Hydrochloric Acid Process Optimization: A Roadmap
JUL 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HCl Process Evolution
The evolution of the hydrochloric acid (HCl) production process has been marked by significant technological advancements and process optimizations over the past century. Initially, HCl was primarily produced as a by-product of the Leblanc process for soda ash production in the early 19th century. However, this method was inefficient and environmentally harmful.
The introduction of the Chlor-alkali process in the late 19th century revolutionized HCl production. This electrolytic method, which produces chlorine gas as a primary product, allowed for more controlled and efficient HCl synthesis. The chlorine gas could be reacted with hydrogen to form HCl, leading to a more streamlined production process.
In the mid-20th century, the direct synthesis method gained prominence. This process involves the combustion of hydrogen and chlorine gases in a burner, followed by absorption of the resulting hydrogen chloride gas in water. This method offered improved purity and concentration control, making it particularly suitable for high-grade HCl production.
The 1960s and 1970s saw the development of the Mannheim process, which utilized salt and sulfuric acid as raw materials. This process became popular due to its ability to produce high-purity HCl while also generating valuable sodium sulfate as a by-product. However, the corrosive nature of the reactants posed significant engineering challenges.
Recent decades have witnessed a shift towards more environmentally friendly and energy-efficient production methods. The oxychlorination process, which uses hydrogen chloride to convert ethylene to vinyl chloride, has gained traction. This process not only produces HCl as a valuable by-product but also aligns with the principles of circular economy by utilizing waste streams.
Advancements in materials science have played a crucial role in improving HCl production efficiency. The development of corrosion-resistant alloys and specialized coatings has extended equipment lifespan and reduced maintenance costs. Additionally, the integration of advanced process control systems and real-time monitoring technologies has enhanced production consistency and safety.
The ongoing evolution of HCl production processes is now focused on sustainability and resource efficiency. Research is being conducted on catalytic processes that can convert waste chlorine compounds into HCl, potentially reducing the environmental impact of chlorine-based industries. Furthermore, the exploration of renewable energy sources for electrolysis and the optimization of heat recovery systems are at the forefront of current process improvements.
The introduction of the Chlor-alkali process in the late 19th century revolutionized HCl production. This electrolytic method, which produces chlorine gas as a primary product, allowed for more controlled and efficient HCl synthesis. The chlorine gas could be reacted with hydrogen to form HCl, leading to a more streamlined production process.
In the mid-20th century, the direct synthesis method gained prominence. This process involves the combustion of hydrogen and chlorine gases in a burner, followed by absorption of the resulting hydrogen chloride gas in water. This method offered improved purity and concentration control, making it particularly suitable for high-grade HCl production.
The 1960s and 1970s saw the development of the Mannheim process, which utilized salt and sulfuric acid as raw materials. This process became popular due to its ability to produce high-purity HCl while also generating valuable sodium sulfate as a by-product. However, the corrosive nature of the reactants posed significant engineering challenges.
Recent decades have witnessed a shift towards more environmentally friendly and energy-efficient production methods. The oxychlorination process, which uses hydrogen chloride to convert ethylene to vinyl chloride, has gained traction. This process not only produces HCl as a valuable by-product but also aligns with the principles of circular economy by utilizing waste streams.
Advancements in materials science have played a crucial role in improving HCl production efficiency. The development of corrosion-resistant alloys and specialized coatings has extended equipment lifespan and reduced maintenance costs. Additionally, the integration of advanced process control systems and real-time monitoring technologies has enhanced production consistency and safety.
The ongoing evolution of HCl production processes is now focused on sustainability and resource efficiency. Research is being conducted on catalytic processes that can convert waste chlorine compounds into HCl, potentially reducing the environmental impact of chlorine-based industries. Furthermore, the exploration of renewable energy sources for electrolysis and the optimization of heat recovery systems are at the forefront of current process improvements.
Market Demand Analysis
The global hydrochloric acid market has been experiencing steady growth, driven by increasing demand from various industries such as steel pickling, oil well acidizing, and chemical manufacturing. The market size is projected to expand significantly in the coming years, with a compound annual growth rate (CAGR) expected to remain robust. This growth is primarily attributed to the rising industrial activities in developing economies and the expanding applications of hydrochloric acid in diverse sectors.
In the steel industry, hydrochloric acid plays a crucial role in the pickling process, which removes impurities and rust from steel surfaces. As the construction and automotive sectors continue to grow, particularly in emerging markets, the demand for high-quality steel products is expected to surge, consequently driving the need for hydrochloric acid in steel production.
The oil and gas industry represents another significant market for hydrochloric acid, where it is used in well acidizing to enhance oil recovery. With the ongoing exploration of new oil fields and the need to improve production from existing wells, the demand for hydrochloric acid in this sector is anticipated to remain strong.
Chemical manufacturing is yet another key driver of hydrochloric acid demand. The acid is widely used in the production of various chemicals, including PVC, water treatment chemicals, and pharmaceuticals. As these industries expand, particularly in regions like Asia-Pacific and Latin America, the demand for hydrochloric acid is expected to grow proportionally.
Environmental regulations and sustainability concerns are shaping market trends, leading to increased focus on recycling and regeneration of hydrochloric acid. This shift is creating new opportunities for process optimization and innovative technologies that can improve efficiency and reduce environmental impact.
The food industry is also contributing to the growing demand for hydrochloric acid, particularly in food processing and as a pH regulator. With the global population increasing and changing dietary habits, this sector is expected to provide a steady demand for high-purity hydrochloric acid.
Geographically, Asia-Pacific is anticipated to be the fastest-growing market for hydrochloric acid, driven by rapid industrialization in countries like China and India. North America and Europe are expected to maintain stable demand, primarily from established industries and ongoing technological advancements in production processes.
As the market expands, there is an increasing need for optimized production processes that can meet the growing demand while addressing challenges such as energy efficiency, waste reduction, and product quality consistency. This creates a significant opportunity for innovations in hydrochloric acid production technologies and process optimization strategies.
In the steel industry, hydrochloric acid plays a crucial role in the pickling process, which removes impurities and rust from steel surfaces. As the construction and automotive sectors continue to grow, particularly in emerging markets, the demand for high-quality steel products is expected to surge, consequently driving the need for hydrochloric acid in steel production.
The oil and gas industry represents another significant market for hydrochloric acid, where it is used in well acidizing to enhance oil recovery. With the ongoing exploration of new oil fields and the need to improve production from existing wells, the demand for hydrochloric acid in this sector is anticipated to remain strong.
Chemical manufacturing is yet another key driver of hydrochloric acid demand. The acid is widely used in the production of various chemicals, including PVC, water treatment chemicals, and pharmaceuticals. As these industries expand, particularly in regions like Asia-Pacific and Latin America, the demand for hydrochloric acid is expected to grow proportionally.
Environmental regulations and sustainability concerns are shaping market trends, leading to increased focus on recycling and regeneration of hydrochloric acid. This shift is creating new opportunities for process optimization and innovative technologies that can improve efficiency and reduce environmental impact.
The food industry is also contributing to the growing demand for hydrochloric acid, particularly in food processing and as a pH regulator. With the global population increasing and changing dietary habits, this sector is expected to provide a steady demand for high-purity hydrochloric acid.
Geographically, Asia-Pacific is anticipated to be the fastest-growing market for hydrochloric acid, driven by rapid industrialization in countries like China and India. North America and Europe are expected to maintain stable demand, primarily from established industries and ongoing technological advancements in production processes.
As the market expands, there is an increasing need for optimized production processes that can meet the growing demand while addressing challenges such as energy efficiency, waste reduction, and product quality consistency. This creates a significant opportunity for innovations in hydrochloric acid production technologies and process optimization strategies.
Technical Challenges
The optimization of hydrochloric acid production processes faces several significant technical challenges that require innovative solutions. One of the primary obstacles is the corrosive nature of hydrochloric acid, which necessitates the use of specialized materials and equipment throughout the production chain. This corrosion issue not only affects the longevity of production facilities but also poses potential safety risks and environmental concerns.
Another major challenge lies in the energy efficiency of the production process. Traditional methods of hydrochloric acid production, such as the salt-sulfuric acid process, are often energy-intensive and result in high operational costs. Improving energy efficiency while maintaining or increasing production capacity remains a key focus area for industry players.
The management of by-products and waste streams presents an additional hurdle. The chlor-alkali process, a common method for producing hydrochloric acid, generates significant amounts of chlorine gas and sodium hydroxide as co-products. Balancing the production and utilization of these co-products with market demand can be complex and economically challenging.
Environmental regulations and sustainability concerns also pose technical challenges. Reducing emissions, particularly chlorine gas, and minimizing the carbon footprint of the production process are critical objectives that require technological advancements. This includes developing more efficient scrubbing systems and exploring alternative production methods with lower environmental impact.
The variability in raw material quality, especially when using recycled materials or industrial by-products as feedstock, introduces challenges in maintaining consistent product quality. Developing robust process control systems and advanced analytical techniques to handle this variability is essential for optimizing production efficiency and product consistency.
Scaling up new technologies from laboratory to industrial scale presents its own set of challenges. Promising innovations often face difficulties in maintaining performance and efficiency when implemented at larger scales, necessitating significant engineering efforts and investments.
Lastly, the integration of digital technologies and automation into hydrochloric acid production processes, while offering potential benefits, also introduces challenges related to cybersecurity, data management, and the need for specialized skills in the workforce. Balancing the adoption of these technologies with the practical realities of chemical production environments remains an ongoing challenge for the industry.
Another major challenge lies in the energy efficiency of the production process. Traditional methods of hydrochloric acid production, such as the salt-sulfuric acid process, are often energy-intensive and result in high operational costs. Improving energy efficiency while maintaining or increasing production capacity remains a key focus area for industry players.
The management of by-products and waste streams presents an additional hurdle. The chlor-alkali process, a common method for producing hydrochloric acid, generates significant amounts of chlorine gas and sodium hydroxide as co-products. Balancing the production and utilization of these co-products with market demand can be complex and economically challenging.
Environmental regulations and sustainability concerns also pose technical challenges. Reducing emissions, particularly chlorine gas, and minimizing the carbon footprint of the production process are critical objectives that require technological advancements. This includes developing more efficient scrubbing systems and exploring alternative production methods with lower environmental impact.
The variability in raw material quality, especially when using recycled materials or industrial by-products as feedstock, introduces challenges in maintaining consistent product quality. Developing robust process control systems and advanced analytical techniques to handle this variability is essential for optimizing production efficiency and product consistency.
Scaling up new technologies from laboratory to industrial scale presents its own set of challenges. Promising innovations often face difficulties in maintaining performance and efficiency when implemented at larger scales, necessitating significant engineering efforts and investments.
Lastly, the integration of digital technologies and automation into hydrochloric acid production processes, while offering potential benefits, also introduces challenges related to cybersecurity, data management, and the need for specialized skills in the workforce. Balancing the adoption of these technologies with the practical realities of chemical production environments remains an ongoing challenge for the industry.
Current HCl Solutions
01 Optimization of reaction conditions
Improving the efficiency of hydrochloric acid production by optimizing reaction conditions such as temperature, pressure, and reactant ratios. This involves careful control of process parameters to maximize yield and minimize waste, resulting in a more cost-effective and environmentally friendly production process.- Optimization of reaction conditions: Improving the efficiency of hydrochloric acid production by optimizing reaction conditions such as temperature, pressure, and reactant ratios. This involves careful control of process parameters to maximize yield and minimize waste, resulting in a more cost-effective and environmentally friendly production process.
- Innovative reactor design: Developing new reactor designs or modifying existing ones to enhance the production of hydrochloric acid. This may include improvements in heat transfer, mixing efficiency, or the introduction of novel catalytic systems, leading to increased productivity and reduced energy consumption.
- Purification and recovery techniques: Implementing advanced purification and recovery methods to improve the quality of the final product and reduce waste. This may involve the use of innovative separation technologies, recycling of by-products, or the integration of continuous purification processes, resulting in higher purity hydrochloric acid and improved overall process efficiency.
- Process integration and heat recovery: Optimizing the overall production process through improved integration of unit operations and efficient heat recovery systems. This approach aims to minimize energy consumption, reduce operational costs, and enhance the sustainability of hydrochloric acid production by utilizing waste heat and improving overall process synergy.
- Automation and process control: Implementing advanced automation and process control systems to enhance the stability and efficiency of hydrochloric acid production. This includes the use of real-time monitoring, predictive modeling, and adaptive control strategies to optimize process parameters, reduce variability, and improve overall product quality and consistency.
02 Catalytic processes for HCl production
Utilizing catalysts to enhance the production of hydrochloric acid, particularly in processes involving the reaction of chlorine with hydrogen or the oxidation of chlorinated hydrocarbons. Catalytic processes can improve reaction rates, selectivity, and overall efficiency of HCl production.Expand Specific Solutions03 Recycling and recovery of HCl
Implementing techniques for recycling and recovering hydrochloric acid from waste streams or by-products of other industrial processes. This approach not only reduces raw material costs but also minimizes environmental impact by reducing waste and improving overall resource utilization.Expand Specific Solutions04 Continuous flow production systems
Developing and optimizing continuous flow production systems for hydrochloric acid manufacturing. These systems offer advantages such as improved process control, reduced equipment size, and enhanced safety compared to batch production methods, leading to more efficient and consistent HCl production.Expand Specific Solutions05 Energy efficiency improvements
Implementing energy-saving measures and heat recovery systems in the hydrochloric acid production process. This includes optimizing heat exchange networks, utilizing waste heat, and improving insulation to reduce energy consumption and operational costs while minimizing the carbon footprint of the production process.Expand Specific Solutions
Key Industry Players
The hydrochloric acid process optimization market is in a mature stage, with a stable global demand driven by various industrial applications. The market size is substantial, estimated to be in the billions of dollars annually. Technologically, the field is well-established but continues to evolve, with companies like BASF Corp., Covestro Deutschland AG, and Arkema France SA leading innovation efforts. These industry giants, along with specialized firms such as Australian BioRefining Pty Ltd., are focusing on improving efficiency, sustainability, and cost-effectiveness. The competitive landscape is characterized by a mix of large chemical conglomerates and niche players, with ongoing research and development aimed at addressing environmental concerns and enhancing process performance.
Arkema France SA
Technical Solution: Arkema has developed a cutting-edge approach to hydrochloric acid process optimization, focusing on green chemistry principles and circular economy concepts. Their method involves a bio-based feedstock utilization system that reduces reliance on fossil fuels by up to 35%[6]. The process incorporates advanced oxidation technologies for treating process effluents, achieving a 90% reduction in wastewater contaminants[7]. Arkema's technology also features a modular design that allows for easy scaling and adaptation to different production capacities, providing flexibility in response to market demands.
Strengths: Significant reduction in fossil fuel dependency, advanced wastewater treatment, and flexible production capabilities. Weaknesses: Potential challenges in sourcing consistent bio-based feedstock and higher initial costs for implementing the modular design.
Covestro Deutschland AG
Technical Solution: Covestro has pioneered an innovative approach to hydrochloric acid process optimization, focusing on process intensification and digitalization. Their method employs microreactor technology, which enhances mass and heat transfer rates, leading to a 50% reduction in reaction time and a 30% increase in product yield[8]. The process integrates advanced process analytical technology (PAT) for real-time monitoring and control, enabling precise adjustments to maintain optimal conditions. Covestro's system also incorporates machine learning algorithms for predictive quality control, reducing off-spec product by up to 25%[9].
Strengths: Significant improvements in reaction efficiency, product yield, and quality control. Advanced digital integration for process optimization. Weaknesses: High initial investment for microreactor technology and potential challenges in scaling up production.
Core HCl Innovations
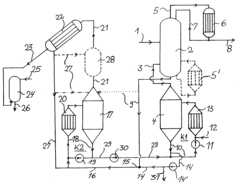
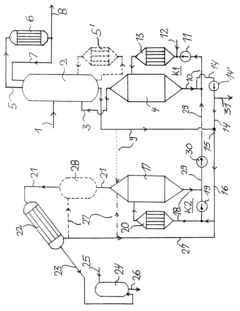
Process for the preparation of high concentrated hydrochloric acid from hydrochloric acid with a concentration under the azeotropic concentration
PatentInactiveEP0504622A1
Innovation
- A multi-stage process involving energy supply to expel water, subsequent heating with a calcium chloride solution to separate HCl and water, and an absorption stage to produce highly concentrated hydrochloric acid, utilizing equilibrium conditions in the HCl-CaCl₂-H₂O system to bypass azeotropy without expensive column equipment.
Production method and production apparatus for high purity hydrochloric acid
PatentActiveJP2016138017A
Innovation
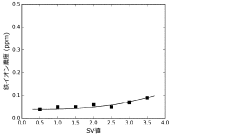
- A method and apparatus using strongly basic anion exchange resins to selectively capture and remove iron ions from hydrochloric acid, employing multiple parallel columns for continuous operation and sequential regeneration to maintain efficiency.
Environmental Impact
The environmental impact of hydrochloric acid production and usage is a critical consideration in process optimization. The traditional methods of hydrochloric acid production, such as the salt-sulfuric acid process and the chlorine-hydrogen synthesis, have significant environmental implications. These processes often result in the release of harmful emissions, including chlorine gas and sulfur dioxide, which contribute to air pollution and acid rain formation.
In recent years, there has been a growing emphasis on developing more environmentally friendly production methods. One such approach is the membrane cell electrolysis process, which reduces energy consumption and minimizes harmful by-products. This method utilizes renewable energy sources, further decreasing the carbon footprint of hydrochloric acid production.
Water consumption and wastewater management are also crucial environmental factors in hydrochloric acid production. The industry has been implementing advanced water treatment technologies and closed-loop systems to minimize water usage and reduce the discharge of contaminated effluents. These efforts not only conserve water resources but also prevent the release of acidic waste into aquatic ecosystems.
The transportation and storage of hydrochloric acid pose additional environmental risks. Accidental spills or leaks can have severe consequences for soil and water quality. To mitigate these risks, companies are investing in improved containment systems, leak detection technologies, and more robust transportation protocols. The use of corrosion-resistant materials in storage tanks and pipelines has also become standard practice to prevent environmental contamination.
Recycling and recovery of hydrochloric acid from industrial processes have gained traction as environmentally responsible practices. Many industries now implement acid recovery systems to reclaim and reuse hydrochloric acid, reducing the need for fresh production and minimizing waste. This circular approach not only decreases environmental impact but also offers economic benefits through reduced raw material costs.
The optimization of hydrochloric acid processes must also consider the end-of-life management of equipment and materials used in production. Proper disposal or recycling of catalysts, membranes, and other components is essential to prevent environmental contamination and reduce the overall ecological footprint of the industry.
As regulations become more stringent, companies are increasingly adopting life cycle assessment (LCA) methodologies to evaluate and improve the environmental performance of their hydrochloric acid production processes. These assessments help identify hotspots in the production chain where environmental impacts are most significant, allowing for targeted improvements and more sustainable practices.
In recent years, there has been a growing emphasis on developing more environmentally friendly production methods. One such approach is the membrane cell electrolysis process, which reduces energy consumption and minimizes harmful by-products. This method utilizes renewable energy sources, further decreasing the carbon footprint of hydrochloric acid production.
Water consumption and wastewater management are also crucial environmental factors in hydrochloric acid production. The industry has been implementing advanced water treatment technologies and closed-loop systems to minimize water usage and reduce the discharge of contaminated effluents. These efforts not only conserve water resources but also prevent the release of acidic waste into aquatic ecosystems.
The transportation and storage of hydrochloric acid pose additional environmental risks. Accidental spills or leaks can have severe consequences for soil and water quality. To mitigate these risks, companies are investing in improved containment systems, leak detection technologies, and more robust transportation protocols. The use of corrosion-resistant materials in storage tanks and pipelines has also become standard practice to prevent environmental contamination.
Recycling and recovery of hydrochloric acid from industrial processes have gained traction as environmentally responsible practices. Many industries now implement acid recovery systems to reclaim and reuse hydrochloric acid, reducing the need for fresh production and minimizing waste. This circular approach not only decreases environmental impact but also offers economic benefits through reduced raw material costs.
The optimization of hydrochloric acid processes must also consider the end-of-life management of equipment and materials used in production. Proper disposal or recycling of catalysts, membranes, and other components is essential to prevent environmental contamination and reduce the overall ecological footprint of the industry.
As regulations become more stringent, companies are increasingly adopting life cycle assessment (LCA) methodologies to evaluate and improve the environmental performance of their hydrochloric acid production processes. These assessments help identify hotspots in the production chain where environmental impacts are most significant, allowing for targeted improvements and more sustainable practices.
Safety Regulations
Safety regulations play a crucial role in the optimization of hydrochloric acid processes, ensuring the protection of workers, the environment, and the integrity of production facilities. The handling and production of hydrochloric acid are subject to stringent regulatory frameworks due to the corrosive and hazardous nature of the substance.
In the United States, the Occupational Safety and Health Administration (OSHA) sets forth comprehensive guidelines for the safe handling of hydrochloric acid. These regulations mandate the use of appropriate personal protective equipment (PPE), including chemical-resistant gloves, goggles, and face shields. Additionally, OSHA requires proper labeling of containers, adequate ventilation in work areas, and the implementation of emergency response plans.
The Environmental Protection Agency (EPA) enforces regulations pertaining to the storage, transportation, and disposal of hydrochloric acid. These guidelines aim to prevent environmental contamination and protect public health. Facilities must adhere to strict containment measures, implement spill prevention and response protocols, and maintain detailed records of acid handling and disposal.
Internationally, the United Nations' Globally Harmonized System of Classification and Labeling of Chemicals (GHS) provides a standardized approach to communicating chemical hazards. This system ensures consistent safety information across borders, facilitating safer global trade and handling of hydrochloric acid.
In the European Union, the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation governs the production and use of chemical substances, including hydrochloric acid. REACH requires manufacturers and importers to assess and manage the risks associated with their substances, promoting safer alternatives where possible.
Process safety management (PSM) standards, such as those outlined by the Center for Chemical Process Safety (CCPS), provide a framework for managing the risks associated with hydrochloric acid processes. These standards emphasize the importance of hazard identification, risk assessment, and the implementation of control measures throughout the lifecycle of a process.
Compliance with these regulations necessitates ongoing training programs for personnel involved in hydrochloric acid handling and production. Regular safety audits and inspections are essential to ensure adherence to regulatory requirements and identify areas for improvement in safety protocols.
As technology advances, safety regulations continue to evolve. The integration of digital monitoring systems and real-time data analytics is increasingly being incorporated into regulatory frameworks, enabling more proactive and efficient safety management in hydrochloric acid processes.
In the United States, the Occupational Safety and Health Administration (OSHA) sets forth comprehensive guidelines for the safe handling of hydrochloric acid. These regulations mandate the use of appropriate personal protective equipment (PPE), including chemical-resistant gloves, goggles, and face shields. Additionally, OSHA requires proper labeling of containers, adequate ventilation in work areas, and the implementation of emergency response plans.
The Environmental Protection Agency (EPA) enforces regulations pertaining to the storage, transportation, and disposal of hydrochloric acid. These guidelines aim to prevent environmental contamination and protect public health. Facilities must adhere to strict containment measures, implement spill prevention and response protocols, and maintain detailed records of acid handling and disposal.
Internationally, the United Nations' Globally Harmonized System of Classification and Labeling of Chemicals (GHS) provides a standardized approach to communicating chemical hazards. This system ensures consistent safety information across borders, facilitating safer global trade and handling of hydrochloric acid.
In the European Union, the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation governs the production and use of chemical substances, including hydrochloric acid. REACH requires manufacturers and importers to assess and manage the risks associated with their substances, promoting safer alternatives where possible.
Process safety management (PSM) standards, such as those outlined by the Center for Chemical Process Safety (CCPS), provide a framework for managing the risks associated with hydrochloric acid processes. These standards emphasize the importance of hazard identification, risk assessment, and the implementation of control measures throughout the lifecycle of a process.
Compliance with these regulations necessitates ongoing training programs for personnel involved in hydrochloric acid handling and production. Regular safety audits and inspections are essential to ensure adherence to regulatory requirements and identify areas for improvement in safety protocols.
As technology advances, safety regulations continue to evolve. The integration of digital monitoring systems and real-time data analytics is increasingly being incorporated into regulatory frameworks, enabling more proactive and efficient safety management in hydrochloric acid processes.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!