How UHMWPE Enhances Longevity in Orthopedic Devices
AUG 6, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
UHMWPE in Orthopedics: Background and Objectives
Ultra-high-molecular-weight polyethylene (UHMWPE) has revolutionized the field of orthopedics since its introduction in the 1960s. This remarkable material has become the gold standard for articulating surfaces in joint replacement implants, particularly in hip and knee prostheses. The journey of UHMWPE in orthopedics began with its initial application as a bearing material in total hip arthroplasty, where it demonstrated superior wear resistance and biocompatibility compared to previously used materials.
Over the decades, UHMWPE has undergone significant advancements to address challenges such as wear, oxidation, and particle-induced osteolysis. These improvements have led to the development of highly cross-linked polyethylene (HXLPE) and vitamin E-infused UHMWPE, which exhibit enhanced wear resistance and oxidative stability. The evolution of UHMWPE technology has been driven by the increasing demand for longer-lasting orthopedic implants, particularly in light of the growing number of younger and more active patients requiring joint replacements.
The primary objective of UHMWPE research and development in orthopedics is to further extend the longevity of implants while maintaining or improving their performance characteristics. This goal is crucial given the rising life expectancy and the desire for patients to maintain active lifestyles well into their later years. Researchers and manufacturers are focusing on optimizing the material properties of UHMWPE to reduce wear rates, enhance mechanical strength, and improve resistance to oxidation and fatigue.
Current research efforts are exploring novel manufacturing techniques, such as 3D printing of UHMWPE components, which could allow for patient-specific implant designs. Additionally, there is ongoing investigation into the incorporation of nanoparticles and surface modifications to further enhance the material's properties. The development of hybrid materials that combine UHMWPE with other advanced polymers or ceramics is also an area of active research.
As we look to the future, the objectives for UHMWPE in orthopedics include achieving wear rates that approach zero, developing self-healing capabilities to mitigate damage, and creating "smart" implants that can adapt to the patient's physiology and activity levels. These ambitious goals reflect the continuous drive to improve patient outcomes and reduce the need for revision surgeries, ultimately enhancing the quality of life for millions of individuals worldwide who rely on orthopedic implants.
Over the decades, UHMWPE has undergone significant advancements to address challenges such as wear, oxidation, and particle-induced osteolysis. These improvements have led to the development of highly cross-linked polyethylene (HXLPE) and vitamin E-infused UHMWPE, which exhibit enhanced wear resistance and oxidative stability. The evolution of UHMWPE technology has been driven by the increasing demand for longer-lasting orthopedic implants, particularly in light of the growing number of younger and more active patients requiring joint replacements.
The primary objective of UHMWPE research and development in orthopedics is to further extend the longevity of implants while maintaining or improving their performance characteristics. This goal is crucial given the rising life expectancy and the desire for patients to maintain active lifestyles well into their later years. Researchers and manufacturers are focusing on optimizing the material properties of UHMWPE to reduce wear rates, enhance mechanical strength, and improve resistance to oxidation and fatigue.
Current research efforts are exploring novel manufacturing techniques, such as 3D printing of UHMWPE components, which could allow for patient-specific implant designs. Additionally, there is ongoing investigation into the incorporation of nanoparticles and surface modifications to further enhance the material's properties. The development of hybrid materials that combine UHMWPE with other advanced polymers or ceramics is also an area of active research.
As we look to the future, the objectives for UHMWPE in orthopedics include achieving wear rates that approach zero, developing self-healing capabilities to mitigate damage, and creating "smart" implants that can adapt to the patient's physiology and activity levels. These ambitious goals reflect the continuous drive to improve patient outcomes and reduce the need for revision surgeries, ultimately enhancing the quality of life for millions of individuals worldwide who rely on orthopedic implants.
Market Demand for Long-lasting Orthopedic Implants
The demand for long-lasting orthopedic implants has been steadily increasing due to several factors, including an aging global population, rising prevalence of orthopedic conditions, and advancements in medical technology. As people live longer and maintain active lifestyles well into their later years, the need for durable and reliable orthopedic devices has become more critical than ever.
The orthopedic implant market has experienced significant growth in recent years, with a particular emphasis on joint replacement devices. Hip and knee replacements, in particular, have seen a surge in demand as they offer improved mobility and quality of life for patients suffering from conditions such as osteoarthritis, rheumatoid arthritis, and traumatic injuries.
One of the key drivers of this market demand is the growing awareness among patients and healthcare providers about the benefits of long-lasting implants. Patients are increasingly seeking solutions that can provide extended functionality and reduce the need for revision surgeries, which can be both costly and risky.
Healthcare systems and insurance providers are also recognizing the economic benefits of long-lasting orthopedic implants. By reducing the frequency of revision surgeries and associated complications, these devices can significantly lower long-term healthcare costs and improve patient outcomes.
The market for orthopedic implants has also been influenced by technological advancements in materials science and manufacturing processes. Innovations in biomaterials, such as ultra-high-molecular-weight polyethylene (UHMWPE), have played a crucial role in enhancing the longevity and performance of orthopedic devices.
Geographically, North America and Europe have been the largest markets for long-lasting orthopedic implants, primarily due to their aging populations and well-established healthcare systems. However, emerging markets in Asia-Pacific and Latin America are showing rapid growth potential as healthcare infrastructure improves and awareness of advanced orthopedic treatments increases.
The COVID-19 pandemic temporarily disrupted the orthopedic implant market, with many elective surgeries being postponed. However, the backlog of procedures and the ongoing need for joint replacements have led to a strong recovery and renewed focus on developing more durable and efficient implant solutions.
Looking ahead, the market demand for long-lasting orthopedic implants is expected to continue its upward trajectory. Factors such as the increasing prevalence of obesity, which can exacerbate joint problems, and the growing popularity of sports and fitness activities among all age groups are likely to further drive the need for advanced, durable orthopedic devices.
The orthopedic implant market has experienced significant growth in recent years, with a particular emphasis on joint replacement devices. Hip and knee replacements, in particular, have seen a surge in demand as they offer improved mobility and quality of life for patients suffering from conditions such as osteoarthritis, rheumatoid arthritis, and traumatic injuries.
One of the key drivers of this market demand is the growing awareness among patients and healthcare providers about the benefits of long-lasting implants. Patients are increasingly seeking solutions that can provide extended functionality and reduce the need for revision surgeries, which can be both costly and risky.
Healthcare systems and insurance providers are also recognizing the economic benefits of long-lasting orthopedic implants. By reducing the frequency of revision surgeries and associated complications, these devices can significantly lower long-term healthcare costs and improve patient outcomes.
The market for orthopedic implants has also been influenced by technological advancements in materials science and manufacturing processes. Innovations in biomaterials, such as ultra-high-molecular-weight polyethylene (UHMWPE), have played a crucial role in enhancing the longevity and performance of orthopedic devices.
Geographically, North America and Europe have been the largest markets for long-lasting orthopedic implants, primarily due to their aging populations and well-established healthcare systems. However, emerging markets in Asia-Pacific and Latin America are showing rapid growth potential as healthcare infrastructure improves and awareness of advanced orthopedic treatments increases.
The COVID-19 pandemic temporarily disrupted the orthopedic implant market, with many elective surgeries being postponed. However, the backlog of procedures and the ongoing need for joint replacements have led to a strong recovery and renewed focus on developing more durable and efficient implant solutions.
Looking ahead, the market demand for long-lasting orthopedic implants is expected to continue its upward trajectory. Factors such as the increasing prevalence of obesity, which can exacerbate joint problems, and the growing popularity of sports and fitness activities among all age groups are likely to further drive the need for advanced, durable orthopedic devices.
Current State and Challenges of UHMWPE in Orthopedics
Ultra-high-molecular-weight polyethylene (UHMWPE) has become a cornerstone material in orthopedic devices, particularly in joint replacement implants. Its current state in orthopedics is characterized by widespread adoption and continuous improvement efforts to enhance its performance and longevity.
UHMWPE is primarily used as a bearing surface in total joint arthroplasty, most notably in hip and knee replacements. Its popularity stems from its excellent wear resistance, low friction coefficient, and biocompatibility. The material's high molecular weight and long polymer chains contribute to its superior mechanical properties, making it ideal for withstanding the repetitive loading and motion experienced in joint replacements.
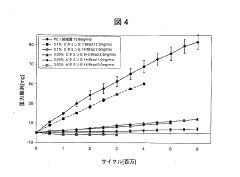
Recent advancements in UHMWPE technology have focused on improving its wear resistance and oxidation stability. Cross-linked UHMWPE, developed in the late 1990s, has shown significant improvements in wear rates compared to conventional UHMWPE. This innovation involves irradiating the material to create cross-links between polymer chains, resulting in enhanced mechanical properties and wear resistance.
Despite these improvements, UHMWPE still faces several challenges in orthopedic applications. One of the primary concerns is long-term oxidation, which can lead to material degradation and reduced mechanical properties over time. This issue is particularly problematic in vivo, where the implant is exposed to a complex biological environment.
Another challenge is the generation of wear particles. Although cross-linking has reduced wear rates, the particles produced can still trigger an inflammatory response in the body, potentially leading to osteolysis and implant loosening. Balancing wear resistance with other mechanical properties, such as fatigue strength and toughness, remains an ongoing challenge for researchers and manufacturers.
The incorporation of antioxidants, particularly vitamin E, into UHMWPE has emerged as a promising solution to combat oxidation. However, optimizing the concentration and distribution of antioxidants without compromising other material properties presents its own set of challenges.
Manufacturing consistency and quality control are also critical issues in UHMWPE production for orthopedic devices. Ensuring uniform molecular weight distribution, crystallinity, and cross-linking across batches is essential for predictable and reliable implant performance.
As the demand for joint replacements continues to rise, particularly in younger and more active patients, there is an increasing need for UHMWPE to perform under more demanding conditions and for longer durations. This necessitates ongoing research into improving the material's properties and understanding its long-term behavior in vivo.
In conclusion, while UHMWPE has made significant strides in enhancing the longevity of orthopedic devices, addressing these challenges remains crucial for further improving patient outcomes and implant durability. The field continues to evolve, with researchers and manufacturers exploring novel modifications and processing techniques to overcome current limitations and push the boundaries of UHMWPE performance in orthopedic applications.
UHMWPE is primarily used as a bearing surface in total joint arthroplasty, most notably in hip and knee replacements. Its popularity stems from its excellent wear resistance, low friction coefficient, and biocompatibility. The material's high molecular weight and long polymer chains contribute to its superior mechanical properties, making it ideal for withstanding the repetitive loading and motion experienced in joint replacements.
Recent advancements in UHMWPE technology have focused on improving its wear resistance and oxidation stability. Cross-linked UHMWPE, developed in the late 1990s, has shown significant improvements in wear rates compared to conventional UHMWPE. This innovation involves irradiating the material to create cross-links between polymer chains, resulting in enhanced mechanical properties and wear resistance.
Despite these improvements, UHMWPE still faces several challenges in orthopedic applications. One of the primary concerns is long-term oxidation, which can lead to material degradation and reduced mechanical properties over time. This issue is particularly problematic in vivo, where the implant is exposed to a complex biological environment.
Another challenge is the generation of wear particles. Although cross-linking has reduced wear rates, the particles produced can still trigger an inflammatory response in the body, potentially leading to osteolysis and implant loosening. Balancing wear resistance with other mechanical properties, such as fatigue strength and toughness, remains an ongoing challenge for researchers and manufacturers.
The incorporation of antioxidants, particularly vitamin E, into UHMWPE has emerged as a promising solution to combat oxidation. However, optimizing the concentration and distribution of antioxidants without compromising other material properties presents its own set of challenges.
Manufacturing consistency and quality control are also critical issues in UHMWPE production for orthopedic devices. Ensuring uniform molecular weight distribution, crystallinity, and cross-linking across batches is essential for predictable and reliable implant performance.
As the demand for joint replacements continues to rise, particularly in younger and more active patients, there is an increasing need for UHMWPE to perform under more demanding conditions and for longer durations. This necessitates ongoing research into improving the material's properties and understanding its long-term behavior in vivo.
In conclusion, while UHMWPE has made significant strides in enhancing the longevity of orthopedic devices, addressing these challenges remains crucial for further improving patient outcomes and implant durability. The field continues to evolve, with researchers and manufacturers exploring novel modifications and processing techniques to overcome current limitations and push the boundaries of UHMWPE performance in orthopedic applications.
Existing UHMWPE Enhancement Techniques
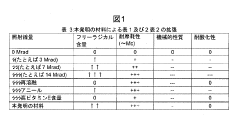
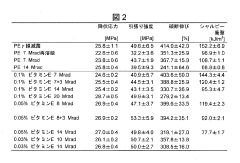
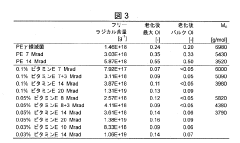
01 Crosslinking techniques for improved longevity
Various crosslinking methods are employed to enhance the longevity of UHMWPE. These techniques include radiation-induced crosslinking, chemical crosslinking, and thermal treatments. Crosslinking improves the wear resistance and mechanical properties of UHMWPE, leading to increased durability and longevity in applications such as joint implants.- Crosslinking techniques for improved longevity: Various crosslinking methods are employed to enhance the longevity of UHMWPE. These techniques include radiation-induced crosslinking, chemical crosslinking, and thermal treatments. Crosslinking improves the wear resistance and mechanical properties of UHMWPE, leading to increased durability and longevity in applications such as joint replacements and industrial components.
- Antioxidant incorporation for oxidation resistance: The addition of antioxidants to UHMWPE formulations significantly enhances its resistance to oxidation, a major factor affecting longevity. Antioxidants such as vitamin E and other stabilizers are incorporated during processing or post-processing to prevent degradation and maintain the material's properties over time, particularly in medical implants and high-stress applications.
- Surface modification for enhanced performance: Surface modification techniques are applied to UHMWPE to improve its longevity and functionality. These include plasma treatments, ion implantation, and surface coatings. Such modifications can enhance wear resistance, reduce friction, and improve biocompatibility in medical applications, contributing to the overall longevity of UHMWPE-based products.
- Nanocomposite formulations for improved properties: Incorporating nanoparticles or nanofibers into UHMWPE creates nanocomposites with enhanced mechanical and thermal properties. These formulations can significantly improve the material's strength, wear resistance, and thermal stability, leading to increased longevity in various applications, from industrial components to advanced medical devices.
- Processing techniques for optimized molecular structure: Advanced processing techniques are developed to optimize the molecular structure of UHMWPE, enhancing its longevity. These include controlled crystallization, orientation of polymer chains, and specialized molding processes. Such techniques result in improved mechanical properties, better wear resistance, and increased overall durability of UHMWPE products.
02 Antioxidant incorporation for oxidation resistance
The addition of antioxidants to UHMWPE formulations significantly enhances its oxidation resistance and long-term stability. Antioxidants such as vitamin E (α-tocopherol) and other stabilizers are incorporated into the polymer matrix to scavenge free radicals and prevent oxidative degradation, thereby extending the material's lifespan.Expand Specific Solutions03 Surface modification for enhanced performance
Surface modification techniques are applied to UHMWPE to improve its tribological properties and longevity. These methods include plasma treatment, ion implantation, and surface coatings. Modified surfaces exhibit reduced friction, improved wear resistance, and enhanced biocompatibility, contributing to the material's extended lifespan in various applications.Expand Specific Solutions04 Nanocomposite formulations for improved properties
UHMWPE nanocomposites are developed by incorporating nanofillers such as carbon nanotubes, graphene, and ceramic nanoparticles. These nanocomposites exhibit enhanced mechanical properties, wear resistance, and thermal stability compared to pure UHMWPE, leading to improved longevity in demanding applications.Expand Specific Solutions05 Processing techniques for optimized performance
Advanced processing techniques are employed to optimize the performance and longevity of UHMWPE. These methods include compression molding, ram extrusion, and gel spinning. Careful control of processing parameters such as temperature, pressure, and cooling rates results in improved crystallinity, molecular orientation, and overall material properties, contributing to enhanced longevity.Expand Specific Solutions
Key Players in UHMWPE Orthopedic Materials
The market for UHMWPE in orthopedic devices is in a mature growth stage, with a significant global market size driven by an aging population and increasing demand for joint replacements. The technology is well-established, with major players like Smith & Nephew, Zimmer, and DePuy Synthes leading innovation. These companies, along with research institutions such as MIT and USC, continue to refine UHMWPE's properties, focusing on enhancing wear resistance and longevity. The competitive landscape is characterized by ongoing research and development efforts to improve implant performance and patient outcomes, with a trend towards customized and 3D-printed solutions incorporating advanced UHMWPE materials.
Smith & Nephew Orthopaedics GmbH
Technical Solution: Smith & Nephew has developed VERILAST™ Technology, which combines their OXINIUM™ oxidized zirconium alloy with XLPE (crosslinked polyethylene) for hip and knee implants. The XLPE component is a highly crosslinked UHMWPE that undergoes a proprietary manufacturing process involving irradiation and thermal treatments[10]. This process aims to improve wear resistance while maintaining mechanical strength. Smith & Nephew's research has shown that VERILAST Technology can reduce wear by up to 81% compared to traditional implant materials[11]. Additionally, they have introduced VISIONAIRE™ Patient-Matched Technology, which combines advanced imaging with VERILAST materials to create personalized cutting blocks for knee replacements, potentially improving implant longevity through optimized alignment[12].
Strengths: Combination of advanced materials (OXINIUM and XLPE), personalized surgical solutions, and significant wear reduction. Weaknesses: Higher cost of specialized materials and patient-matched technology, potential limitations in revision scenarios due to the hardness of OXINIUM.
Zimmer, Inc.
Technical Solution: Zimmer Biomet has developed Vivacit-E® UHMWPE, a highly crosslinked polyethylene blended with vitamin E. This material undergoes a proprietary manufacturing process that includes electron beam irradiation and thermal treatments[4]. The addition of vitamin E acts as an antioxidant, reducing oxidation and wear while maintaining mechanical strength[5]. Zimmer's UHMWPE technology is used in various orthopedic applications, including hip, knee, and shoulder implants. The company has also introduced Trabecular Metal™ Technology, which combines UHMWPE with a porous metal structure to enhance osseointegration and implant stability[6].
Strengths: Vitamin E integration for improved oxidation resistance, versatile applications across joint replacements. Weaknesses: Complexity in manufacturing process, potential long-term effects of vitamin E on implant performance still under study.
Core Innovations in UHMWPE Material Science
Melt-stabilized ultra high molecular weight polyethylene
PatentWO2016153925A1
Innovation
- A method involving coating UHMWPE with antioxidants like protected vitamin E, pre-irradiative heating to diffuse the antioxidants, irradiation for crosslinking, and post-irradiative heating to melt and solidify the material, reducing oxidative degradation and minimizing surface oxidation, thereby reducing waste and processing costs.
Ultra-high molecular weight polyethylene for joint surface
PatentActiveJP2017201037A
Innovation
- A method involving the addition of trace amounts (0.02 to 0.12 wt%) of vitamin E to UHMWPE powder before molding, followed by gamma irradiation at doses between 5 and 20 Mrad, without subsequent heat treatment, to create a composition with improved wear resistance, oxidative stability, and mechanical properties.
Biocompatibility and Safety Considerations
Ultra-high-molecular-weight polyethylene (UHMWPE) has become a cornerstone material in orthopedic devices, particularly in joint replacement implants. Its biocompatibility and safety profile are crucial factors contributing to its widespread adoption and success in enhancing the longevity of orthopedic devices.
UHMWPE demonstrates excellent biocompatibility, which is essential for long-term implantation in the human body. The material's inert nature minimizes adverse reactions with surrounding tissues, reducing the risk of inflammation and rejection. This biocompatibility is attributed to its stable chemical structure and resistance to degradation in the physiological environment.
The safety of UHMWPE in orthopedic applications is further enhanced by its low wear rate and high resistance to fatigue. These properties significantly reduce the generation of wear particles, which is a critical factor in preventing osteolysis and aseptic loosening of implants. The reduction in wear particles also minimizes the risk of adverse local tissue reactions and systemic effects.
Extensive research and clinical studies have consistently demonstrated the long-term safety of UHMWPE in orthopedic devices. The material has shown minimal toxicity and carcinogenicity, with no significant evidence of adverse systemic effects over extended periods of implantation. This safety profile is crucial for patients who require long-term or permanent orthopedic solutions.
The manufacturing process of UHMWPE for medical applications adheres to stringent quality control measures to ensure the highest levels of purity and safety. Advanced sterilization techniques, such as gamma irradiation or ethylene oxide treatment, are employed to eliminate potential pathogens without compromising the material's mechanical properties or biocompatibility.
Recent advancements in UHMWPE technology, including the development of highly crosslinked and vitamin E-infused variants, have further improved its safety profile. These innovations have led to enhanced oxidation resistance and reduced wear rates, addressing some of the long-term concerns associated with traditional UHMWPE.
Regulatory bodies, including the FDA and European health authorities, have extensively evaluated UHMWPE and approved its use in various orthopedic applications. This regulatory scrutiny provides additional assurance of the material's safety and suitability for long-term implantation.
While UHMWPE has demonstrated an excellent safety record, ongoing surveillance and post-market studies continue to monitor its long-term performance and safety. This vigilance ensures that any potential risks or complications are identified and addressed promptly, maintaining the high standards of patient safety in orthopedic care.
UHMWPE demonstrates excellent biocompatibility, which is essential for long-term implantation in the human body. The material's inert nature minimizes adverse reactions with surrounding tissues, reducing the risk of inflammation and rejection. This biocompatibility is attributed to its stable chemical structure and resistance to degradation in the physiological environment.
The safety of UHMWPE in orthopedic applications is further enhanced by its low wear rate and high resistance to fatigue. These properties significantly reduce the generation of wear particles, which is a critical factor in preventing osteolysis and aseptic loosening of implants. The reduction in wear particles also minimizes the risk of adverse local tissue reactions and systemic effects.
Extensive research and clinical studies have consistently demonstrated the long-term safety of UHMWPE in orthopedic devices. The material has shown minimal toxicity and carcinogenicity, with no significant evidence of adverse systemic effects over extended periods of implantation. This safety profile is crucial for patients who require long-term or permanent orthopedic solutions.
The manufacturing process of UHMWPE for medical applications adheres to stringent quality control measures to ensure the highest levels of purity and safety. Advanced sterilization techniques, such as gamma irradiation or ethylene oxide treatment, are employed to eliminate potential pathogens without compromising the material's mechanical properties or biocompatibility.
Recent advancements in UHMWPE technology, including the development of highly crosslinked and vitamin E-infused variants, have further improved its safety profile. These innovations have led to enhanced oxidation resistance and reduced wear rates, addressing some of the long-term concerns associated with traditional UHMWPE.
Regulatory bodies, including the FDA and European health authorities, have extensively evaluated UHMWPE and approved its use in various orthopedic applications. This regulatory scrutiny provides additional assurance of the material's safety and suitability for long-term implantation.
While UHMWPE has demonstrated an excellent safety record, ongoing surveillance and post-market studies continue to monitor its long-term performance and safety. This vigilance ensures that any potential risks or complications are identified and addressed promptly, maintaining the high standards of patient safety in orthopedic care.
Cost-Benefit Analysis of UHMWPE in Orthopedics
The cost-benefit analysis of Ultra-High Molecular Weight Polyethylene (UHMWPE) in orthopedics reveals a compelling case for its widespread adoption. Initially, the implementation of UHMWPE in orthopedic devices may incur higher upfront costs compared to traditional materials. However, the long-term benefits significantly outweigh these initial expenses.
UHMWPE's exceptional wear resistance and durability contribute to extended implant longevity, reducing the frequency of revision surgeries. This translates to substantial cost savings for healthcare systems and patients alike. Studies have shown that UHMWPE-based implants can last up to 20 years or more, compared to the 10-15 year lifespan of conventional implants.
The reduced wear rate of UHMWPE also minimizes the release of wear particles, which are associated with osteolysis and implant loosening. This leads to fewer complications and a decreased need for follow-up treatments, further reducing long-term healthcare costs.
From a patient perspective, the enhanced longevity of UHMWPE implants results in improved quality of life and reduced physical and emotional stress associated with revision surgeries. This translates to increased patient satisfaction and potentially lower costs related to pain management and rehabilitation.
Healthcare providers benefit from reduced operating room time and resources allocated to revision surgeries. This efficiency allows for more effective utilization of medical facilities and personnel, potentially increasing the number of primary surgeries that can be performed.
The manufacturing process of UHMWPE has become more cost-effective over time, with advancements in production techniques and economies of scale. This has helped to offset the initial higher material costs, making UHMWPE more economically viable for widespread use in orthopedic devices.
Insurance companies and healthcare systems also stand to benefit from the long-term cost savings associated with UHMWPE implants. The reduced need for revision surgeries and complications management can lead to lower overall healthcare expenditures, potentially resulting in more favorable insurance premiums for patients.
In conclusion, while the initial costs of implementing UHMWPE in orthopedic devices may be higher, the long-term economic benefits are substantial. The extended implant longevity, reduced complications, and improved patient outcomes justify the investment in this advanced material for orthopedic applications.
UHMWPE's exceptional wear resistance and durability contribute to extended implant longevity, reducing the frequency of revision surgeries. This translates to substantial cost savings for healthcare systems and patients alike. Studies have shown that UHMWPE-based implants can last up to 20 years or more, compared to the 10-15 year lifespan of conventional implants.
The reduced wear rate of UHMWPE also minimizes the release of wear particles, which are associated with osteolysis and implant loosening. This leads to fewer complications and a decreased need for follow-up treatments, further reducing long-term healthcare costs.
From a patient perspective, the enhanced longevity of UHMWPE implants results in improved quality of life and reduced physical and emotional stress associated with revision surgeries. This translates to increased patient satisfaction and potentially lower costs related to pain management and rehabilitation.
Healthcare providers benefit from reduced operating room time and resources allocated to revision surgeries. This efficiency allows for more effective utilization of medical facilities and personnel, potentially increasing the number of primary surgeries that can be performed.
The manufacturing process of UHMWPE has become more cost-effective over time, with advancements in production techniques and economies of scale. This has helped to offset the initial higher material costs, making UHMWPE more economically viable for widespread use in orthopedic devices.
Insurance companies and healthcare systems also stand to benefit from the long-term cost savings associated with UHMWPE implants. The reduced need for revision surgeries and complications management can lead to lower overall healthcare expenditures, potentially resulting in more favorable insurance premiums for patients.
In conclusion, while the initial costs of implementing UHMWPE in orthopedic devices may be higher, the long-term economic benefits are substantial. The extended implant longevity, reduced complications, and improved patient outcomes justify the investment in this advanced material for orthopedic applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!