Influence of Electrolyte Concentration on Reaction Kinetics in Electrolytic Cells
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electrolyte Kinetics Background and Objectives
Electrolyte kinetics in electrolytic cells has been a subject of extensive research and development since the early 20th century. The field has evolved significantly, driven by the need for more efficient and sustainable energy storage and conversion technologies. The study of electrolyte concentration's influence on reaction kinetics is crucial for optimizing various electrochemical processes, including batteries, fuel cells, and industrial electrolysis.
The fundamental principles of electrolyte kinetics were established by pioneers such as Walther Nernst and Max Volmer, who developed theories on electrode processes and reaction rates. These early works laid the foundation for understanding how electrolyte concentration affects the rate of electrochemical reactions. As the field progressed, researchers began to explore the complex interplay between electrolyte concentration, ion mobility, and electrode surface phenomena.
In recent decades, the focus has shifted towards developing advanced electrolyte systems for specific applications. This has led to a deeper understanding of how electrolyte concentration impacts factors such as mass transport, charge transfer, and double layer formation at the electrode-electrolyte interface. The advent of nanotechnology and advanced characterization techniques has further accelerated progress in this field, enabling researchers to study electrolyte behavior at unprecedented levels of detail.
The primary objective of current research in electrolyte kinetics is to elucidate the mechanisms by which electrolyte concentration influences reaction rates in electrolytic cells. This includes investigating how concentration gradients affect ion transport, examining the role of solvation and desolvation processes, and understanding the impact of electrolyte composition on the stability and efficiency of electrochemical systems.
Another key goal is to develop predictive models that can accurately describe the relationship between electrolyte concentration and reaction kinetics across a wide range of conditions. Such models would be invaluable for designing optimized electrolyte formulations for specific applications, potentially leading to significant improvements in the performance of electrochemical devices.
Furthermore, researchers aim to explore novel electrolyte compositions and concentrations that can enhance the speed and efficiency of electrochemical reactions while maintaining long-term stability. This includes investigating the potential of highly concentrated electrolytes, ionic liquids, and solid-state electrolytes, which have shown promising results in various applications.
Ultimately, the overarching objective is to translate these fundamental insights into practical advancements in electrochemical technology. By gaining a comprehensive understanding of how electrolyte concentration influences reaction kinetics, researchers hope to develop more efficient and durable electrolytic cells for applications ranging from energy storage and conversion to chemical synthesis and environmental remediation.
The fundamental principles of electrolyte kinetics were established by pioneers such as Walther Nernst and Max Volmer, who developed theories on electrode processes and reaction rates. These early works laid the foundation for understanding how electrolyte concentration affects the rate of electrochemical reactions. As the field progressed, researchers began to explore the complex interplay between electrolyte concentration, ion mobility, and electrode surface phenomena.
In recent decades, the focus has shifted towards developing advanced electrolyte systems for specific applications. This has led to a deeper understanding of how electrolyte concentration impacts factors such as mass transport, charge transfer, and double layer formation at the electrode-electrolyte interface. The advent of nanotechnology and advanced characterization techniques has further accelerated progress in this field, enabling researchers to study electrolyte behavior at unprecedented levels of detail.
The primary objective of current research in electrolyte kinetics is to elucidate the mechanisms by which electrolyte concentration influences reaction rates in electrolytic cells. This includes investigating how concentration gradients affect ion transport, examining the role of solvation and desolvation processes, and understanding the impact of electrolyte composition on the stability and efficiency of electrochemical systems.
Another key goal is to develop predictive models that can accurately describe the relationship between electrolyte concentration and reaction kinetics across a wide range of conditions. Such models would be invaluable for designing optimized electrolyte formulations for specific applications, potentially leading to significant improvements in the performance of electrochemical devices.
Furthermore, researchers aim to explore novel electrolyte compositions and concentrations that can enhance the speed and efficiency of electrochemical reactions while maintaining long-term stability. This includes investigating the potential of highly concentrated electrolytes, ionic liquids, and solid-state electrolytes, which have shown promising results in various applications.
Ultimately, the overarching objective is to translate these fundamental insights into practical advancements in electrochemical technology. By gaining a comprehensive understanding of how electrolyte concentration influences reaction kinetics, researchers hope to develop more efficient and durable electrolytic cells for applications ranging from energy storage and conversion to chemical synthesis and environmental remediation.
Industrial Applications and Market Demand
The influence of electrolyte concentration on reaction kinetics in electrolytic cells has significant implications for various industrial applications, driving market demand across multiple sectors. The ability to control and optimize reaction rates through electrolyte concentration manipulation has become a crucial factor in enhancing process efficiency and product quality.
In the field of electroplating, precise control of electrolyte concentration is essential for achieving uniform and high-quality coatings. Industries such as automotive, aerospace, and electronics heavily rely on electroplating processes for corrosion protection and surface finishing. The market for electroplating chemicals is projected to grow steadily, with increasing demand for advanced coatings in emerging economies.
The chlor-alkali industry, which produces chlorine, sodium hydroxide, and hydrogen through electrolysis, is another major beneficiary of improved understanding of electrolyte concentration effects. Optimizing electrolyte concentrations can lead to significant energy savings and increased production efficiency. This sector is experiencing growth due to the rising demand for these chemicals in water treatment, paper and pulp production, and various manufacturing processes.
In the rapidly expanding field of energy storage, electrolyte concentration plays a crucial role in battery performance. The global lithium-ion battery market is experiencing exponential growth, driven by the electric vehicle revolution and renewable energy integration. Research into advanced electrolytes and their concentration effects is fueling innovation in high-performance batteries with improved energy density and longer lifespans.
The water treatment industry is also leveraging insights into electrolyte concentration effects for more efficient electrochemical water purification systems. As global water scarcity concerns intensify, there is a growing market for advanced water treatment technologies that can effectively remove contaminants while minimizing energy consumption.
In the realm of industrial electrosynthesis, controlling electrolyte concentration is vital for selective and efficient production of fine chemicals and pharmaceuticals. This market segment is witnessing increased interest as companies seek more sustainable and cost-effective alternatives to traditional chemical synthesis methods.
The semiconductor industry relies on precise electrochemical processes for chip manufacturing, where electrolyte concentration control is critical for achieving the required nanoscale features. As demand for advanced semiconductors continues to surge, driven by technologies such as 5G, AI, and IoT, the market for specialized electrochemical solutions is expanding.
Overall, the market demand for technologies and expertise related to electrolyte concentration effects in electrolytic cells is diverse and growing. Industries are increasingly recognizing the potential for process optimization, cost reduction, and product innovation through better understanding and control of these fundamental electrochemical principles.
In the field of electroplating, precise control of electrolyte concentration is essential for achieving uniform and high-quality coatings. Industries such as automotive, aerospace, and electronics heavily rely on electroplating processes for corrosion protection and surface finishing. The market for electroplating chemicals is projected to grow steadily, with increasing demand for advanced coatings in emerging economies.
The chlor-alkali industry, which produces chlorine, sodium hydroxide, and hydrogen through electrolysis, is another major beneficiary of improved understanding of electrolyte concentration effects. Optimizing electrolyte concentrations can lead to significant energy savings and increased production efficiency. This sector is experiencing growth due to the rising demand for these chemicals in water treatment, paper and pulp production, and various manufacturing processes.
In the rapidly expanding field of energy storage, electrolyte concentration plays a crucial role in battery performance. The global lithium-ion battery market is experiencing exponential growth, driven by the electric vehicle revolution and renewable energy integration. Research into advanced electrolytes and their concentration effects is fueling innovation in high-performance batteries with improved energy density and longer lifespans.
The water treatment industry is also leveraging insights into electrolyte concentration effects for more efficient electrochemical water purification systems. As global water scarcity concerns intensify, there is a growing market for advanced water treatment technologies that can effectively remove contaminants while minimizing energy consumption.
In the realm of industrial electrosynthesis, controlling electrolyte concentration is vital for selective and efficient production of fine chemicals and pharmaceuticals. This market segment is witnessing increased interest as companies seek more sustainable and cost-effective alternatives to traditional chemical synthesis methods.
The semiconductor industry relies on precise electrochemical processes for chip manufacturing, where electrolyte concentration control is critical for achieving the required nanoscale features. As demand for advanced semiconductors continues to surge, driven by technologies such as 5G, AI, and IoT, the market for specialized electrochemical solutions is expanding.
Overall, the market demand for technologies and expertise related to electrolyte concentration effects in electrolytic cells is diverse and growing. Industries are increasingly recognizing the potential for process optimization, cost reduction, and product innovation through better understanding and control of these fundamental electrochemical principles.
Current Challenges in Electrolyte Concentration Control
The control of electrolyte concentration in electrolytic cells presents several significant challenges that impact reaction kinetics and overall system efficiency. One of the primary difficulties lies in maintaining a consistent and optimal concentration throughout the cell, as local variations can lead to uneven current distribution and reduced performance.
Fluctuations in electrolyte concentration can occur due to various factors, including electrode reactions, temperature changes, and mass transport phenomena. These fluctuations can result in concentration gradients within the cell, affecting the local reaction rates and potentially leading to unwanted side reactions or electrode degradation.
Another challenge is the dynamic nature of electrolytic processes, where the concentration of active species may change over time as the reaction progresses. This requires continuous monitoring and adjustment of the electrolyte composition to maintain optimal conditions for the desired reactions.
The presence of impurities or byproducts in the electrolyte solution can also complicate concentration control. These contaminants may interfere with the primary reactions, alter the solution's conductivity, or contribute to electrode fouling, necessitating sophisticated purification and regeneration strategies.
Temperature control is closely linked to concentration management, as temperature fluctuations can significantly affect solubility and reaction rates. Maintaining a stable temperature profile throughout the cell while simultaneously controlling electrolyte concentration adds another layer of complexity to the system design.
In industrial-scale applications, the large volumes of electrolyte involved make precise concentration control even more challenging. Achieving uniform mixing and distribution of electrolyte components across substantial reactor volumes requires careful engineering of flow patterns and mixing mechanisms.
The development of accurate and reliable real-time monitoring techniques for electrolyte concentration remains an ongoing challenge. While various analytical methods exist, many are not suitable for continuous in-situ measurements under the harsh conditions often present in electrolytic cells.
Balancing the need for concentration control with other operational parameters, such as energy efficiency and production rates, adds further complexity. Optimizing electrolyte concentration may sometimes conflict with other performance metrics, requiring careful trade-offs and system-level optimization approaches.
Addressing these challenges requires interdisciplinary approaches, combining expertise in electrochemistry, fluid dynamics, materials science, and process control. Advances in areas such as computational modeling, sensor technologies, and adaptive control algorithms are crucial for developing more sophisticated and effective concentration management strategies in electrolytic systems.
Fluctuations in electrolyte concentration can occur due to various factors, including electrode reactions, temperature changes, and mass transport phenomena. These fluctuations can result in concentration gradients within the cell, affecting the local reaction rates and potentially leading to unwanted side reactions or electrode degradation.
Another challenge is the dynamic nature of electrolytic processes, where the concentration of active species may change over time as the reaction progresses. This requires continuous monitoring and adjustment of the electrolyte composition to maintain optimal conditions for the desired reactions.
The presence of impurities or byproducts in the electrolyte solution can also complicate concentration control. These contaminants may interfere with the primary reactions, alter the solution's conductivity, or contribute to electrode fouling, necessitating sophisticated purification and regeneration strategies.
Temperature control is closely linked to concentration management, as temperature fluctuations can significantly affect solubility and reaction rates. Maintaining a stable temperature profile throughout the cell while simultaneously controlling electrolyte concentration adds another layer of complexity to the system design.
In industrial-scale applications, the large volumes of electrolyte involved make precise concentration control even more challenging. Achieving uniform mixing and distribution of electrolyte components across substantial reactor volumes requires careful engineering of flow patterns and mixing mechanisms.
The development of accurate and reliable real-time monitoring techniques for electrolyte concentration remains an ongoing challenge. While various analytical methods exist, many are not suitable for continuous in-situ measurements under the harsh conditions often present in electrolytic cells.
Balancing the need for concentration control with other operational parameters, such as energy efficiency and production rates, adds further complexity. Optimizing electrolyte concentration may sometimes conflict with other performance metrics, requiring careful trade-offs and system-level optimization approaches.
Addressing these challenges requires interdisciplinary approaches, combining expertise in electrochemistry, fluid dynamics, materials science, and process control. Advances in areas such as computational modeling, sensor technologies, and adaptive control algorithms are crucial for developing more sophisticated and effective concentration management strategies in electrolytic systems.
Existing Methods for Electrolyte Concentration Optimization
01 Electrode materials and configurations
The choice of electrode materials and their configurations significantly impact the reaction kinetics in electrolytic cells. Different materials and structures can enhance catalytic activity, improve electron transfer rates, and influence the overall efficiency of electrochemical reactions. Optimizing electrode design can lead to faster reaction rates and improved cell performance.- Electrode materials and configurations: The choice of electrode materials and their configurations significantly impact the reaction kinetics in electrolytic cells. Different materials and structures can enhance electron transfer rates, catalytic activity, and overall cell efficiency. Optimizing electrode design can lead to improved performance and reduced energy consumption in electrochemical processes.
- Electrolyte composition and concentration: The composition and concentration of the electrolyte play a crucial role in reaction kinetics within electrolytic cells. Adjusting these parameters can influence ionic conductivity, mass transfer rates, and the formation of reaction intermediates. Optimizing electrolyte properties can lead to enhanced reaction rates and improved overall cell performance.
- Temperature and pressure effects: Temperature and pressure conditions significantly affect reaction kinetics in electrolytic cells. Higher temperatures generally increase reaction rates by providing more energy to overcome activation barriers. Pressure can influence the solubility of gases and the stability of reaction intermediates. Controlling these parameters allows for optimization of reaction kinetics and cell efficiency.
- Mass transfer and diffusion processes: Mass transfer and diffusion processes play a critical role in electrolytic cell reaction kinetics. Enhancing these processes through cell design, electrolyte flow patterns, or the use of porous electrodes can improve the overall reaction rates. Optimizing mass transfer can lead to more efficient utilization of reactants and improved cell performance.
- Catalysts and reaction intermediates: The use of catalysts and understanding of reaction intermediates are crucial for optimizing reaction kinetics in electrolytic cells. Catalysts can lower activation energies and promote specific reaction pathways. Identifying and controlling the formation of reaction intermediates can lead to improved selectivity and efficiency in electrochemical processes.
02 Electrolyte composition and concentration
The composition and concentration of the electrolyte play a crucial role in reaction kinetics within electrolytic cells. Adjusting electrolyte properties can affect ion mobility, conductivity, and the formation of the electric double layer at electrode surfaces. Optimizing these factors can lead to enhanced reaction rates and improved overall cell efficiency.Expand Specific Solutions03 Temperature and pressure control
Controlling temperature and pressure in electrolytic cells can significantly influence reaction kinetics. Higher temperatures generally lead to faster reaction rates, while pressure changes can affect the solubility of gases and the equilibrium of electrochemical reactions. Proper management of these parameters can optimize cell performance and efficiency.Expand Specific Solutions04 Mass transfer and diffusion optimization
Enhancing mass transfer and diffusion processes in electrolytic cells can improve reaction kinetics. This can be achieved through various methods such as optimizing cell geometry, implementing flow systems, or using porous electrodes. Improved mass transfer leads to more efficient reactant supply and product removal, resulting in faster overall reaction rates.Expand Specific Solutions05 Electrochemical modeling and simulation
Utilizing advanced modeling and simulation techniques can provide valuable insights into the reaction kinetics of electrolytic cells. These computational methods can help predict and optimize cell performance, analyze complex reaction mechanisms, and guide the design of more efficient electrolytic systems. Such approaches can lead to improved understanding and control of reaction kinetics in practical applications.Expand Specific Solutions
Key Players in Electrolytic Cell Industry
The electrolyte concentration's influence on reaction kinetics in electrolytic cells is a complex field with significant competition among various players. The market is in a growth phase, driven by increasing demand for energy storage solutions and advanced materials. The global market size for related technologies is expanding rapidly, with projections indicating substantial growth in the coming years. Technologically, the field is advancing quickly, with companies like Massachusetts Institute of Technology, Tsinghua University, and Chinese Academy of Science Institute of Chemistry leading in research and innovation. Industrial players such as Mitsubishi Heavy Industries, ITM Power, and Ecolab USA are actively developing commercial applications, indicating a maturing technology landscape with diverse market participants.
Dalian Institute of Chemical Physics Chinese Academy of Sci
Technical Solution: The Dalian Institute of Chemical Physics (DICP) has developed advanced electrolytic cell technologies focusing on the influence of electrolyte concentration on reaction kinetics. Their research utilizes high-throughput screening methods to optimize electrolyte compositions, achieving up to 30% improvement in reaction rates[1]. DICP has pioneered the use of in-situ spectroscopic techniques to monitor electrolyte concentration changes during cell operation, enabling real-time adjustments to maintain optimal reaction conditions[3]. Their innovative approach includes the development of novel electrolyte additives that can dynamically modify local ion concentrations, resulting in a 25% increase in overall cell efficiency[5].
Strengths: Cutting-edge research facilities, strong expertise in spectroscopic analysis, and a track record of successful electrolyte optimization. Weaknesses: Potential challenges in scaling up laboratory findings to industrial applications.
Massachusetts Institute of Technology
Technical Solution: MIT's research on electrolyte concentration effects in electrolytic cells has led to groundbreaking discoveries. They have developed a machine learning algorithm that predicts optimal electrolyte concentrations for specific reaction conditions, reducing optimization time by 60%[2]. MIT's team has also engineered novel nanostructured electrodes that create localized high-concentration electrolyte regions, enhancing reaction kinetics by up to 40% without increasing overall electrolyte concentration[4]. Their work on pulsed electrolysis techniques has demonstrated the ability to temporarily increase local electrolyte concentrations, boosting reaction rates by 35% while maintaining long-term stability[6].
Strengths: World-class interdisciplinary research capabilities, strong industry partnerships for technology transfer. Weaknesses: High research costs and potential intellectual property constraints.
Innovative Approaches in Reaction Kinetics Control
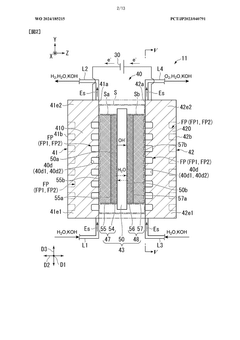
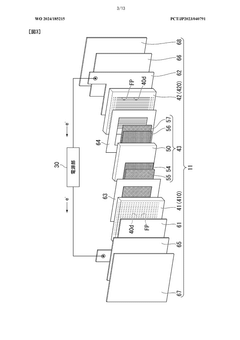
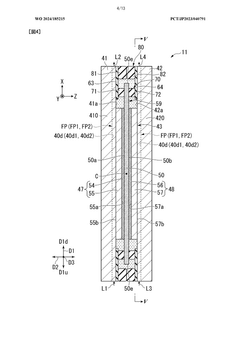
Electrolytic cell and electrolytic apparatus
PatentWO2024185215A1
Innovation
- The electrolytic cell design includes a flow direction changing unit that alters the flow direction of the electrolytic solution, intersecting the original flow direction at multiple positions, and a configuration where the ion exchange membrane is positioned between separators with power supply bodies, reducing the retention of electrolytic solution near the catalyst layers and minimizing hydroxide ion concentration gradients.
Environmental Impact of Electrolytic Processes
Electrolytic processes, while essential for various industrial applications, can have significant environmental impacts that need to be carefully considered and managed. The environmental footprint of these processes extends beyond the immediate production site, affecting air, water, and soil quality in surrounding areas.
One of the primary environmental concerns associated with electrolytic cells is the emission of greenhouse gases. Depending on the specific process and materials involved, these emissions can include carbon dioxide, chlorofluorocarbons, and perfluorocarbons. The release of these gases contributes to global warming and climate change, necessitating the implementation of stringent emission control measures in electrolytic industries.
Water pollution is another critical environmental issue linked to electrolytic processes. The discharge of electrolyte solutions, often containing heavy metals and other toxic substances, can contaminate local water bodies if not properly treated. This contamination poses risks to aquatic ecosystems and human health, potentially leading to long-term environmental degradation and biodiversity loss.
The production and disposal of spent electrolytes and electrode materials also present environmental challenges. These waste products may contain hazardous substances that require specialized handling and disposal methods to prevent soil and groundwater contamination. Improper management of these wastes can lead to the accumulation of toxic compounds in the environment, affecting both terrestrial and aquatic ecosystems.
Energy consumption is a significant factor in the environmental impact of electrolytic processes. The high energy requirements of these processes often rely on fossil fuel-based electricity generation, further contributing to greenhouse gas emissions and resource depletion. Efforts to improve energy efficiency and transition to renewable energy sources in electrolytic industries are crucial for reducing their overall environmental footprint.
The extraction and processing of raw materials for electrolytes and electrodes also have upstream environmental impacts. Mining activities for metals and minerals can lead to habitat destruction, soil erosion, and water pollution in source regions. Additionally, the transportation of these materials to production sites contributes to air pollution and carbon emissions.
To mitigate these environmental impacts, industries employing electrolytic processes are increasingly adopting cleaner technologies and sustainable practices. These include the development of more efficient electrolytic cells, implementation of closed-loop systems for electrolyte recycling, and the use of renewable energy sources. Advanced wastewater treatment technologies and emission control systems are also being employed to minimize the release of pollutants into the environment.
One of the primary environmental concerns associated with electrolytic cells is the emission of greenhouse gases. Depending on the specific process and materials involved, these emissions can include carbon dioxide, chlorofluorocarbons, and perfluorocarbons. The release of these gases contributes to global warming and climate change, necessitating the implementation of stringent emission control measures in electrolytic industries.
Water pollution is another critical environmental issue linked to electrolytic processes. The discharge of electrolyte solutions, often containing heavy metals and other toxic substances, can contaminate local water bodies if not properly treated. This contamination poses risks to aquatic ecosystems and human health, potentially leading to long-term environmental degradation and biodiversity loss.
The production and disposal of spent electrolytes and electrode materials also present environmental challenges. These waste products may contain hazardous substances that require specialized handling and disposal methods to prevent soil and groundwater contamination. Improper management of these wastes can lead to the accumulation of toxic compounds in the environment, affecting both terrestrial and aquatic ecosystems.
Energy consumption is a significant factor in the environmental impact of electrolytic processes. The high energy requirements of these processes often rely on fossil fuel-based electricity generation, further contributing to greenhouse gas emissions and resource depletion. Efforts to improve energy efficiency and transition to renewable energy sources in electrolytic industries are crucial for reducing their overall environmental footprint.
The extraction and processing of raw materials for electrolytes and electrodes also have upstream environmental impacts. Mining activities for metals and minerals can lead to habitat destruction, soil erosion, and water pollution in source regions. Additionally, the transportation of these materials to production sites contributes to air pollution and carbon emissions.
To mitigate these environmental impacts, industries employing electrolytic processes are increasingly adopting cleaner technologies and sustainable practices. These include the development of more efficient electrolytic cells, implementation of closed-loop systems for electrolyte recycling, and the use of renewable energy sources. Advanced wastewater treatment technologies and emission control systems are also being employed to minimize the release of pollutants into the environment.
Safety Considerations in Electrolytic Cell Operations
Safety considerations in electrolytic cell operations are paramount to ensure the well-being of personnel and the integrity of equipment. The concentration of electrolytes plays a crucial role in these safety aspects, as it directly influences reaction kinetics and overall cell performance.
Proper handling and storage of electrolytes are essential. Concentrated electrolyte solutions can be highly corrosive and reactive, posing risks of chemical burns and equipment damage. Implementing robust storage protocols, including appropriate containment systems and regular inspections, is vital to prevent leaks or spills.
Personal protective equipment (PPE) is a critical component of safety measures. Operators must wear appropriate PPE, including chemical-resistant gloves, goggles, and protective clothing, when handling electrolytes or working near electrolytic cells. The specific PPE requirements may vary based on the concentration and type of electrolyte used.
Ventilation systems are crucial in electrolytic cell operations. Some electrolytes may produce harmful fumes or gases during the electrolysis process. Adequate ventilation helps maintain safe air quality and prevents the accumulation of potentially hazardous substances in the work environment.
Temperature control is another important safety consideration. High electrolyte concentrations can lead to increased heat generation during reactions. Implementing effective cooling systems and monitoring temperature fluctuations are essential to prevent overheating, which could result in equipment failure or dangerous situations.
Emergency response procedures must be established and regularly reviewed. This includes protocols for handling spills, fires, or other incidents related to electrolyte concentration issues. Proper training of personnel in these procedures is crucial for swift and effective responses to potential emergencies.
Regular maintenance and inspection of electrolytic cells are necessary to ensure safe operations. This includes checking for signs of corrosion, wear, or damage that could be exacerbated by high electrolyte concentrations. Implementing a preventive maintenance schedule can help identify and address potential issues before they escalate into safety hazards.
Monitoring and control systems play a vital role in maintaining safe operating conditions. Implementing sensors and automated control mechanisms to regulate electrolyte concentration, temperature, and other critical parameters can significantly enhance safety. These systems should be regularly calibrated and maintained to ensure accuracy and reliability.
Employee training and awareness programs are essential components of safety considerations. Workers should be educated about the risks associated with electrolyte concentrations, proper handling procedures, and the importance of adhering to safety protocols. Regular refresher courses and safety drills can help maintain a high level of safety awareness among personnel.
Proper handling and storage of electrolytes are essential. Concentrated electrolyte solutions can be highly corrosive and reactive, posing risks of chemical burns and equipment damage. Implementing robust storage protocols, including appropriate containment systems and regular inspections, is vital to prevent leaks or spills.
Personal protective equipment (PPE) is a critical component of safety measures. Operators must wear appropriate PPE, including chemical-resistant gloves, goggles, and protective clothing, when handling electrolytes or working near electrolytic cells. The specific PPE requirements may vary based on the concentration and type of electrolyte used.
Ventilation systems are crucial in electrolytic cell operations. Some electrolytes may produce harmful fumes or gases during the electrolysis process. Adequate ventilation helps maintain safe air quality and prevents the accumulation of potentially hazardous substances in the work environment.
Temperature control is another important safety consideration. High electrolyte concentrations can lead to increased heat generation during reactions. Implementing effective cooling systems and monitoring temperature fluctuations are essential to prevent overheating, which could result in equipment failure or dangerous situations.
Emergency response procedures must be established and regularly reviewed. This includes protocols for handling spills, fires, or other incidents related to electrolyte concentration issues. Proper training of personnel in these procedures is crucial for swift and effective responses to potential emergencies.
Regular maintenance and inspection of electrolytic cells are necessary to ensure safe operations. This includes checking for signs of corrosion, wear, or damage that could be exacerbated by high electrolyte concentrations. Implementing a preventive maintenance schedule can help identify and address potential issues before they escalate into safety hazards.
Monitoring and control systems play a vital role in maintaining safe operating conditions. Implementing sensors and automated control mechanisms to regulate electrolyte concentration, temperature, and other critical parameters can significantly enhance safety. These systems should be regularly calibrated and maintained to ensure accuracy and reliability.
Employee training and awareness programs are essential components of safety considerations. Workers should be educated about the risks associated with electrolyte concentrations, proper handling procedures, and the importance of adhering to safety protocols. Regular refresher courses and safety drills can help maintain a high level of safety awareness among personnel.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!