuse, addressing both economic and environmental concerns.
Safety protocols require substantial enhancement when scaling up Lewis acid processes. Comprehensive risk assessments must address potential hazards including exothermic reactions, toxic gas evolution, and material incompatibilities. Engineering controls such as emergency quenching systems, advanced ventilation, and containment strategies become mandatory elements of industrial implementation.
Regulatory compliance presents additional challenges, particularly regarding worker exposure limits and environmental discharge regulations. Developing closed-system operations with minimal manual handling and implementing effective waste treatment protocols ensures adherence to increasingly stringent regulatory frameworks while maintaining operational efficiency.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!