Hydrochloric Acid and Its Role in Nanotechnology Applications
JUL 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HCl in Nanotech: Background and Objectives
Hydrochloric acid (HCl) has emerged as a crucial component in the rapidly evolving field of nanotechnology. This versatile chemical compound, known for its strong acidic properties, has found numerous applications in the synthesis, modification, and characterization of nanomaterials. The integration of HCl in nanotechnology represents a significant advancement in materials science and engineering, offering new possibilities for creating and manipulating structures at the nanoscale.
The historical use of hydrochloric acid in industrial processes dates back centuries, but its role in nanotechnology has only been fully realized in recent decades. As researchers and engineers sought to develop more precise and controlled methods for nanomaterial synthesis, HCl's unique properties became increasingly valuable. Its ability to etch surfaces, catalyze reactions, and modify surface properties at the nanoscale has made it an indispensable tool in the nanotechnologist's arsenal.
The evolution of HCl's applications in nanotechnology has been driven by the growing demand for advanced materials with tailored properties. From semiconductor manufacturing to the production of nanoparticles for drug delivery systems, HCl has demonstrated its versatility across a wide range of nanotechnology applications. This has led to a surge in research and development efforts aimed at optimizing HCl-based processes for nanomaterial synthesis and modification.
As we look towards the future, the objectives for HCl in nanotechnology are multifaceted. One primary goal is to enhance the precision and control of nanomaterial synthesis using HCl, enabling the creation of more complex and functional nanostructures. Researchers are also focusing on developing environmentally friendly and sustainable methods for incorporating HCl in nanotechnology processes, addressing concerns about the chemical's potential environmental impact.
Another key objective is to expand the range of materials that can be manipulated using HCl-based nanotechnology techniques. This includes exploring new applications in emerging fields such as quantum computing, advanced energy storage, and next-generation electronics. Additionally, there is a growing interest in leveraging HCl's properties to develop novel surface modification techniques that can impart unique functionalities to nanomaterials.
The integration of HCl with other advanced technologies, such as artificial intelligence and machine learning, represents another exciting frontier. These technologies could potentially optimize HCl-based processes in nanotechnology, leading to more efficient and innovative manufacturing techniques. As research in this area progresses, we can expect to see new breakthroughs that further cement HCl's role as a cornerstone of modern nanotechnology.
The historical use of hydrochloric acid in industrial processes dates back centuries, but its role in nanotechnology has only been fully realized in recent decades. As researchers and engineers sought to develop more precise and controlled methods for nanomaterial synthesis, HCl's unique properties became increasingly valuable. Its ability to etch surfaces, catalyze reactions, and modify surface properties at the nanoscale has made it an indispensable tool in the nanotechnologist's arsenal.
The evolution of HCl's applications in nanotechnology has been driven by the growing demand for advanced materials with tailored properties. From semiconductor manufacturing to the production of nanoparticles for drug delivery systems, HCl has demonstrated its versatility across a wide range of nanotechnology applications. This has led to a surge in research and development efforts aimed at optimizing HCl-based processes for nanomaterial synthesis and modification.
As we look towards the future, the objectives for HCl in nanotechnology are multifaceted. One primary goal is to enhance the precision and control of nanomaterial synthesis using HCl, enabling the creation of more complex and functional nanostructures. Researchers are also focusing on developing environmentally friendly and sustainable methods for incorporating HCl in nanotechnology processes, addressing concerns about the chemical's potential environmental impact.
Another key objective is to expand the range of materials that can be manipulated using HCl-based nanotechnology techniques. This includes exploring new applications in emerging fields such as quantum computing, advanced energy storage, and next-generation electronics. Additionally, there is a growing interest in leveraging HCl's properties to develop novel surface modification techniques that can impart unique functionalities to nanomaterials.
The integration of HCl with other advanced technologies, such as artificial intelligence and machine learning, represents another exciting frontier. These technologies could potentially optimize HCl-based processes in nanotechnology, leading to more efficient and innovative manufacturing techniques. As research in this area progresses, we can expect to see new breakthroughs that further cement HCl's role as a cornerstone of modern nanotechnology.
Market Analysis for HCl-Based Nanotech Solutions
The market for HCl-based nanotechnology solutions is experiencing significant growth, driven by the increasing demand for advanced materials and processes in various industries. Hydrochloric acid plays a crucial role in nanotechnology applications, particularly in the synthesis and modification of nanomaterials, etching processes, and surface treatments.
In the semiconductor industry, HCl-based solutions are essential for cleaning and etching silicon wafers, contributing to the production of smaller and more efficient microchips. As the demand for miniaturization in electronics continues to rise, the market for HCl-based nanotech solutions in this sector is expected to expand substantially.
The healthcare and pharmaceutical industries are also driving market growth, with HCl-based nanotech solutions being utilized in drug delivery systems, biosensors, and diagnostic tools. The ability to create nanostructures with precise control over size and surface properties has opened up new possibilities for targeted drug delivery and improved diagnostic accuracy.
Environmental applications represent another significant market segment for HCl-based nanotech solutions. These include water purification systems, air filtration technologies, and remediation of contaminated soil. The unique properties of nanomaterials synthesized using HCl enable more efficient and cost-effective environmental solutions.
The energy sector is increasingly adopting HCl-based nanotech solutions for applications such as fuel cells, batteries, and solar panels. Nanomaterials produced using HCl can enhance energy storage capacity, improve conductivity, and increase the overall efficiency of energy conversion devices.
Geographically, North America and Europe currently dominate the market for HCl-based nanotech solutions, owing to their advanced research infrastructure and strong presence of key industry players. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by rapid industrialization, increasing R&D investments, and growing adoption of nanotechnology across various sectors.
Key market players in this space include established chemical companies expanding their nanotech portfolios, as well as specialized nanotechnology firms focusing on specific applications. Collaborations between academic institutions and industry partners are also contributing to market growth by accelerating the development and commercialization of novel HCl-based nanotech solutions.
Despite the promising outlook, challenges such as regulatory uncertainties, potential environmental and health concerns, and high initial investment costs may impact market growth. However, ongoing research and development efforts are addressing these challenges, paving the way for wider adoption of HCl-based nanotech solutions across industries.
In the semiconductor industry, HCl-based solutions are essential for cleaning and etching silicon wafers, contributing to the production of smaller and more efficient microchips. As the demand for miniaturization in electronics continues to rise, the market for HCl-based nanotech solutions in this sector is expected to expand substantially.
The healthcare and pharmaceutical industries are also driving market growth, with HCl-based nanotech solutions being utilized in drug delivery systems, biosensors, and diagnostic tools. The ability to create nanostructures with precise control over size and surface properties has opened up new possibilities for targeted drug delivery and improved diagnostic accuracy.
Environmental applications represent another significant market segment for HCl-based nanotech solutions. These include water purification systems, air filtration technologies, and remediation of contaminated soil. The unique properties of nanomaterials synthesized using HCl enable more efficient and cost-effective environmental solutions.
The energy sector is increasingly adopting HCl-based nanotech solutions for applications such as fuel cells, batteries, and solar panels. Nanomaterials produced using HCl can enhance energy storage capacity, improve conductivity, and increase the overall efficiency of energy conversion devices.
Geographically, North America and Europe currently dominate the market for HCl-based nanotech solutions, owing to their advanced research infrastructure and strong presence of key industry players. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by rapid industrialization, increasing R&D investments, and growing adoption of nanotechnology across various sectors.
Key market players in this space include established chemical companies expanding their nanotech portfolios, as well as specialized nanotechnology firms focusing on specific applications. Collaborations between academic institutions and industry partners are also contributing to market growth by accelerating the development and commercialization of novel HCl-based nanotech solutions.
Despite the promising outlook, challenges such as regulatory uncertainties, potential environmental and health concerns, and high initial investment costs may impact market growth. However, ongoing research and development efforts are addressing these challenges, paving the way for wider adoption of HCl-based nanotech solutions across industries.
Current Challenges in HCl Nanotech Applications
Despite the significant advancements in nanotechnology applications utilizing hydrochloric acid (HCl), several challenges persist in this field. One of the primary issues is the corrosive nature of HCl, which can lead to material degradation and equipment damage. This necessitates the development of more resistant materials and protective coatings to ensure the longevity of nanotech devices and manufacturing equipment.
Another challenge lies in the precise control of HCl concentration and pH levels during nanofabrication processes. Even slight variations can significantly impact the quality and uniformity of nanostructures, affecting their performance and reliability. Achieving consistent and reproducible results across different batches and scales remains a hurdle for many researchers and manufacturers.
The environmental impact of HCl usage in nanotechnology applications is also a growing concern. As the industry scales up, the safe disposal and neutralization of acid waste become increasingly important. Developing eco-friendly alternatives or closed-loop systems for acid recycling is crucial for sustainable nanotech production.
Furthermore, the interaction between HCl and various nanomaterials is not fully understood. This knowledge gap hampers the optimization of etching processes and the development of novel nanostructures. More comprehensive studies on the chemical mechanisms and kinetics of HCl-nanoparticle interactions are needed to advance the field.
Safety considerations pose another significant challenge. The handling and storage of HCl in nanotech facilities require stringent protocols and specialized equipment. Ensuring worker safety while maintaining production efficiency is a delicate balance that many companies struggle to achieve.
In the realm of characterization and quality control, current analytical techniques sometimes fall short in accurately measuring HCl concentrations and its effects at the nanoscale. This limitation hinders the development of more sophisticated nanotech applications and slows down the optimization of manufacturing processes.
Lastly, the integration of HCl-based nanotech processes with other manufacturing steps remains challenging. Compatibility issues between acid-treated components and subsequent processing stages can lead to defects or reduced performance in final products. Developing seamless integration strategies is essential for the widespread adoption of HCl-enabled nanotechnologies across various industries.
Another challenge lies in the precise control of HCl concentration and pH levels during nanofabrication processes. Even slight variations can significantly impact the quality and uniformity of nanostructures, affecting their performance and reliability. Achieving consistent and reproducible results across different batches and scales remains a hurdle for many researchers and manufacturers.
The environmental impact of HCl usage in nanotechnology applications is also a growing concern. As the industry scales up, the safe disposal and neutralization of acid waste become increasingly important. Developing eco-friendly alternatives or closed-loop systems for acid recycling is crucial for sustainable nanotech production.
Furthermore, the interaction between HCl and various nanomaterials is not fully understood. This knowledge gap hampers the optimization of etching processes and the development of novel nanostructures. More comprehensive studies on the chemical mechanisms and kinetics of HCl-nanoparticle interactions are needed to advance the field.
Safety considerations pose another significant challenge. The handling and storage of HCl in nanotech facilities require stringent protocols and specialized equipment. Ensuring worker safety while maintaining production efficiency is a delicate balance that many companies struggle to achieve.
In the realm of characterization and quality control, current analytical techniques sometimes fall short in accurately measuring HCl concentrations and its effects at the nanoscale. This limitation hinders the development of more sophisticated nanotech applications and slows down the optimization of manufacturing processes.
Lastly, the integration of HCl-based nanotech processes with other manufacturing steps remains challenging. Compatibility issues between acid-treated components and subsequent processing stages can lead to defects or reduced performance in final products. Developing seamless integration strategies is essential for the widespread adoption of HCl-enabled nanotechnologies across various industries.
Existing HCl Nanotech Solutions
01 Production and purification of hydrochloric acid
Various methods and systems for producing and purifying hydrochloric acid are described. These include processes for manufacturing high-purity hydrochloric acid, as well as techniques for removing impurities and contaminants from the acid. The production methods may involve chemical reactions or industrial processes to generate hydrochloric acid efficiently.- Production and purification of hydrochloric acid: Various methods and systems for producing and purifying hydrochloric acid are described. These include processes for manufacturing high-purity hydrochloric acid, as well as techniques for removing impurities and contaminants from the acid. The methods often involve distillation, absorption, or other separation processes to achieve the desired purity levels.
- Applications of hydrochloric acid in chemical processes: Hydrochloric acid is widely used in various chemical processes and industrial applications. It serves as a key reagent in the production of other chemicals, metal treatment, and as a pH regulator. The acid's properties make it valuable in processes such as chlorination, hydrometallurgy, and as a catalyst in organic synthesis reactions.
- Handling and storage of hydrochloric acid: Specialized equipment and methods for handling and storing hydrochloric acid are crucial due to its corrosive nature. This includes the design of storage tanks, transportation containers, and safety systems to prevent leaks and protect workers. Innovations in materials resistant to hydrochloric acid corrosion are also important in this context.
- Environmental and safety considerations: Addressing environmental concerns and safety issues related to hydrochloric acid use is critical. This involves developing methods for neutralizing and disposing of waste acid, implementing emission control systems, and creating protocols for safe handling and emergency response. Innovations in this area aim to minimize environmental impact and protect human health.
- Recovery and recycling of hydrochloric acid: Techniques for recovering and recycling hydrochloric acid from industrial processes are important for sustainability and cost-efficiency. These methods often involve capturing and purifying acid vapors or solutions from waste streams, allowing the acid to be reused in various applications. Such processes can significantly reduce raw material consumption and waste generation.
02 Applications of hydrochloric acid in chemical processes
Hydrochloric acid is widely used in various chemical processes and industrial applications. It serves as a key reagent in the production of other chemicals, metal treatment, and as a catalyst in certain reactions. The acid's properties make it valuable in diverse fields such as pharmaceuticals, petrochemicals, and water treatment.Expand Specific Solutions03 Storage and handling of hydrochloric acid
Specialized equipment and methods for storing, transporting, and handling hydrochloric acid are crucial due to its corrosive nature. This includes the design of storage tanks, safety measures, and containment systems to prevent leaks and ensure safe handling. Proper materials selection for equipment in contact with the acid is also important.Expand Specific Solutions04 Environmental and safety considerations
Addressing environmental concerns and safety issues related to hydrochloric acid use is essential. This involves developing methods for neutralizing and disposing of the acid, implementing pollution control measures, and establishing safety protocols for workers handling the substance. Techniques for reducing emissions and managing waste are also important aspects.Expand Specific Solutions05 Innovative applications of hydrochloric acid
Novel uses and applications of hydrochloric acid are being explored in various fields. These include its use in advanced materials processing, energy storage systems, and specialized chemical synthesis. Researchers are also investigating ways to enhance the efficiency and sustainability of processes involving hydrochloric acid.Expand Specific Solutions
Key Players in HCl Nanotech Industry
The hydrochloric acid market in nanotechnology applications is in a growth phase, driven by increasing demand in various industries. The market size is expanding, with a projected CAGR of 5-7% over the next five years. Technologically, the field is advancing rapidly, with companies like Fluid Energy Group Ltd. and Merck Patent GmbH leading innovation in specialized formulations. Emerging players such as Enlighten Innovations, Inc. and Aquaox, Inc. are developing novel applications, while established firms like BASF Corp. and 3M Innovative Properties Co. are leveraging their R&D capabilities to maintain market share. Universities like Southeast University and Central South University are contributing to fundamental research, potentially opening new avenues for commercial exploitation in the near future.
Southeast University
Technical Solution: Southeast University has made notable advancements in utilizing hydrochloric acid for nanotechnology applications, particularly in the field of energy storage and conversion. Researchers at the university have developed a novel method for synthesizing nanostructured electrode materials for supercapacitors using HCl-assisted etching. This technique allows for the creation of hierarchical porous structures with high surface area and improved ion transport properties[7]. Furthermore, the university has pioneered the use of HCl in the fabrication of nanostructured catalysts for fuel cells. Their approach involves using controlled HCl etching to expose active sites on metal nanoparticles, significantly enhancing catalytic activity and stability[8].
Strengths: Cutting-edge research in energy-related nanotechnology, innovative synthesis methods for nanostructured materials, and strong academic collaborations. Weaknesses: Potential challenges in scaling up laboratory processes for industrial applications and securing funding for commercialization.
Merck Patent GmbH
Technical Solution: Merck Patent GmbH has made significant strides in utilizing hydrochloric acid for nanotechnology applications in the pharmaceutical and life sciences sectors. The company has developed a novel approach for synthesizing drug-loaded nanocarriers using HCl-mediated pH-responsive assembly. This technique allows for precise control over drug release kinetics by manipulating the nanocarrier's structure through HCl concentration gradients[5]. Additionally, Merck has pioneered the use of HCl in the production of quantum dots with enhanced optical properties. Their method involves using HCl as a surface etching agent to fine-tune the size and shape of semiconductor nanocrystals, resulting in improved quantum yield and stability[6].
Strengths: Expertise in pharmaceutical nanotechnology, advanced drug delivery systems, and high-quality quantum dot production. Weaknesses: Regulatory challenges associated with nanomedicine and potential scalability issues for some specialized nanotech processes.
Innovative HCl Nanotech Patents and Research
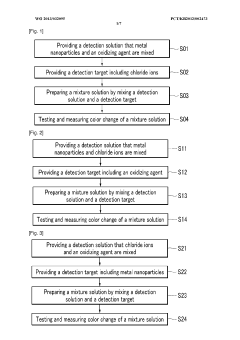
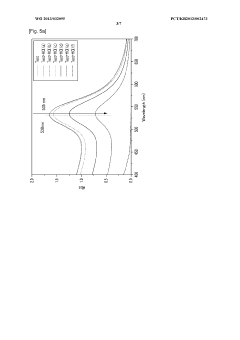
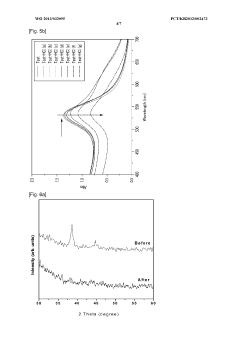
Detection method using colorimetric analysis
PatentWO2013032095A1
Innovation
- A colorimetric detection method using metal nanoparticles, specifically gold or silver nanoparticles, in combination with an oxidizing agent like nitric acid or hydrogen peroxide, to detect chloride ions in water samples, where the color change indicates the presence and concentration of hydrochloric acid.
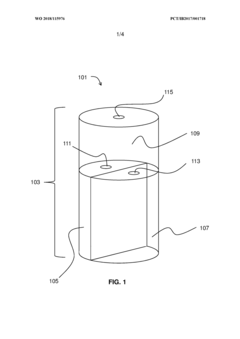
Multi-chambered storage and delivery container
PatentWO2018115976A1
Innovation
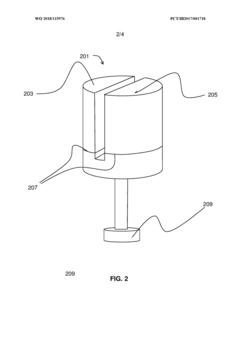
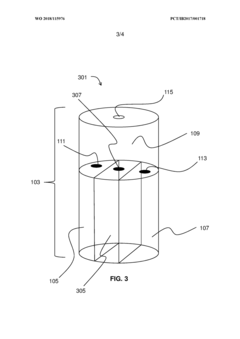
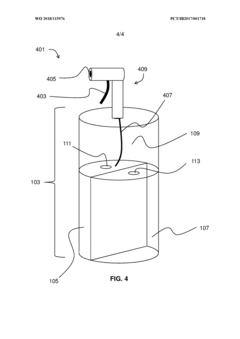
- A multi-chambered container system that separates and stabilizes the components for producing hypochlorous acid, using one-way valves and buffering agents to maintain a stable pH and prevent air exposure, allowing for on-site preparation and long-term storage of hypochlorous acid compositions, which are substantially free of chloride and metal ions.
Environmental Impact of HCl in Nanotech
The environmental impact of hydrochloric acid (HCl) in nanotechnology applications is a critical consideration for sustainable development in this field. HCl plays a significant role in various nanotech processes, including the synthesis of nanoparticles, surface modification, and etching. However, its use also raises concerns about potential environmental risks and long-term ecological effects.
One of the primary environmental concerns associated with HCl in nanotech is its potential for soil and water contamination. When improperly handled or disposed of, HCl can alter the pH of soil and aquatic ecosystems, leading to adverse effects on flora and fauna. This acidification can disrupt nutrient cycles, harm microbial communities, and impact the overall biodiversity of affected areas.
Atmospheric emissions of HCl during nanotech manufacturing processes pose another environmental challenge. These emissions can contribute to acid rain formation, which has far-reaching consequences for terrestrial and aquatic ecosystems, as well as human-made structures. The corrosive nature of HCl vapors can also lead to air quality degradation in industrial areas, potentially affecting human health and local ecosystems.
The production and transportation of HCl for nanotech applications also have environmental implications. The energy-intensive processes involved in HCl manufacture contribute to greenhouse gas emissions, while transportation risks include potential spills and accidents that could result in localized environmental damage.
However, it is important to note that the nanotech industry has been making strides in mitigating these environmental risks. Advanced containment and treatment systems are increasingly being employed to minimize HCl releases into the environment. Closed-loop systems and recycling processes are being developed to reduce the overall consumption of HCl and minimize waste generation.
Furthermore, research is ongoing to find more environmentally friendly alternatives to HCl in certain nanotech applications. Green chemistry principles are being applied to develop less hazardous acids or entirely new processes that achieve similar results without the use of corrosive substances.
The environmental impact of HCl in nanotech also extends to the end-of-life management of nanomaterials. As nanoparticles and nanostructures produced using HCl-based processes enter the waste stream, there are concerns about their potential to release residual acid or interact with the environment in unforeseen ways. This has led to increased focus on developing safe disposal methods and lifecycle assessments for nanomaterials.
In conclusion, while HCl remains an essential component in many nanotechnology applications, its environmental impact is a significant concern that requires ongoing attention and innovation. Balancing the technological benefits with environmental stewardship will be crucial for the sustainable growth of the nanotech industry.
One of the primary environmental concerns associated with HCl in nanotech is its potential for soil and water contamination. When improperly handled or disposed of, HCl can alter the pH of soil and aquatic ecosystems, leading to adverse effects on flora and fauna. This acidification can disrupt nutrient cycles, harm microbial communities, and impact the overall biodiversity of affected areas.
Atmospheric emissions of HCl during nanotech manufacturing processes pose another environmental challenge. These emissions can contribute to acid rain formation, which has far-reaching consequences for terrestrial and aquatic ecosystems, as well as human-made structures. The corrosive nature of HCl vapors can also lead to air quality degradation in industrial areas, potentially affecting human health and local ecosystems.
The production and transportation of HCl for nanotech applications also have environmental implications. The energy-intensive processes involved in HCl manufacture contribute to greenhouse gas emissions, while transportation risks include potential spills and accidents that could result in localized environmental damage.
However, it is important to note that the nanotech industry has been making strides in mitigating these environmental risks. Advanced containment and treatment systems are increasingly being employed to minimize HCl releases into the environment. Closed-loop systems and recycling processes are being developed to reduce the overall consumption of HCl and minimize waste generation.
Furthermore, research is ongoing to find more environmentally friendly alternatives to HCl in certain nanotech applications. Green chemistry principles are being applied to develop less hazardous acids or entirely new processes that achieve similar results without the use of corrosive substances.
The environmental impact of HCl in nanotech also extends to the end-of-life management of nanomaterials. As nanoparticles and nanostructures produced using HCl-based processes enter the waste stream, there are concerns about their potential to release residual acid or interact with the environment in unforeseen ways. This has led to increased focus on developing safe disposal methods and lifecycle assessments for nanomaterials.
In conclusion, while HCl remains an essential component in many nanotechnology applications, its environmental impact is a significant concern that requires ongoing attention and innovation. Balancing the technological benefits with environmental stewardship will be crucial for the sustainable growth of the nanotech industry.
Safety Protocols for HCl Nanotech Research
The implementation of safety protocols is paramount in hydrochloric acid (HCl) nanotechnology research due to the corrosive and potentially hazardous nature of the acid. Proper safety measures are essential to protect researchers, equipment, and the environment from potential risks associated with HCl handling and usage in nanoscale applications.
Personal protective equipment (PPE) forms the first line of defense in HCl nanotech research. Researchers must wear appropriate chemical-resistant gloves, lab coats, and safety goggles or face shields to prevent skin and eye contact with HCl. Closed-toe shoes and long pants are also mandatory to minimize exposure risks. Additionally, proper respiratory protection, such as fume hoods or respirators with acid gas cartridges, should be used when working with HCl vapors.
Proper storage and handling procedures are crucial for maintaining a safe research environment. HCl should be stored in well-ventilated areas, away from incompatible materials and heat sources. Containers must be clearly labeled and regularly inspected for signs of corrosion or leakage. When transferring or diluting HCl, researchers should work in fume hoods and use appropriate equipment, such as glass or acid-resistant plastic containers.
Emergency response protocols must be established and communicated to all personnel involved in HCl nanotech research. This includes the location and proper use of safety showers, eyewash stations, and spill kits. Regular safety drills and training sessions should be conducted to ensure all researchers are familiar with emergency procedures and can respond quickly and effectively in case of accidents.
Waste management is another critical aspect of HCl safety protocols in nanotechnology research. Proper neutralization and disposal methods must be implemented to prevent environmental contamination. This may involve the use of specialized waste containers, neutralization procedures, and adherence to local and national regulations regarding hazardous waste disposal.
Regular safety audits and risk assessments should be conducted to identify potential hazards and improve existing safety protocols. This includes evaluating the effectiveness of current safety measures, identifying areas for improvement, and staying updated on new safety technologies and best practices in HCl handling for nanotechnology applications.
Training and education play a vital role in maintaining a safe research environment. All personnel involved in HCl nanotech research should receive comprehensive safety training, covering topics such as proper handling techniques, emergency procedures, and the specific risks associated with HCl in nanoscale applications. Ongoing education and refresher courses should be provided to ensure researchers remain up-to-date on safety protocols and best practices.
Personal protective equipment (PPE) forms the first line of defense in HCl nanotech research. Researchers must wear appropriate chemical-resistant gloves, lab coats, and safety goggles or face shields to prevent skin and eye contact with HCl. Closed-toe shoes and long pants are also mandatory to minimize exposure risks. Additionally, proper respiratory protection, such as fume hoods or respirators with acid gas cartridges, should be used when working with HCl vapors.
Proper storage and handling procedures are crucial for maintaining a safe research environment. HCl should be stored in well-ventilated areas, away from incompatible materials and heat sources. Containers must be clearly labeled and regularly inspected for signs of corrosion or leakage. When transferring or diluting HCl, researchers should work in fume hoods and use appropriate equipment, such as glass or acid-resistant plastic containers.
Emergency response protocols must be established and communicated to all personnel involved in HCl nanotech research. This includes the location and proper use of safety showers, eyewash stations, and spill kits. Regular safety drills and training sessions should be conducted to ensure all researchers are familiar with emergency procedures and can respond quickly and effectively in case of accidents.
Waste management is another critical aspect of HCl safety protocols in nanotechnology research. Proper neutralization and disposal methods must be implemented to prevent environmental contamination. This may involve the use of specialized waste containers, neutralization procedures, and adherence to local and national regulations regarding hazardous waste disposal.
Regular safety audits and risk assessments should be conducted to identify potential hazards and improve existing safety protocols. This includes evaluating the effectiveness of current safety measures, identifying areas for improvement, and staying updated on new safety technologies and best practices in HCl handling for nanotechnology applications.
Training and education play a vital role in maintaining a safe research environment. All personnel involved in HCl nanotech research should receive comprehensive safety training, covering topics such as proper handling techniques, emergency procedures, and the specific risks associated with HCl in nanoscale applications. Ongoing education and refresher courses should be provided to ensure researchers remain up-to-date on safety protocols and best practices.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!