Lewis Acid Efficiency in Low-Temperature Reactions
AUG 26, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lewis Acid Catalysis Background and Objectives
Lewis acid catalysis has evolved significantly since the pioneering work of Gilbert N. Lewis in 1923, who first conceptualized acids as electron pair acceptors. This fundamental definition has led to the development of a diverse array of Lewis acid catalysts that play crucial roles in modern synthetic chemistry. The historical trajectory shows a progression from simple metal halides like AlCl3 and BF3 to more sophisticated, structurally complex catalysts designed for specific reaction environments and substrates.
The evolution of Lewis acid catalysis has been particularly notable in the realm of low-temperature reactions, where traditional thermal activation methods face significant limitations. Low-temperature reactions (typically below 0°C) present unique challenges for catalyst efficiency, including decreased molecular mobility, altered reaction kinetics, and solvent limitations. However, they also offer substantial benefits such as enhanced selectivity, reduced side reactions, and energy conservation.
Current technological trends in this field are moving toward the development of highly selective Lewis acid catalysts that can operate efficiently under mild conditions. The integration of computational chemistry and high-throughput experimentation has accelerated the discovery of novel catalyst structures with optimized electronic and steric properties. Additionally, there is growing interest in sustainable catalysis, with research focusing on recyclable catalysts, reduced metal loading, and environmentally benign reaction conditions.
The global push toward greener chemical processes has further intensified research into Lewis acid catalysis at low temperatures, as these conditions often allow for more atom-economical transformations with reduced waste generation. Recent advances in asymmetric catalysis have also highlighted the potential of chiral Lewis acids to enable enantioselective transformations at reduced temperatures, opening new avenues for pharmaceutical and fine chemical synthesis.
The primary technical objectives of current research in this domain include enhancing catalyst activity at low temperatures without compromising selectivity, developing robust catalytic systems that maintain performance across varying reaction conditions, and designing catalysts with broader substrate scope. There is also significant interest in understanding the fundamental mechanisms of Lewis acid activation at low temperatures, particularly the interplay between electronic effects, coordination geometry, and solvent interactions.
Looking forward, the field aims to establish predictive models for catalyst performance, enabling rational design rather than empirical optimization. This includes developing comprehensive structure-activity relationships that account for the unique challenges of low-temperature environments and creating unified theoretical frameworks that can guide the next generation of catalyst innovation.
The evolution of Lewis acid catalysis has been particularly notable in the realm of low-temperature reactions, where traditional thermal activation methods face significant limitations. Low-temperature reactions (typically below 0°C) present unique challenges for catalyst efficiency, including decreased molecular mobility, altered reaction kinetics, and solvent limitations. However, they also offer substantial benefits such as enhanced selectivity, reduced side reactions, and energy conservation.
Current technological trends in this field are moving toward the development of highly selective Lewis acid catalysts that can operate efficiently under mild conditions. The integration of computational chemistry and high-throughput experimentation has accelerated the discovery of novel catalyst structures with optimized electronic and steric properties. Additionally, there is growing interest in sustainable catalysis, with research focusing on recyclable catalysts, reduced metal loading, and environmentally benign reaction conditions.
The global push toward greener chemical processes has further intensified research into Lewis acid catalysis at low temperatures, as these conditions often allow for more atom-economical transformations with reduced waste generation. Recent advances in asymmetric catalysis have also highlighted the potential of chiral Lewis acids to enable enantioselective transformations at reduced temperatures, opening new avenues for pharmaceutical and fine chemical synthesis.
The primary technical objectives of current research in this domain include enhancing catalyst activity at low temperatures without compromising selectivity, developing robust catalytic systems that maintain performance across varying reaction conditions, and designing catalysts with broader substrate scope. There is also significant interest in understanding the fundamental mechanisms of Lewis acid activation at low temperatures, particularly the interplay between electronic effects, coordination geometry, and solvent interactions.
Looking forward, the field aims to establish predictive models for catalyst performance, enabling rational design rather than empirical optimization. This includes developing comprehensive structure-activity relationships that account for the unique challenges of low-temperature environments and creating unified theoretical frameworks that can guide the next generation of catalyst innovation.
Market Applications for Low-Temperature Lewis Acid Catalysis
The market for low-temperature Lewis acid catalysis spans multiple high-value industrial sectors, with pharmaceutical manufacturing representing the most significant application area. In pharmaceutical synthesis, these catalysts enable selective transformations at reduced temperatures (typically below 0°C), preserving sensitive functional groups while enhancing stereoselectivity in complex molecule synthesis. This capability directly translates to higher yields of enantiopure compounds, reduced waste streams, and lower energy consumption—all critical factors in an industry where production efficiency directly impacts medication affordability.
Fine chemical production constitutes another substantial market, where low-temperature Lewis acid catalysis facilitates precise control over reaction pathways in the synthesis of specialty chemicals, flavors, fragrances, and agricultural compounds. The ability to conduct reactions at lower temperatures provides manufacturers with improved product consistency and reduced byproduct formation, addressing growing regulatory pressures regarding chemical purity specifications.
The polymer industry has increasingly adopted low-temperature Lewis acid catalysts for controlled polymerization processes. These catalysts enable the production of polymers with narrower molecular weight distributions and more uniform structures, resulting in materials with superior mechanical properties. Notable applications include the production of specialty elastomers, high-performance thermoplastics, and advanced composite materials used in automotive and aerospace sectors.
Emerging applications in green chemistry represent a rapidly expanding market segment. Low-temperature Lewis acid catalysis aligns with sustainability initiatives by reducing energy requirements and enabling reactions in environmentally benign solvents. This technology facilitates the valorization of biomass-derived feedstocks, supporting the transition from petroleum-based to bio-based chemical manufacturing pathways.
The electronic materials sector utilizes these catalysts in the production of high-purity precursors for semiconductor manufacturing and advanced display technologies. The precision afforded by low-temperature catalysis is particularly valuable for creating materials with stringent purity requirements and defined molecular architectures.
Market analysis indicates that specialty catalyst manufacturers focusing on low-temperature Lewis acid systems have experienced compound annual growth rates exceeding the broader catalyst market average. This growth is driven by increasing demand for energy-efficient processes, stricter product specifications, and the ongoing shift toward more complex, high-value chemical products across multiple industries.
Fine chemical production constitutes another substantial market, where low-temperature Lewis acid catalysis facilitates precise control over reaction pathways in the synthesis of specialty chemicals, flavors, fragrances, and agricultural compounds. The ability to conduct reactions at lower temperatures provides manufacturers with improved product consistency and reduced byproduct formation, addressing growing regulatory pressures regarding chemical purity specifications.
The polymer industry has increasingly adopted low-temperature Lewis acid catalysts for controlled polymerization processes. These catalysts enable the production of polymers with narrower molecular weight distributions and more uniform structures, resulting in materials with superior mechanical properties. Notable applications include the production of specialty elastomers, high-performance thermoplastics, and advanced composite materials used in automotive and aerospace sectors.
Emerging applications in green chemistry represent a rapidly expanding market segment. Low-temperature Lewis acid catalysis aligns with sustainability initiatives by reducing energy requirements and enabling reactions in environmentally benign solvents. This technology facilitates the valorization of biomass-derived feedstocks, supporting the transition from petroleum-based to bio-based chemical manufacturing pathways.
The electronic materials sector utilizes these catalysts in the production of high-purity precursors for semiconductor manufacturing and advanced display technologies. The precision afforded by low-temperature catalysis is particularly valuable for creating materials with stringent purity requirements and defined molecular architectures.
Market analysis indicates that specialty catalyst manufacturers focusing on low-temperature Lewis acid systems have experienced compound annual growth rates exceeding the broader catalyst market average. This growth is driven by increasing demand for energy-efficient processes, stricter product specifications, and the ongoing shift toward more complex, high-value chemical products across multiple industries.
Current Challenges in Low-Temperature Lewis Acid Reactions
Despite significant advancements in Lewis acid catalysis, several critical challenges persist in low-temperature reaction environments that limit industrial applications and research progress. The primary obstacle remains catalyst activity at reduced temperatures, where many conventional Lewis acids exhibit dramatically decreased reaction rates or complete deactivation below certain thermal thresholds. This temperature-dependent efficiency decline creates a fundamental barrier to energy-efficient chemical processes.
Solubility and homogeneity issues present another significant challenge. As temperatures decrease, many Lewis acids demonstrate poor solubility in common organic solvents, leading to heterogeneous reaction conditions that compromise catalytic performance through reduced substrate-catalyst interactions. This often necessitates higher catalyst loadings, diminishing both economic and environmental sustainability.
Moisture sensitivity represents a persistent technical hurdle, particularly pronounced at lower temperatures where water removal becomes more difficult. Many potent Lewis acids undergo rapid hydrolysis or form inactive hydrated complexes when exposed to even trace moisture, requiring stringent anhydrous conditions that complicate scalability and increase operational costs.
Selectivity control at low temperatures introduces complex kinetic considerations. While reduced thermal energy can enhance stereoselectivity in certain reactions, it may simultaneously promote undesired side reactions or alter reaction pathways entirely. This unpredictable behavior necessitates extensive optimization for each specific transformation, impeding the development of broadly applicable catalytic systems.
Catalyst recovery and recycling efficiency decrease substantially at lower temperatures, particularly for homogeneous systems. Separation processes become more energy-intensive and time-consuming, often resulting in significant catalyst degradation or loss. This challenge directly impacts the economic viability of industrial applications, especially for precious metal-based Lewis acid catalysts.
Mechanistic understanding remains incomplete for many low-temperature Lewis acid interactions. The altered coordination environments, modified substrate activation modes, and different solvation effects at reduced temperatures are inadequately characterized, hampering rational catalyst design. This knowledge gap forces researchers to rely heavily on empirical approaches rather than predictive models.
Compatibility with green solvents presents an emerging challenge, as many environmentally preferable reaction media exhibit different solvation properties at low temperatures, often diminishing Lewis acid performance through competitive coordination or altered dielectric properties. This creates tension between sustainability goals and reaction efficiency in modern chemical process development.
Solubility and homogeneity issues present another significant challenge. As temperatures decrease, many Lewis acids demonstrate poor solubility in common organic solvents, leading to heterogeneous reaction conditions that compromise catalytic performance through reduced substrate-catalyst interactions. This often necessitates higher catalyst loadings, diminishing both economic and environmental sustainability.
Moisture sensitivity represents a persistent technical hurdle, particularly pronounced at lower temperatures where water removal becomes more difficult. Many potent Lewis acids undergo rapid hydrolysis or form inactive hydrated complexes when exposed to even trace moisture, requiring stringent anhydrous conditions that complicate scalability and increase operational costs.
Selectivity control at low temperatures introduces complex kinetic considerations. While reduced thermal energy can enhance stereoselectivity in certain reactions, it may simultaneously promote undesired side reactions or alter reaction pathways entirely. This unpredictable behavior necessitates extensive optimization for each specific transformation, impeding the development of broadly applicable catalytic systems.
Catalyst recovery and recycling efficiency decrease substantially at lower temperatures, particularly for homogeneous systems. Separation processes become more energy-intensive and time-consuming, often resulting in significant catalyst degradation or loss. This challenge directly impacts the economic viability of industrial applications, especially for precious metal-based Lewis acid catalysts.
Mechanistic understanding remains incomplete for many low-temperature Lewis acid interactions. The altered coordination environments, modified substrate activation modes, and different solvation effects at reduced temperatures are inadequately characterized, hampering rational catalyst design. This knowledge gap forces researchers to rely heavily on empirical approaches rather than predictive models.
Compatibility with green solvents presents an emerging challenge, as many environmentally preferable reaction media exhibit different solvation properties at low temperatures, often diminishing Lewis acid performance through competitive coordination or altered dielectric properties. This creates tension between sustainability goals and reaction efficiency in modern chemical process development.
Contemporary Low-Temperature Lewis Acid Systems
01 Lewis Acids in Catalytic Reactions
Lewis acids serve as effective catalysts in various chemical reactions, enhancing reaction rates and selectivity. They function by accepting electron pairs from substrates, facilitating bond formation or cleavage. Common Lewis acid catalysts include metal halides, metal triflates, and organometallic compounds. Their catalytic efficiency depends on factors such as electronic properties, steric hindrance, and coordination ability, making them valuable in industrial synthesis processes.- Lewis acids in catalytic reactions: Lewis acids serve as effective catalysts in various chemical reactions, enhancing reaction rates and selectivity. They function by accepting electron pairs from substrates, facilitating bond formation or cleavage. The efficiency of Lewis acid catalysts depends on their electron-accepting strength, coordination ability, and compatibility with reaction conditions. These catalysts are particularly valuable in organic synthesis for promoting transformations like alkylation, acylation, and cyclization reactions.
- Metal-based Lewis acids for industrial processes: Metal-based Lewis acids, including aluminum, titanium, zinc, and boron compounds, demonstrate high efficiency in industrial chemical processes. These compounds exhibit strong electron-accepting properties and can be tuned for specific applications by modifying their ligand environment. Their catalytic efficiency is often enhanced through structural modifications that improve stability, selectivity, and recyclability. These Lewis acids are widely employed in polymerization reactions, pharmaceutical synthesis, and petrochemical processing.
- Lewis acid efficiency in polymerization reactions: Lewis acids play a crucial role in controlling polymerization reactions, affecting polymer molecular weight, distribution, and stereochemistry. Their efficiency in polymerization processes depends on factors such as coordination strength, steric hindrance, and counter-ion effects. By carefully selecting and optimizing Lewis acid catalysts, researchers can achieve higher conversion rates, better selectivity, and improved polymer properties. Advanced Lewis acid systems often incorporate co-catalysts or activators to further enhance their efficiency.
- Novel Lewis acid structures and formulations: Innovative Lewis acid structures and formulations have been developed to overcome limitations of traditional systems. These include supported Lewis acids, Lewis acid-surfactant combined catalysts, and heterogeneous Lewis acid systems. Novel formulations often feature enhanced stability under reaction conditions, improved recyclability, and reduced environmental impact. Structural modifications such as immobilization on solid supports or incorporation into metal-organic frameworks can significantly improve the efficiency and practical applicability of Lewis acid catalysts.
- Lewis acids in environmentally friendly processes: Lewis acids are increasingly being optimized for environmentally friendly chemical processes. This includes the development of water-tolerant Lewis acids, recyclable catalyst systems, and Lewis acids derived from sustainable resources. These environmentally conscious Lewis acid catalysts maintain high efficiency while reducing waste generation and energy consumption. Recent advances focus on creating Lewis acid systems that operate effectively under mild conditions, in green solvents, or in solvent-free environments, contributing to more sustainable chemical manufacturing processes.
02 Lewis Acids in Polymerization Processes
Lewis acids play a crucial role in polymerization reactions, particularly in cationic and coordination polymerization. They activate monomers by forming complexes that facilitate chain propagation and control molecular weight distribution. The efficiency of Lewis acids in polymerization depends on their strength, concentration, and compatibility with the reaction medium. Optimizing these parameters leads to polymers with desired properties and structural characteristics.Expand Specific Solutions03 Novel Lewis Acid Structures and Formulations
Research has led to the development of novel Lewis acid structures with enhanced efficiency and selectivity. These include supported Lewis acids, Lewis acid-surfactant combined catalysts, and heterogeneous Lewis acid systems. Structural modifications such as ligand design and metal center selection can significantly improve catalytic performance. These innovations allow for easier catalyst recovery, reduced environmental impact, and application in continuous flow processes.Expand Specific Solutions04 Lewis Acids in Organic Synthesis Applications
Lewis acids are widely employed in organic synthesis for reactions including Friedel-Crafts alkylation, Diels-Alder reactions, and aldol condensations. Their efficiency in these applications depends on matching the Lewis acid strength to the specific reaction requirements. Factors affecting their performance include solvent compatibility, temperature stability, and functional group tolerance. Strategic selection of Lewis acids can lead to improved yields, reduced side reactions, and enhanced stereoselectivity.Expand Specific Solutions05 Lewis Acid Efficiency Enhancement Techniques
Various techniques have been developed to enhance Lewis acid efficiency, including immobilization on solid supports, use of ionic liquids as reaction media, and development of water-tolerant Lewis acids. Additional approaches involve combining Lewis acids with co-catalysts, using Lewis acid-base pairs, and designing recyclable Lewis acid systems. These enhancement methods address traditional limitations such as sensitivity to moisture, difficulty in separation, and catalyst deactivation, resulting in more sustainable and economical processes.Expand Specific Solutions
Leading Research Groups and Industrial Players
The Lewis acid efficiency in low-temperature reactions market is currently in a growth phase, with increasing applications across pharmaceutical and chemical industries. The global market size is expanding as industries seek more energy-efficient catalytic processes. Technologically, significant advancements have been made by key players including BASF Corp. and Dow Silicones Corp., who lead commercial applications with proprietary catalyst systems. Academic institutions like Fudan University and Jilin University are driving fundamental research, while pharmaceutical companies such as Takeda and Otsuka are implementing these technologies in drug synthesis. Wanhua Chemical and Arkema France represent emerging players developing specialized Lewis acid catalysts for niche applications. The field is characterized by collaboration between industry and academia, with competition focused on catalyst stability and selectivity at increasingly lower temperatures.
BASF Corp.
Technical Solution: BASF has developed advanced Lewis acid catalysts for low-temperature reactions, particularly focusing on metal-organic frameworks (MOFs) with tunable Lewis acidity. Their proprietary CuBTC (copper benzene-1,3,5-tricarboxylate) framework demonstrates exceptional activity at temperatures as low as -20°C for various transformations including Diels-Alder reactions and selective oxidations. BASF's technology incorporates coordinatively unsaturated metal sites that maintain catalytic activity even at reduced thermal energy conditions. Their recent innovations include aluminum-based Lewis acid catalysts that exhibit high efficiency in carbonyl activation reactions at temperatures between -78°C and 0°C, with conversion rates exceeding 90% in many applications. BASF has also pioneered the development of supported Lewis acid systems that combine homogeneous-like selectivity with heterogeneous recoverability, allowing for multiple reaction cycles without significant activity loss even at sub-ambient temperatures.
Strengths: Superior catalyst stability at low temperatures with minimal deactivation; excellent selectivity control; industrial scalability with established manufacturing processes. Weaknesses: Some systems require specialized handling due to moisture sensitivity; higher production costs compared to traditional catalysts; potential mass transfer limitations in heterogeneous systems at very low temperatures.
Arkema France SA
Technical Solution: Arkema has pioneered innovative Lewis acid technologies specifically designed for low-temperature polymerization and fine chemical synthesis. Their proprietary ALCAT™ system features aluminum-based Lewis acids with modified ligand environments that maintain catalytic activity at temperatures as low as -40°C. These catalysts incorporate fluorinated aryl groups that enhance Lewis acidity while providing stability against deactivation pathways common at reduced temperatures. Arkema's technology enables controlled ring-opening polymerizations with narrow molecular weight distributions (PDI < 1.2) even at temperatures where traditional catalysts become inactive. Their recent developments include immobilized Lewis acid systems on proprietary support materials that combine the selectivity advantages of homogeneous catalysts with the practical benefits of heterogeneous systems, particularly valuable in continuous flow applications at low temperatures. Arkema has also developed dual-function Lewis acid catalysts that can simultaneously activate multiple reaction components, allowing for cascade transformations to occur efficiently at temperatures between -20°C and 0°C.
Strengths: Exceptional selectivity control in stereospecific reactions; compatibility with sensitive functional groups; reduced side reactions at low temperatures leading to higher product purity. Weaknesses: Some systems show sensitivity to trace impurities; higher catalyst loadings sometimes required compared to elevated temperature processes; limited substrate scope for certain catalyst variants.
Key Patents and Breakthroughs in Lewis Acid Chemistry
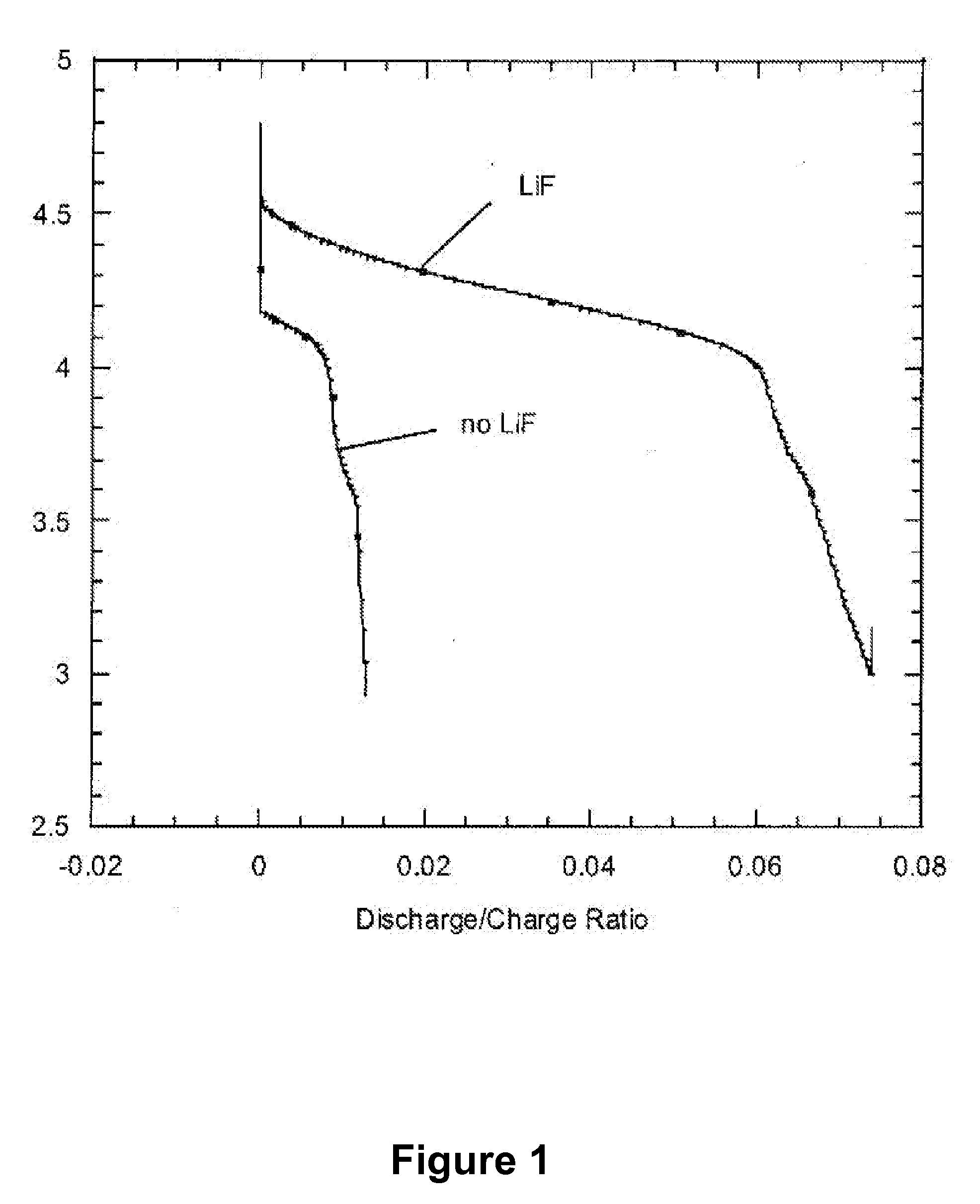
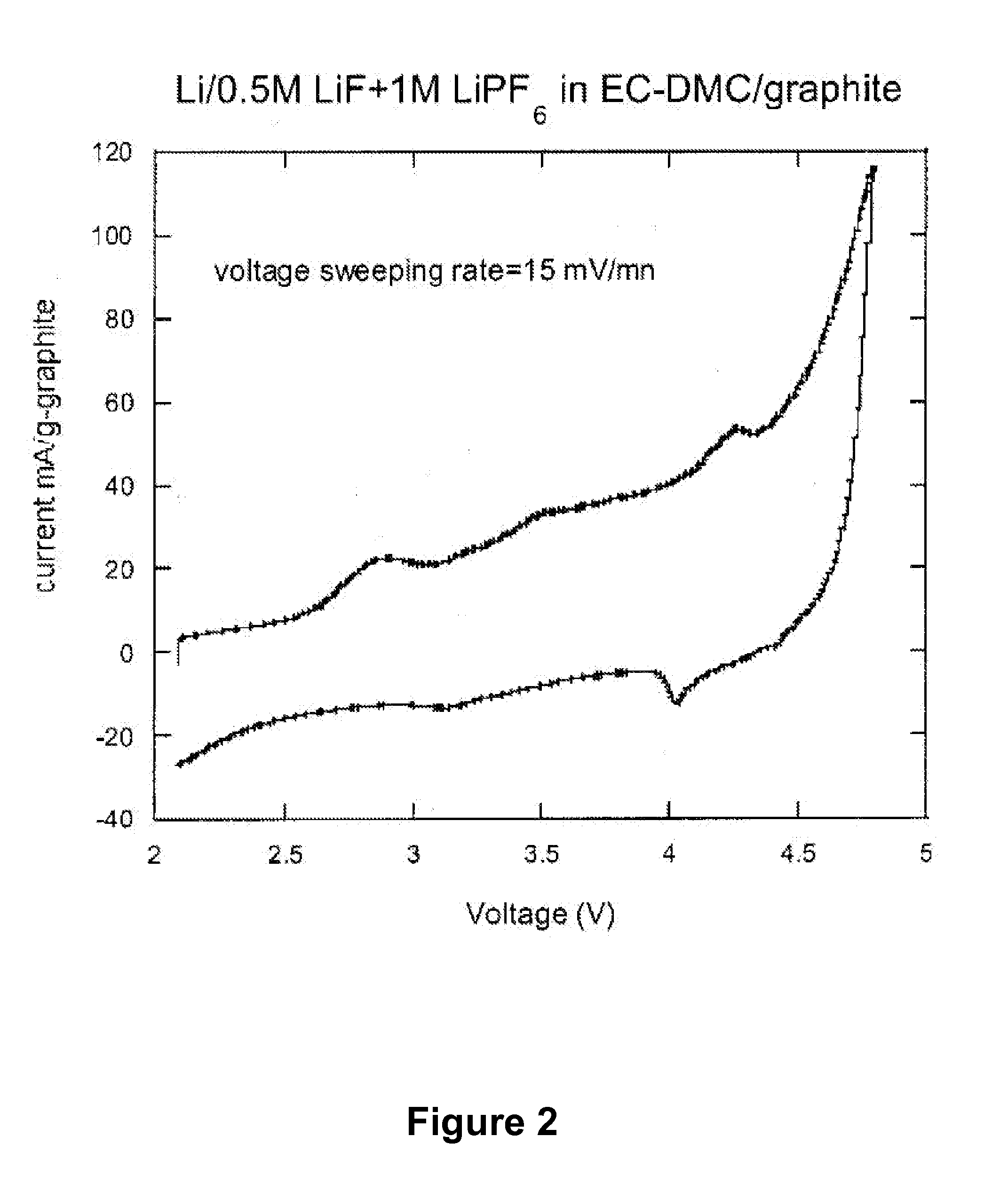
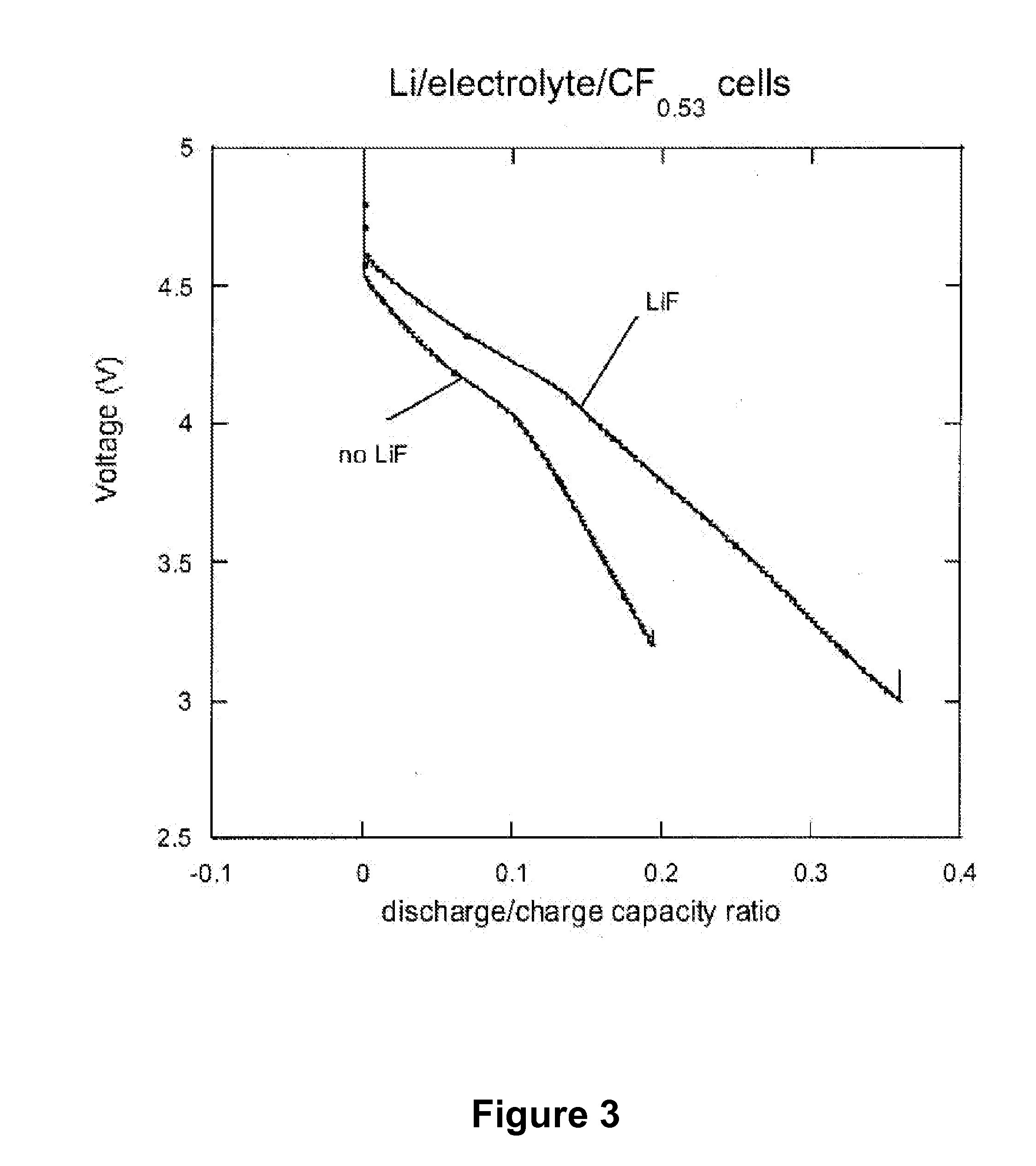
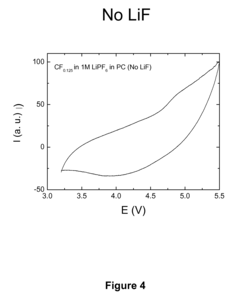
Dissociating agents, formulations and methods providing enhanced solubility of fluorides
PatentActiveUS20080171268A1
Innovation
- Incorporation of dissociating agents such as Lewis acids, Lewis bases, anion receptors, and cation receptors into the electrolyte formulations to enhance the dissolution and solubility of lithium salts, particularly lithium fluoride, in nonaqueous organic solvents, thereby increasing ionic conductivity and stability.
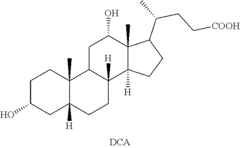
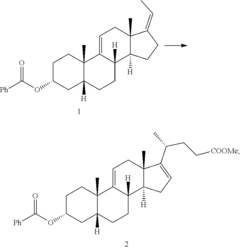
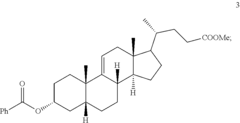
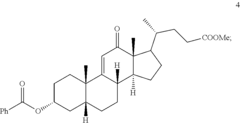
Methods for preparing deoxycholic acid
PatentInactiveUS20190077826A1
Innovation
- A chemical synthesis method involving a series of reactions, including reacting compound 1 with methyl acrylate in the presence of a Lewis acid, followed by hydrogenation, oxidation, and reduction steps, culminating in deprotection or hydrolysis to produce deoxycholic acid or its ester or pharmaceutically acceptable salts.
Environmental Impact and Green Chemistry Considerations
The integration of Lewis acid catalysis with green chemistry principles represents a significant opportunity for sustainable chemical manufacturing. Traditional Lewis acid reactions often involve harsh conditions, toxic reagents, and generate substantial waste. However, low-temperature Lewis acid catalysis offers promising environmental benefits through reduced energy consumption, decreased side reactions, and improved atom economy.
When operating at lower temperatures, Lewis acid catalysts typically require less energy input, directly reducing the carbon footprint of chemical processes. This energy efficiency aligns with the first principle of green chemistry: energy conservation. Studies indicate that reducing reaction temperatures by just 20-30°C can decrease energy requirements by 15-25%, depending on the specific process and scale.
Waste reduction constitutes another critical environmental advantage. Low-temperature Lewis acid reactions generally produce fewer by-products due to enhanced selectivity, resulting in higher yields and reduced purification requirements. This improvement addresses multiple green chemistry principles simultaneously: waste prevention, atom economy, and safer chemistry.
The solvent systems employed in low-temperature Lewis acid catalysis also merit environmental consideration. Traditional approaches often rely on chlorinated or other environmentally problematic solvents. Recent innovations have demonstrated successful implementation of greener alternatives, including bio-derived solvents, ionic liquids, and in some cases, solvent-free conditions. These alternatives significantly reduce the environmental impact associated with solvent disposal and potential atmospheric emissions.
Catalyst recovery and reusability represent another dimension of environmental sustainability. Novel supported Lewis acid catalysts designed for low-temperature applications have demonstrated impressive recyclability, with some systems maintaining over 90% activity after five cycles. This extended catalyst lifetime reduces both waste generation and the environmental burden of catalyst production.
Risk assessment frameworks specifically developed for low-temperature Lewis acid processes indicate reduced hazard profiles compared to conventional approaches. Lower reaction temperatures inherently decrease risks associated with runaway reactions, pressure build-up, and volatile emissions. This safety improvement translates to reduced environmental risk from accidental releases or operational incidents.
Life cycle assessment (LCA) studies comparing traditional and low-temperature Lewis acid processes have documented substantial environmental benefits across multiple impact categories. A comprehensive analysis of a model Friedel-Crafts acylation revealed that low-temperature alternatives reduced global warming potential by approximately 35% and ecotoxicity impacts by over 40% when compared to conventional high-temperature approaches using traditional Lewis acids.
When operating at lower temperatures, Lewis acid catalysts typically require less energy input, directly reducing the carbon footprint of chemical processes. This energy efficiency aligns with the first principle of green chemistry: energy conservation. Studies indicate that reducing reaction temperatures by just 20-30°C can decrease energy requirements by 15-25%, depending on the specific process and scale.
Waste reduction constitutes another critical environmental advantage. Low-temperature Lewis acid reactions generally produce fewer by-products due to enhanced selectivity, resulting in higher yields and reduced purification requirements. This improvement addresses multiple green chemistry principles simultaneously: waste prevention, atom economy, and safer chemistry.
The solvent systems employed in low-temperature Lewis acid catalysis also merit environmental consideration. Traditional approaches often rely on chlorinated or other environmentally problematic solvents. Recent innovations have demonstrated successful implementation of greener alternatives, including bio-derived solvents, ionic liquids, and in some cases, solvent-free conditions. These alternatives significantly reduce the environmental impact associated with solvent disposal and potential atmospheric emissions.
Catalyst recovery and reusability represent another dimension of environmental sustainability. Novel supported Lewis acid catalysts designed for low-temperature applications have demonstrated impressive recyclability, with some systems maintaining over 90% activity after five cycles. This extended catalyst lifetime reduces both waste generation and the environmental burden of catalyst production.
Risk assessment frameworks specifically developed for low-temperature Lewis acid processes indicate reduced hazard profiles compared to conventional approaches. Lower reaction temperatures inherently decrease risks associated with runaway reactions, pressure build-up, and volatile emissions. This safety improvement translates to reduced environmental risk from accidental releases or operational incidents.
Life cycle assessment (LCA) studies comparing traditional and low-temperature Lewis acid processes have documented substantial environmental benefits across multiple impact categories. A comprehensive analysis of a model Friedel-Crafts acylation revealed that low-temperature alternatives reduced global warming potential by approximately 35% and ecotoxicity impacts by over 40% when compared to conventional high-temperature approaches using traditional Lewis acids.
Scale-up and Industrial Implementation Strategies
Scaling up Lewis acid catalyzed reactions from laboratory to industrial scale presents unique challenges, particularly in low-temperature environments. The transition requires careful engineering considerations to maintain reaction efficiency while addressing economic and practical constraints. Industrial implementation typically begins with pilot-scale testing, where reaction parameters are optimized under conditions that more closely resemble full production environments.
Continuous flow reactors have emerged as preferred systems for large-scale Lewis acid catalyzed reactions at low temperatures. These systems offer superior heat transfer capabilities, critical for maintaining precise temperature control in exothermic reactions. Additionally, they enable more efficient catalyst utilization and recovery, addressing one of the primary cost factors in industrial implementation. Companies like BASF and Dow Chemical have successfully deployed modified tubular reactors with enhanced mixing zones specifically designed for Lewis acid catalysis.
Material selection becomes increasingly important at industrial scale, as Lewis acids often exhibit corrosive properties. Specialized alloys, glass-lined equipment, or fluoropolymer coatings are commonly employed to extend equipment lifespan while preventing contamination of reaction products. For extremely sensitive processes, hastelloy or tantalum-lined reactors may be necessary, though these significantly impact capital expenditure calculations.
Catalyst immobilization strategies represent a critical advancement for industrial implementation. Heterogeneous systems using supported Lewis acids on silica, alumina, or polymeric substrates allow for continuous operation with simplified catalyst separation. Recent innovations include magnetic nanoparticle-supported Lewis acids that combine high surface area with easy recovery through magnetic separation, reducing downtime between production cycles.
Energy management systems must be carefully engineered for low-temperature reactions at scale. Cryogenic cooling infrastructure represents a substantial investment and ongoing operational cost. Advanced heat exchanger networks that recover cooling capacity from process streams can significantly improve economic viability. Some facilities have implemented cascade refrigeration systems that utilize multiple refrigerants to achieve efficient cooling across different temperature ranges.
Monitoring and control systems require special consideration for industrial implementation. In-line analytical techniques such as FTIR, Raman spectroscopy, or specialized calorimetry enable real-time reaction monitoring, critical for maintaining product quality and safety at scale. Advanced process control algorithms can dynamically adjust reaction parameters to compensate for inevitable variations in industrial environments, maintaining optimal Lewis acid efficiency despite fluctuations in feed composition or ambient conditions.
Regulatory compliance and safety protocols must be integrated into scale-up strategies, particularly when handling reactive Lewis acids like aluminum chloride or boron trifluoride. Closed handling systems, automated dosing equipment, and comprehensive containment measures are essential components of industrial implementation plans.
Continuous flow reactors have emerged as preferred systems for large-scale Lewis acid catalyzed reactions at low temperatures. These systems offer superior heat transfer capabilities, critical for maintaining precise temperature control in exothermic reactions. Additionally, they enable more efficient catalyst utilization and recovery, addressing one of the primary cost factors in industrial implementation. Companies like BASF and Dow Chemical have successfully deployed modified tubular reactors with enhanced mixing zones specifically designed for Lewis acid catalysis.
Material selection becomes increasingly important at industrial scale, as Lewis acids often exhibit corrosive properties. Specialized alloys, glass-lined equipment, or fluoropolymer coatings are commonly employed to extend equipment lifespan while preventing contamination of reaction products. For extremely sensitive processes, hastelloy or tantalum-lined reactors may be necessary, though these significantly impact capital expenditure calculations.
Catalyst immobilization strategies represent a critical advancement for industrial implementation. Heterogeneous systems using supported Lewis acids on silica, alumina, or polymeric substrates allow for continuous operation with simplified catalyst separation. Recent innovations include magnetic nanoparticle-supported Lewis acids that combine high surface area with easy recovery through magnetic separation, reducing downtime between production cycles.
Energy management systems must be carefully engineered for low-temperature reactions at scale. Cryogenic cooling infrastructure represents a substantial investment and ongoing operational cost. Advanced heat exchanger networks that recover cooling capacity from process streams can significantly improve economic viability. Some facilities have implemented cascade refrigeration systems that utilize multiple refrigerants to achieve efficient cooling across different temperature ranges.
Monitoring and control systems require special consideration for industrial implementation. In-line analytical techniques such as FTIR, Raman spectroscopy, or specialized calorimetry enable real-time reaction monitoring, critical for maintaining product quality and safety at scale. Advanced process control algorithms can dynamically adjust reaction parameters to compensate for inevitable variations in industrial environments, maintaining optimal Lewis acid efficiency despite fluctuations in feed composition or ambient conditions.
Regulatory compliance and safety protocols must be integrated into scale-up strategies, particularly when handling reactive Lewis acids like aluminum chloride or boron trifluoride. Closed handling systems, automated dosing equipment, and comprehensive containment measures are essential components of industrial implementation plans.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!