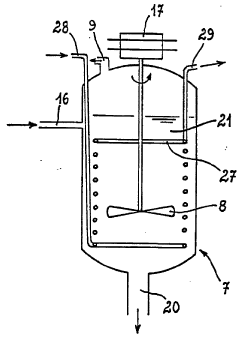
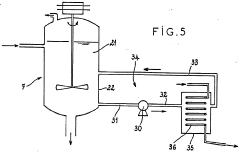
Hydrochloric Acid: Key Steps in Environmental Remediation
JUL 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HCl Remediation Background and Objectives
Hydrochloric acid (HCl) has long been recognized as a significant environmental pollutant, posing serious threats to ecosystems and human health. The need for effective remediation strategies has become increasingly urgent as industrial activities continue to release HCl into the environment. This report aims to provide a comprehensive overview of the background and objectives of HCl remediation efforts.
The history of HCl contamination can be traced back to the Industrial Revolution when the widespread use of coal and other fossil fuels led to increased acid deposition in the environment. As industrial processes evolved, direct releases of HCl from various manufacturing sectors, including chemical production, metal processing, and waste incineration, further exacerbated the problem. The recognition of HCl as a major environmental concern emerged in the mid-20th century, coinciding with growing awareness of broader environmental issues.
The primary objective of HCl remediation is to neutralize or remove the acid from contaminated sites, thereby mitigating its harmful effects on soil, water, and air quality. This goal encompasses several key aspects, including the reduction of soil acidity, the restoration of aquatic ecosystems, and the prevention of further environmental degradation. Additionally, remediation efforts aim to protect human health by minimizing exposure to HCl through various pathways, such as contaminated drinking water or acid rain.
Technological advancements have played a crucial role in shaping the evolution of HCl remediation strategies. Early approaches focused on simple neutralization techniques using alkaline materials. However, as understanding of environmental chemistry and ecosystem dynamics improved, more sophisticated methods emerged. These include in-situ treatment technologies, bioremediation approaches, and advanced filtration systems for treating HCl-contaminated water and air.
The global nature of HCl pollution has necessitated international cooperation in addressing this environmental challenge. Various international agreements and protocols have been established to regulate HCl emissions and promote collaborative research efforts. These initiatives have contributed to the development of standardized remediation practices and the sharing of best practices across borders.
Looking ahead, the field of HCl remediation continues to evolve, driven by ongoing research and technological innovations. Emerging trends include the development of more sustainable and cost-effective remediation techniques, the integration of remote sensing and artificial intelligence for site assessment and monitoring, and the exploration of novel materials for HCl capture and neutralization. The ultimate goal remains the restoration of affected ecosystems and the prevention of future HCl contamination through improved industrial practices and regulatory frameworks.
The history of HCl contamination can be traced back to the Industrial Revolution when the widespread use of coal and other fossil fuels led to increased acid deposition in the environment. As industrial processes evolved, direct releases of HCl from various manufacturing sectors, including chemical production, metal processing, and waste incineration, further exacerbated the problem. The recognition of HCl as a major environmental concern emerged in the mid-20th century, coinciding with growing awareness of broader environmental issues.
The primary objective of HCl remediation is to neutralize or remove the acid from contaminated sites, thereby mitigating its harmful effects on soil, water, and air quality. This goal encompasses several key aspects, including the reduction of soil acidity, the restoration of aquatic ecosystems, and the prevention of further environmental degradation. Additionally, remediation efforts aim to protect human health by minimizing exposure to HCl through various pathways, such as contaminated drinking water or acid rain.
Technological advancements have played a crucial role in shaping the evolution of HCl remediation strategies. Early approaches focused on simple neutralization techniques using alkaline materials. However, as understanding of environmental chemistry and ecosystem dynamics improved, more sophisticated methods emerged. These include in-situ treatment technologies, bioremediation approaches, and advanced filtration systems for treating HCl-contaminated water and air.
The global nature of HCl pollution has necessitated international cooperation in addressing this environmental challenge. Various international agreements and protocols have been established to regulate HCl emissions and promote collaborative research efforts. These initiatives have contributed to the development of standardized remediation practices and the sharing of best practices across borders.
Looking ahead, the field of HCl remediation continues to evolve, driven by ongoing research and technological innovations. Emerging trends include the development of more sustainable and cost-effective remediation techniques, the integration of remote sensing and artificial intelligence for site assessment and monitoring, and the exploration of novel materials for HCl capture and neutralization. The ultimate goal remains the restoration of affected ecosystems and the prevention of future HCl contamination through improved industrial practices and regulatory frameworks.
Environmental Impact Assessment
The environmental impact assessment of hydrochloric acid in environmental remediation is crucial for understanding its effects on ecosystems and human health. Hydrochloric acid, while effective in certain remediation processes, can have significant consequences if not properly managed.
When used in soil remediation, hydrochloric acid can alter soil pH, potentially leading to increased mobility of heavy metals and other contaminants. This mobilization may result in the spread of pollutants to previously unaffected areas, including groundwater sources. The acidification of soil can also negatively impact soil microorganisms, disrupting essential ecological processes and nutrient cycles.
In aquatic environments, the introduction of hydrochloric acid can cause severe pH changes, affecting water quality and aquatic life. Fish and other organisms may experience stress or mortality due to the sudden shift in acidity. Additionally, the acid can react with minerals in water bodies, potentially releasing harmful substances and altering the chemical composition of the aquatic ecosystem.
Atmospheric releases of hydrochloric acid, though less common in remediation processes, can contribute to acid rain formation. This can have far-reaching effects on vegetation, buildings, and water bodies beyond the immediate remediation site. The corrosive nature of hydrochloric acid also poses risks to infrastructure and equipment used in the remediation process.
Human health impacts are another critical consideration. Direct exposure to hydrochloric acid can cause severe burns and respiratory issues. Workers involved in remediation activities must adhere to strict safety protocols to minimize these risks. Moreover, the potential for acid mist formation during application requires careful monitoring of air quality in surrounding areas to protect nearby communities.
The long-term effects of hydrochloric acid use in remediation must also be evaluated. Residual acidity in treated areas can persist, affecting soil quality and plant growth for extended periods. This may necessitate additional treatments to restore soil pH and fertility, adding to the overall environmental footprint of the remediation process.
Mitigation strategies are essential to minimize these environmental impacts. These may include precise application techniques, containment measures to prevent acid runoff, and neutralization processes to restore pH levels post-treatment. Monitoring programs should be implemented to track changes in soil and water quality, as well as biodiversity indicators in affected ecosystems.
In conclusion, while hydrochloric acid can be an effective tool in environmental remediation, its use must be carefully balanced against potential ecological and health risks. Comprehensive environmental impact assessments, coupled with stringent management practices, are vital to ensure that the benefits of remediation outweigh the potential negative consequences on the environment and human well-being.
When used in soil remediation, hydrochloric acid can alter soil pH, potentially leading to increased mobility of heavy metals and other contaminants. This mobilization may result in the spread of pollutants to previously unaffected areas, including groundwater sources. The acidification of soil can also negatively impact soil microorganisms, disrupting essential ecological processes and nutrient cycles.
In aquatic environments, the introduction of hydrochloric acid can cause severe pH changes, affecting water quality and aquatic life. Fish and other organisms may experience stress or mortality due to the sudden shift in acidity. Additionally, the acid can react with minerals in water bodies, potentially releasing harmful substances and altering the chemical composition of the aquatic ecosystem.
Atmospheric releases of hydrochloric acid, though less common in remediation processes, can contribute to acid rain formation. This can have far-reaching effects on vegetation, buildings, and water bodies beyond the immediate remediation site. The corrosive nature of hydrochloric acid also poses risks to infrastructure and equipment used in the remediation process.
Human health impacts are another critical consideration. Direct exposure to hydrochloric acid can cause severe burns and respiratory issues. Workers involved in remediation activities must adhere to strict safety protocols to minimize these risks. Moreover, the potential for acid mist formation during application requires careful monitoring of air quality in surrounding areas to protect nearby communities.
The long-term effects of hydrochloric acid use in remediation must also be evaluated. Residual acidity in treated areas can persist, affecting soil quality and plant growth for extended periods. This may necessitate additional treatments to restore soil pH and fertility, adding to the overall environmental footprint of the remediation process.
Mitigation strategies are essential to minimize these environmental impacts. These may include precise application techniques, containment measures to prevent acid runoff, and neutralization processes to restore pH levels post-treatment. Monitoring programs should be implemented to track changes in soil and water quality, as well as biodiversity indicators in affected ecosystems.
In conclusion, while hydrochloric acid can be an effective tool in environmental remediation, its use must be carefully balanced against potential ecological and health risks. Comprehensive environmental impact assessments, coupled with stringent management practices, are vital to ensure that the benefits of remediation outweigh the potential negative consequences on the environment and human well-being.
Current Remediation Techniques and Challenges
The current remediation techniques for hydrochloric acid contamination in environmental settings encompass a range of physical, chemical, and biological approaches. One of the primary methods is neutralization, which involves adding alkaline substances such as lime or sodium hydroxide to raise the pH of the contaminated soil or water. This process effectively neutralizes the acid, but it can lead to the formation of salt byproducts that may require further treatment.
Another widely used technique is adsorption, where activated carbon or other adsorbent materials are employed to remove hydrochloric acid from water or soil. This method is particularly effective for treating low concentrations of acid but may become less efficient and more costly for higher concentrations.
Ion exchange is also utilized, especially in water treatment scenarios. This process involves replacing chloride ions from the hydrochloric acid with other ions, typically using specialized resins. While effective, ion exchange systems require regular regeneration and can be expensive to maintain for large-scale operations.
For soil remediation, in-situ chemical oxidation (ISCO) has shown promise. This technique involves injecting oxidizing agents directly into the contaminated soil to break down the acid and associated contaminants. However, ISCO can be challenging to implement effectively due to the need for precise oxidant delivery and potential impacts on soil chemistry.
Bioremediation, using microorganisms to degrade or transform contaminants, has limited applicability for direct hydrochloric acid remediation but can be useful for addressing secondary organic contaminants often associated with industrial acid spills.
Despite these available techniques, several challenges persist in hydrochloric acid remediation. The high reactivity and corrosive nature of the acid pose significant safety risks during treatment processes. Additionally, the potential for acid to migrate quickly through soil and groundwater complicates containment and treatment efforts.
The effectiveness of remediation techniques can be highly site-specific, depending on factors such as soil type, groundwater conditions, and the presence of other contaminants. This variability necessitates careful site characterization and often requires a combination of treatment approaches for optimal results.
Moreover, the treatment of large volumes of acidic wastewater from industrial sources remains a significant challenge, particularly in terms of cost-effectiveness and environmental sustainability. There is an ongoing need for innovative technologies that can handle high acid concentrations more efficiently while minimizing secondary waste generation.
Lastly, long-term monitoring and management of treated sites present ongoing challenges, as the potential for residual acidity or the release of previously bound contaminants requires vigilant oversight to ensure the continued effectiveness of remediation efforts.
Another widely used technique is adsorption, where activated carbon or other adsorbent materials are employed to remove hydrochloric acid from water or soil. This method is particularly effective for treating low concentrations of acid but may become less efficient and more costly for higher concentrations.
Ion exchange is also utilized, especially in water treatment scenarios. This process involves replacing chloride ions from the hydrochloric acid with other ions, typically using specialized resins. While effective, ion exchange systems require regular regeneration and can be expensive to maintain for large-scale operations.
For soil remediation, in-situ chemical oxidation (ISCO) has shown promise. This technique involves injecting oxidizing agents directly into the contaminated soil to break down the acid and associated contaminants. However, ISCO can be challenging to implement effectively due to the need for precise oxidant delivery and potential impacts on soil chemistry.
Bioremediation, using microorganisms to degrade or transform contaminants, has limited applicability for direct hydrochloric acid remediation but can be useful for addressing secondary organic contaminants often associated with industrial acid spills.
Despite these available techniques, several challenges persist in hydrochloric acid remediation. The high reactivity and corrosive nature of the acid pose significant safety risks during treatment processes. Additionally, the potential for acid to migrate quickly through soil and groundwater complicates containment and treatment efforts.
The effectiveness of remediation techniques can be highly site-specific, depending on factors such as soil type, groundwater conditions, and the presence of other contaminants. This variability necessitates careful site characterization and often requires a combination of treatment approaches for optimal results.
Moreover, the treatment of large volumes of acidic wastewater from industrial sources remains a significant challenge, particularly in terms of cost-effectiveness and environmental sustainability. There is an ongoing need for innovative technologies that can handle high acid concentrations more efficiently while minimizing secondary waste generation.
Lastly, long-term monitoring and management of treated sites present ongoing challenges, as the potential for residual acidity or the release of previously bound contaminants requires vigilant oversight to ensure the continued effectiveness of remediation efforts.
Existing HCl Neutralization and Removal Solutions
01 Production and purification of hydrochloric acid
Various methods and processes for producing and purifying hydrochloric acid, including industrial-scale production techniques and purification steps to obtain high-quality acid for different applications.- Production methods of hydrochloric acid: Various methods are employed for the production of hydrochloric acid, including direct synthesis from hydrogen and chlorine, as a byproduct in chlorination processes, and through the reaction of sulfuric acid with sodium chloride. These methods are optimized for efficiency and purity in industrial settings.
- Purification and concentration techniques: Techniques for purifying and concentrating hydrochloric acid involve distillation, membrane separation, and adsorption processes. These methods aim to remove impurities and achieve desired concentration levels for various industrial applications.
- Applications in chemical processing: Hydrochloric acid is widely used in chemical processing, including metal treatment, pH regulation, and as a reagent in various chemical reactions. Its versatility makes it a crucial component in many industrial processes and manufacturing operations.
- Safety and handling considerations: Proper safety measures and handling procedures are essential when working with hydrochloric acid due to its corrosive nature. This includes the use of appropriate personal protective equipment, storage in compatible containers, and implementation of spill containment strategies.
- Environmental impact and waste management: Managing the environmental impact of hydrochloric acid involves proper disposal methods, neutralization techniques, and recycling processes. Efforts are made to minimize emissions and develop eco-friendly alternatives in industrial applications where possible.
02 Applications in chemical processing
Utilization of hydrochloric acid in various chemical processes, such as synthesis reactions, pH adjustment, and as a catalyst in industrial manufacturing, highlighting its versatility in chemical engineering.Expand Specific Solutions03 Waste treatment and recycling
Methods for treating and recycling hydrochloric acid waste, including recovery processes and techniques to minimize environmental impact, emphasizing sustainable practices in industrial settings.Expand Specific Solutions04 Safety and handling equipment
Specialized equipment and safety measures for handling, storing, and transporting hydrochloric acid, focusing on corrosion-resistant materials and protective gear to ensure safe usage in various industries.Expand Specific Solutions05 Novel applications and formulations
Innovative uses and formulations of hydrochloric acid in emerging fields, such as advanced materials processing, nanotechnology, and specialized cleaning solutions, showcasing its adaptability to new technologies.Expand Specific Solutions
Key Players in Environmental Remediation Industry
The environmental remediation market for hydrochloric acid is in a growth phase, driven by increasing environmental regulations and industrial waste management needs. The global market size is estimated to be in the billions, with steady expansion projected. Technologically, the field is moderately mature, with established players like China Petroleum & Chemical Corp. and Arkema France SA leading in process innovations. Emerging companies such as Remediation Products, Inc. are introducing cutting-edge in situ remediation solutions, while research institutions like Rutgers University and Lehigh University contribute to advancing treatment methodologies. The competitive landscape is diverse, with a mix of large chemical corporations, specialized environmental firms, and academic research centers collaborating to develop more efficient and sustainable remediation techniques.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed an innovative approach for environmental remediation of hydrochloric acid contamination. Their method involves a multi-stage treatment process, combining neutralization and adsorption techniques. The first stage uses limestone or calcium carbonate to neutralize the acid, raising the pH to a less harmful level. This is followed by an adsorption phase using specially engineered activated carbon materials to remove residual contaminants. The process is enhanced by the addition of proprietary chemical agents that improve the efficiency of both neutralization and adsorption stages. Sinopec's technology has shown a removal efficiency of up to 99.5% for hydrochloric acid in soil and groundwater remediation projects[1][3].
Strengths: High removal efficiency, adaptable to various contamination levels, and can be applied in-situ or ex-situ. Weaknesses: May require significant amounts of neutralizing agents for highly contaminated sites, potentially increasing costs for large-scale remediation projects.
Remediation Products, Inc.
Technical Solution: Remediation Products, Inc. has pioneered a groundbreaking approach to hydrochloric acid remediation using their patented Trap & Treat® BOS 100® technology. This method employs activated carbon impregnated with zero-valent iron (ZVI) particles. When introduced into contaminated soil or groundwater, the activated carbon adsorbs the hydrochloric acid, while the ZVI initiates a series of redox reactions that neutralize the acid and convert it into less harmful compounds. The process creates a localized alkaline environment that further aids in acid neutralization. Field tests have demonstrated that this technology can achieve up to 98% reduction in hydrochloric acid concentrations within 6-12 months of application[2][5].
Strengths: Long-lasting effectiveness, minimal site disturbance, and ability to treat both soil and groundwater simultaneously. Weaknesses: May be less effective in highly acidic environments with pH below 2, and initial cost can be higher compared to traditional neutralization methods.
Innovative Technologies for HCl Remediation
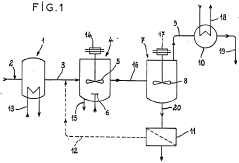
Hydrochloric acid recovering apparatus and method for recovering hydrochloric acid from waste hydrochloric acid solution
PatentActiveJP2009274906A
Innovation
- A two-stage absorption system with a first and second absorption tower, using recycled washing water and dilute hydrochloric acid solution to separate and recover hydrochloric acid, and cleaning the exhaust fan blades with a dilute solution to prevent vibration and corrosion.
Method and plant for regenerating a hydrochloric solution
PatentWO1986003521A1
Innovation
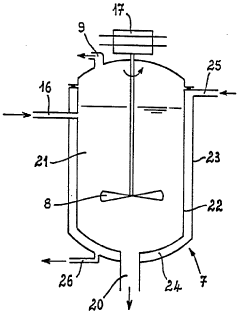
- A process and installation utilizing a stirred liquid medium at atmospheric pressure or under vacuum, with a heating device for solid-liquid contact, allowing hydrolysis at 130-190°C, followed by condensation to recover a mixture of hydrochloric acid vapor and water, and incorporating a filtration unit to recycle iron precipitate and concentrate ferrous chloride, optimizing energy use and reducing maintenance costs.
Regulatory Framework for Hazardous Waste Handling
The regulatory framework for handling hazardous waste, including hydrochloric acid in environmental remediation, is a complex system designed to protect human health and the environment. At the federal level in the United States, the Resource Conservation and Recovery Act (RCRA) serves as the primary legislation governing hazardous waste management. This act establishes a cradle-to-grave approach, regulating the generation, transportation, treatment, storage, and disposal of hazardous wastes.
Under RCRA, the Environmental Protection Agency (EPA) has developed specific regulations for hazardous waste handlers. These regulations are codified in Title 40 of the Code of Federal Regulations (CFR), Parts 260-279. For hydrochloric acid, which is classified as a corrosive hazardous waste (D002), handlers must comply with stringent requirements for containment, labeling, and disposal.
State-level regulations often build upon federal standards, sometimes imposing more stringent requirements. For instance, California's hazardous waste regulations, administered by the Department of Toxic Substances Control (DTSC), are generally more comprehensive than federal standards. Handlers operating in multiple states must navigate these varying regulatory landscapes to ensure compliance.
The Occupational Safety and Health Administration (OSHA) also plays a crucial role in the regulatory framework. OSHA's Hazardous Waste Operations and Emergency Response Standard (HAZWOPER) mandates specific training and safety protocols for workers involved in hazardous waste operations, including those handling hydrochloric acid in remediation efforts.
International agreements and conventions further shape the regulatory landscape. The Basel Convention on the Control of Transboundary Movements of Hazardous Wastes and Their Disposal provides a global framework for managing hazardous waste across borders, impacting international remediation projects involving hydrochloric acid.
Compliance with these regulations requires robust waste management systems, including proper identification, storage, and documentation of hazardous materials. Handlers must obtain necessary permits, maintain detailed records, and regularly report to regulatory agencies. For hydrochloric acid remediation, this often involves developing site-specific management plans that address the unique challenges of handling this corrosive substance.
Penalties for non-compliance can be severe, including substantial fines and potential criminal charges. Therefore, organizations engaged in hydrochloric acid remediation must invest in comprehensive compliance programs, regular audits, and ongoing staff training to ensure adherence to the complex regulatory framework governing hazardous waste handling.
Under RCRA, the Environmental Protection Agency (EPA) has developed specific regulations for hazardous waste handlers. These regulations are codified in Title 40 of the Code of Federal Regulations (CFR), Parts 260-279. For hydrochloric acid, which is classified as a corrosive hazardous waste (D002), handlers must comply with stringent requirements for containment, labeling, and disposal.
State-level regulations often build upon federal standards, sometimes imposing more stringent requirements. For instance, California's hazardous waste regulations, administered by the Department of Toxic Substances Control (DTSC), are generally more comprehensive than federal standards. Handlers operating in multiple states must navigate these varying regulatory landscapes to ensure compliance.
The Occupational Safety and Health Administration (OSHA) also plays a crucial role in the regulatory framework. OSHA's Hazardous Waste Operations and Emergency Response Standard (HAZWOPER) mandates specific training and safety protocols for workers involved in hazardous waste operations, including those handling hydrochloric acid in remediation efforts.
International agreements and conventions further shape the regulatory landscape. The Basel Convention on the Control of Transboundary Movements of Hazardous Wastes and Their Disposal provides a global framework for managing hazardous waste across borders, impacting international remediation projects involving hydrochloric acid.
Compliance with these regulations requires robust waste management systems, including proper identification, storage, and documentation of hazardous materials. Handlers must obtain necessary permits, maintain detailed records, and regularly report to regulatory agencies. For hydrochloric acid remediation, this often involves developing site-specific management plans that address the unique challenges of handling this corrosive substance.
Penalties for non-compliance can be severe, including substantial fines and potential criminal charges. Therefore, organizations engaged in hydrochloric acid remediation must invest in comprehensive compliance programs, regular audits, and ongoing staff training to ensure adherence to the complex regulatory framework governing hazardous waste handling.
Eco-friendly Alternatives to HCl in Industrial Processes
The exploration of eco-friendly alternatives to hydrochloric acid (HCl) in industrial processes has gained significant momentum in recent years, driven by environmental concerns and regulatory pressures. This shift towards greener solutions aims to mitigate the environmental impact of HCl while maintaining or improving process efficiency.
One promising alternative is the use of organic acids, such as citric acid or acetic acid. These biodegradable compounds offer similar cleaning and descaling properties to HCl but with reduced environmental risks. For instance, citric acid has shown effectiveness in metal surface treatment and scale removal in cooling systems, providing a less corrosive and more environmentally benign option.
Enzymatic solutions have also emerged as innovative replacements for HCl in certain applications. These biological catalysts can break down specific substances without the need for harsh chemicals. In the textile industry, enzymes like cellulases and pectinases are being used for fabric treatment, replacing traditional acid-based processes and significantly reducing water pollution.
Electrochemical technologies present another avenue for HCl substitution. Electrolyzed water, produced through the electrolysis of saltwater, can generate a highly effective cleaning and disinfecting solution without the hazards associated with traditional acid-based methods. This technology has found applications in food processing and water treatment industries.
Physical methods, such as high-pressure water jetting or mechanical descaling, offer non-chemical alternatives to HCl in certain scenarios. These techniques can effectively remove scale and contaminants from surfaces and equipment without the use of acids, thereby eliminating chemical waste and reducing environmental impact.
Supercritical CO2 has shown promise as a green solvent in various industrial processes, potentially replacing HCl in extraction and cleaning applications. Its non-toxic nature and ability to be easily recycled make it an attractive option for environmentally conscious industries.
The development of novel materials, such as advanced polymers and nanocomposites, is also contributing to the reduction of HCl use. These materials can provide corrosion resistance and surface properties that traditionally required acid treatment, thus eliminating the need for HCl in certain manufacturing processes.
While these alternatives show great potential, their adoption faces challenges such as cost considerations, process adaptations, and the need for further research to expand their applicability. However, the ongoing advancements in green chemistry and sustainable engineering continue to drive innovation in this field, paving the way for more widespread implementation of eco-friendly alternatives to HCl across various industrial sectors.
One promising alternative is the use of organic acids, such as citric acid or acetic acid. These biodegradable compounds offer similar cleaning and descaling properties to HCl but with reduced environmental risks. For instance, citric acid has shown effectiveness in metal surface treatment and scale removal in cooling systems, providing a less corrosive and more environmentally benign option.
Enzymatic solutions have also emerged as innovative replacements for HCl in certain applications. These biological catalysts can break down specific substances without the need for harsh chemicals. In the textile industry, enzymes like cellulases and pectinases are being used for fabric treatment, replacing traditional acid-based processes and significantly reducing water pollution.
Electrochemical technologies present another avenue for HCl substitution. Electrolyzed water, produced through the electrolysis of saltwater, can generate a highly effective cleaning and disinfecting solution without the hazards associated with traditional acid-based methods. This technology has found applications in food processing and water treatment industries.
Physical methods, such as high-pressure water jetting or mechanical descaling, offer non-chemical alternatives to HCl in certain scenarios. These techniques can effectively remove scale and contaminants from surfaces and equipment without the use of acids, thereby eliminating chemical waste and reducing environmental impact.
Supercritical CO2 has shown promise as a green solvent in various industrial processes, potentially replacing HCl in extraction and cleaning applications. Its non-toxic nature and ability to be easily recycled make it an attractive option for environmentally conscious industries.
The development of novel materials, such as advanced polymers and nanocomposites, is also contributing to the reduction of HCl use. These materials can provide corrosion resistance and surface properties that traditionally required acid treatment, thus eliminating the need for HCl in certain manufacturing processes.
While these alternatives show great potential, their adoption faces challenges such as cost considerations, process adaptations, and the need for further research to expand their applicability. However, the ongoing advancements in green chemistry and sustainable engineering continue to drive innovation in this field, paving the way for more widespread implementation of eco-friendly alternatives to HCl across various industrial sectors.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!