Lewis Acid in Versatile Organic Syntheses
AUG 26, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lewis Acid Evolution and Research Objectives
Lewis acids have evolved significantly since their initial conceptualization by Gilbert N. Lewis in 1923. These electron pair acceptors have transformed from simple theoretical constructs to powerful catalytic tools in modern organic synthesis. The historical trajectory began with simple metal halides like AlCl3 and BF3, which were primarily utilized in Friedel-Crafts reactions. By the mid-20th century, researchers expanded the application scope to include various carbon-carbon bond-forming reactions, establishing Lewis acids as fundamental components in synthetic organic chemistry.
The 1980s marked a pivotal shift with the development of chiral Lewis acids, enabling stereoselective transformations that revolutionized pharmaceutical synthesis. This innovation addressed the growing demand for enantiomerically pure compounds in drug development. Subsequently, the 1990s witnessed the emergence of designer Lewis acids with tunable properties, allowing chemists to optimize reaction conditions for specific transformations with unprecedented precision.
Recent decades have seen remarkable advancements in Lewis acid technology, including the development of solid-supported catalysts that combine high activity with easy recovery and reuse. Additionally, water-compatible Lewis acids have emerged, challenging the traditional limitation of moisture sensitivity and expanding potential applications in green chemistry. The integration of Lewis acids into multifunctional catalytic systems has further enhanced their versatility in complex molecule synthesis.
Current research objectives in Lewis acid chemistry focus on several key areas. First, developing more sustainable catalysts with reduced environmental impact remains a priority, with particular emphasis on replacing rare earth metals with abundant alternatives. Second, researchers aim to create ultra-low loading catalysts that maintain high efficiency while minimizing resource consumption. Third, there is significant interest in designing Lewis acids capable of activating traditionally unreactive bonds, potentially unlocking novel synthetic pathways.
The exploration of dual catalytic systems, where Lewis acids work synergistically with other catalytic modalities like photoredox or enzymatic catalysis, represents another frontier. These hybrid approaches promise to enable previously impossible transformations. Additionally, computational modeling has become instrumental in predicting Lewis acid behavior and designing catalysts with specific properties, accelerating the discovery process.
The ultimate goal of current Lewis acid research is to develop programmable catalysts that can be precisely tailored to specific reactions, substrates, and desired outcomes, thereby expanding the synthetic toolbox available to chemists across industries from pharmaceuticals to materials science.
The 1980s marked a pivotal shift with the development of chiral Lewis acids, enabling stereoselective transformations that revolutionized pharmaceutical synthesis. This innovation addressed the growing demand for enantiomerically pure compounds in drug development. Subsequently, the 1990s witnessed the emergence of designer Lewis acids with tunable properties, allowing chemists to optimize reaction conditions for specific transformations with unprecedented precision.
Recent decades have seen remarkable advancements in Lewis acid technology, including the development of solid-supported catalysts that combine high activity with easy recovery and reuse. Additionally, water-compatible Lewis acids have emerged, challenging the traditional limitation of moisture sensitivity and expanding potential applications in green chemistry. The integration of Lewis acids into multifunctional catalytic systems has further enhanced their versatility in complex molecule synthesis.
Current research objectives in Lewis acid chemistry focus on several key areas. First, developing more sustainable catalysts with reduced environmental impact remains a priority, with particular emphasis on replacing rare earth metals with abundant alternatives. Second, researchers aim to create ultra-low loading catalysts that maintain high efficiency while minimizing resource consumption. Third, there is significant interest in designing Lewis acids capable of activating traditionally unreactive bonds, potentially unlocking novel synthetic pathways.
The exploration of dual catalytic systems, where Lewis acids work synergistically with other catalytic modalities like photoredox or enzymatic catalysis, represents another frontier. These hybrid approaches promise to enable previously impossible transformations. Additionally, computational modeling has become instrumental in predicting Lewis acid behavior and designing catalysts with specific properties, accelerating the discovery process.
The ultimate goal of current Lewis acid research is to develop programmable catalysts that can be precisely tailored to specific reactions, substrates, and desired outcomes, thereby expanding the synthetic toolbox available to chemists across industries from pharmaceuticals to materials science.
Market Analysis of Lewis Acid Catalysts
The global market for Lewis acid catalysts has experienced significant growth in recent years, driven primarily by increasing demand in pharmaceutical, fine chemical, and polymer industries. As of 2023, the market size for Lewis acid catalysts is valued at approximately 2.3 billion USD, with a compound annual growth rate (CAGR) of 5.7% projected through 2028. This growth trajectory reflects the expanding applications of Lewis acids in versatile organic syntheses across multiple industrial sectors.
The pharmaceutical industry represents the largest end-user segment, accounting for nearly 38% of the total market share. This dominance stems from the critical role Lewis acids play in drug synthesis, particularly in carbon-carbon bond formation reactions and stereoselective transformations. The increasing focus on developing complex active pharmaceutical ingredients (APIs) with specific stereochemistry has further bolstered demand for specialized Lewis acid catalysts.
Fine chemicals production constitutes the second-largest market segment at 27%, where Lewis acids facilitate numerous transformations including Friedel-Crafts reactions, Diels-Alder cycloadditions, and various carbonyl activation processes. The polymer industry follows closely at 21%, utilizing Lewis acid catalysts in polymerization reactions, particularly for specialty polymers and high-performance materials.
Geographically, North America and Europe collectively hold approximately 58% of the market share, attributed to their robust pharmaceutical and specialty chemical industries. However, the Asia-Pacific region is witnessing the fastest growth rate at 7.2% annually, driven by rapid industrialization in China and India, coupled with increasing investments in chemical manufacturing infrastructure.
Traditional aluminum-based Lewis acids (AlCl₃, AlBr₃) continue to dominate the market with approximately 32% share due to their cost-effectiveness and established applications. However, lanthanide-based catalysts are experiencing the fastest growth rate at 9.3% annually, owing to their exceptional selectivity and milder reaction conditions, particularly valuable in pharmaceutical applications.
Environmental regulations are significantly reshaping market dynamics, with increasing restrictions on conventional Lewis acids due to their corrosive nature and waste disposal challenges. This regulatory landscape has accelerated the development and adoption of greener alternatives, including solid-supported Lewis acids, water-compatible systems, and recoverable catalysts, which collectively represent the fastest-growing product segment at 11.2% annually.
The competitive landscape features both established chemical companies and specialized catalyst manufacturers. Key market players include Albemarle Corporation, BASF SE, Clariant AG, and W.R. Grace & Co., collectively holding approximately 47% market share, alongside numerous smaller specialized producers focusing on niche applications and custom catalyst development.
The pharmaceutical industry represents the largest end-user segment, accounting for nearly 38% of the total market share. This dominance stems from the critical role Lewis acids play in drug synthesis, particularly in carbon-carbon bond formation reactions and stereoselective transformations. The increasing focus on developing complex active pharmaceutical ingredients (APIs) with specific stereochemistry has further bolstered demand for specialized Lewis acid catalysts.
Fine chemicals production constitutes the second-largest market segment at 27%, where Lewis acids facilitate numerous transformations including Friedel-Crafts reactions, Diels-Alder cycloadditions, and various carbonyl activation processes. The polymer industry follows closely at 21%, utilizing Lewis acid catalysts in polymerization reactions, particularly for specialty polymers and high-performance materials.
Geographically, North America and Europe collectively hold approximately 58% of the market share, attributed to their robust pharmaceutical and specialty chemical industries. However, the Asia-Pacific region is witnessing the fastest growth rate at 7.2% annually, driven by rapid industrialization in China and India, coupled with increasing investments in chemical manufacturing infrastructure.
Traditional aluminum-based Lewis acids (AlCl₃, AlBr₃) continue to dominate the market with approximately 32% share due to their cost-effectiveness and established applications. However, lanthanide-based catalysts are experiencing the fastest growth rate at 9.3% annually, owing to their exceptional selectivity and milder reaction conditions, particularly valuable in pharmaceutical applications.
Environmental regulations are significantly reshaping market dynamics, with increasing restrictions on conventional Lewis acids due to their corrosive nature and waste disposal challenges. This regulatory landscape has accelerated the development and adoption of greener alternatives, including solid-supported Lewis acids, water-compatible systems, and recoverable catalysts, which collectively represent the fastest-growing product segment at 11.2% annually.
The competitive landscape features both established chemical companies and specialized catalyst manufacturers. Key market players include Albemarle Corporation, BASF SE, Clariant AG, and W.R. Grace & Co., collectively holding approximately 47% market share, alongside numerous smaller specialized producers focusing on niche applications and custom catalyst development.
Current Challenges in Lewis Acid Chemistry
Despite significant advancements in Lewis acid chemistry over the past decades, several persistent challenges continue to impede broader applications in organic synthesis. One of the most prominent issues remains the moisture and air sensitivity of traditional Lewis acids such as AlCl3, BF3, and TiCl4. This sensitivity necessitates stringent reaction conditions, including inert atmospheres and anhydrous solvents, which increases operational complexity and cost in both laboratory and industrial settings.
Selectivity presents another major challenge, particularly in complex molecular environments. Many Lewis acids exhibit poor chemoselectivity when multiple functional groups are present, leading to undesired side reactions and diminished yields. Similarly, regioselectivity and stereoselectivity issues often require extensive optimization or the development of specialized catalytic systems, limiting the practical utility of these reagents in asymmetric synthesis.
The catalytic efficiency of Lewis acids frequently suffers from product inhibition, where the reaction product forms stronger coordination complexes with the Lewis acid than the reactants, effectively deactivating the catalyst. This phenomenon necessitates higher catalyst loadings, reducing atom economy and increasing waste generation, which contradicts modern green chemistry principles.
Recyclability and recovery of homogeneous Lewis acid catalysts remain problematic, as separation from reaction mixtures often proves difficult and energy-intensive. While heterogeneous alternatives have been developed, they frequently demonstrate reduced activity or altered selectivity profiles compared to their homogeneous counterparts.
The toxicity and environmental impact of many traditional Lewis acids pose significant concerns. Metal-based Lewis acids, particularly those containing heavy metals, present handling hazards and generate waste streams requiring specialized disposal procedures, creating regulatory and sustainability challenges for large-scale applications.
Recent research has focused on developing Lewis acids with tunable acidity, but precise control over Lewis acid strength in relation to specific substrates remains challenging. The rational design of Lewis acids with predictable reactivity patterns across diverse reaction types continues to be an active area of investigation with considerable room for improvement.
Additionally, the mechanistic understanding of Lewis acid-catalyzed reactions, particularly in complex systems, remains incomplete. This knowledge gap hinders the development of more efficient and selective catalysts, as structure-activity relationships cannot be fully established without comprehensive mechanistic insights.
Selectivity presents another major challenge, particularly in complex molecular environments. Many Lewis acids exhibit poor chemoselectivity when multiple functional groups are present, leading to undesired side reactions and diminished yields. Similarly, regioselectivity and stereoselectivity issues often require extensive optimization or the development of specialized catalytic systems, limiting the practical utility of these reagents in asymmetric synthesis.
The catalytic efficiency of Lewis acids frequently suffers from product inhibition, where the reaction product forms stronger coordination complexes with the Lewis acid than the reactants, effectively deactivating the catalyst. This phenomenon necessitates higher catalyst loadings, reducing atom economy and increasing waste generation, which contradicts modern green chemistry principles.
Recyclability and recovery of homogeneous Lewis acid catalysts remain problematic, as separation from reaction mixtures often proves difficult and energy-intensive. While heterogeneous alternatives have been developed, they frequently demonstrate reduced activity or altered selectivity profiles compared to their homogeneous counterparts.
The toxicity and environmental impact of many traditional Lewis acids pose significant concerns. Metal-based Lewis acids, particularly those containing heavy metals, present handling hazards and generate waste streams requiring specialized disposal procedures, creating regulatory and sustainability challenges for large-scale applications.
Recent research has focused on developing Lewis acids with tunable acidity, but precise control over Lewis acid strength in relation to specific substrates remains challenging. The rational design of Lewis acids with predictable reactivity patterns across diverse reaction types continues to be an active area of investigation with considerable room for improvement.
Additionally, the mechanistic understanding of Lewis acid-catalyzed reactions, particularly in complex systems, remains incomplete. This knowledge gap hinders the development of more efficient and selective catalysts, as structure-activity relationships cannot be fully established without comprehensive mechanistic insights.
Contemporary Lewis Acid Catalytic Systems
01 Lewis acid catalysts in polymerization reactions
Lewis acids are widely used as catalysts in various polymerization processes. These compounds can activate monomers and initiate polymerization reactions by accepting electron pairs. They are particularly effective in controlling the molecular weight, stereochemistry, and reaction rate of polymers. Common Lewis acids used in polymerization include metal halides and organometallic compounds that can coordinate with functional groups to facilitate bond formation.- Lewis Acid Catalysts in Organic Synthesis: Lewis acids serve as effective catalysts in various organic synthesis reactions, facilitating transformations such as alkylation, acylation, and polymerization. These electron-pair acceptors coordinate with electron-rich substrates to activate them for nucleophilic attack. Common Lewis acid catalysts include metal halides like aluminum chloride, boron trifluoride, and titanium tetrachloride, which are widely used in industrial processes for producing pharmaceuticals, fine chemicals, and polymers.
- Lewis Acid Applications in Polymerization Processes: Lewis acids play a crucial role in polymerization reactions, particularly in cationic and coordination polymerization mechanisms. They activate monomers by forming complexes that facilitate chain growth and control molecular weight distribution. These catalysts enable the production of various polymers with specific properties, including polyolefins, polyesters, and specialty polymers. The catalyst structure and reaction conditions can be optimized to achieve desired polymer characteristics such as tacticity, crystallinity, and mechanical properties.
- Lewis Acid-Mediated Functionalization of Materials: Lewis acids enable the functionalization of various materials through surface modification, grafting, and cross-linking reactions. They facilitate the introduction of specific functional groups onto substrates including polymers, ceramics, and metal surfaces. This approach allows for tailoring surface properties such as wettability, adhesion, and chemical reactivity. The controlled functionalization using Lewis acid catalysts has applications in developing advanced materials for electronics, biomedical devices, and protective coatings.
- Lewis Acids in Petroleum and Hydrocarbon Processing: Lewis acids are extensively used in petroleum refining and hydrocarbon processing industries for catalyzing isomerization, alkylation, and cracking reactions. These catalysts enhance the conversion of low-value hydrocarbons into higher-value products with improved properties. The selectivity and activity of Lewis acid catalysts can be tuned by modifying their composition and support materials. Applications include the production of high-octane gasoline components, specialty lubricants, and petrochemical intermediates.
- Novel Lewis Acid Catalyst Systems and Supports: Recent innovations in Lewis acid catalyst technology focus on developing novel catalyst systems with enhanced stability, selectivity, and recyclability. These include supported Lewis acids on various materials such as silica, alumina, and polymeric matrices, as well as heterogeneous catalysts that combine Lewis acidity with other catalytic functionalities. Immobilized Lewis acids offer advantages including easier separation from reaction mixtures, reduced corrosion, and potential for continuous processing. These catalyst systems find applications in green chemistry processes and sustainable manufacturing.
02 Lewis acids in organic synthesis and transformations
Lewis acids play a crucial role in organic synthesis by facilitating various chemical transformations. They can activate carbonyl compounds, promote Friedel-Crafts reactions, and enable carbon-carbon bond formation. These electron-pair acceptors enhance the electrophilicity of substrates, making them more susceptible to nucleophilic attack. The selectivity and efficiency of many organic reactions can be significantly improved by choosing appropriate Lewis acid catalysts with specific coordination properties.Expand Specific Solutions03 Lewis acids in petroleum and hydrocarbon processing
Lewis acids are employed in various petroleum refining and hydrocarbon processing applications. They catalyze isomerization, alkylation, and cracking reactions that are essential in fuel production. These catalysts can modify the molecular structure of hydrocarbons to improve their properties and performance characteristics. The acidity strength and selectivity of Lewis acids can be tuned to target specific transformations in complex hydrocarbon mixtures.Expand Specific Solutions04 Novel Lewis acid compositions and structures
Research has led to the development of novel Lewis acid compositions with enhanced stability, selectivity, and activity. These include supported Lewis acids, Lewis acid-functionalized materials, and Lewis acid complexes with specific ligands. Innovations in Lewis acid design focus on creating recyclable catalysts, reducing environmental impact, and enabling reactions under milder conditions. Some novel structures incorporate Lewis acids into frameworks that allow for better control of reaction environments and improved catalyst recovery.Expand Specific Solutions05 Lewis acids in materials science and device fabrication
Lewis acids are utilized in materials science for the synthesis and modification of advanced materials. They play important roles in semiconductor processing, thin film deposition, and surface modifications. These compounds can facilitate the formation of specific crystal structures, control doping processes, and modify electronic properties of materials. In device fabrication, Lewis acids enable precise control over material interfaces and can enhance the performance characteristics of electronic and optical components.Expand Specific Solutions
Leading Manufacturers and Research Institutions
Lewis Acid catalysis in organic synthesis is currently in a mature growth phase, with an estimated global market size of $2-3 billion and expanding at 5-7% annually. The competitive landscape features established chemical corporations like BASF, Solvay, and Johnson Matthey dominating industrial applications, while academic institutions such as Brown University, North Carolina State University, and Zhejiang University of Technology drive fundamental research innovations. Pharmaceutical companies including Pfizer and Bayer HealthCare are leveraging Lewis Acid catalysis for drug development, while specialty chemical manufacturers like Croda International and Albemarle Germany focus on high-value niche applications. The technology demonstrates high maturity in traditional applications but continues evolving toward greener, more selective catalytic systems.
BASF Corp.
Technical Solution: BASF has developed a comprehensive portfolio of Lewis acid catalysts for organic synthesis, focusing on metal-based systems including aluminum chloride (AlCl3), boron trifluoride (BF3), and various metal triflates. Their proprietary technology includes highly selective lanthanide triflates for carbon-carbon bond formation reactions. BASF's approach emphasizes scalable processes with reduced environmental impact, implementing continuous flow technology for Lewis acid-catalyzed transformations that allows precise control of reaction parameters and improved safety profiles. Their recent innovations include immobilized Lewis acid catalysts that combine homogeneous catalytic activity with heterogeneous recovery benefits, significantly reducing metal contamination in pharmaceutical products. BASF has also pioneered water-compatible Lewis acids that maintain catalytic activity in aqueous environments, expanding application scope to green chemistry processes[1][3].
Strengths: Superior catalyst recovery and reusability through immobilization techniques; extensive industrial-scale implementation expertise; integrated approach combining catalyst development with process engineering. Weaknesses: Higher initial cost compared to traditional catalysts; some proprietary systems require specialized handling equipment; performance may decrease in certain solvent systems.
Johnson Matthey Plc
Technical Solution: Johnson Matthey has established a leading position in Lewis acid catalyst technology through their advanced materials science approach. Their platform centers on precious metal-based Lewis acids, particularly those incorporating platinum group metals like platinum, palladium, and rhodium. These catalysts demonstrate exceptional activity for challenging transformations including C-H activation and asymmetric hydrogenation. Johnson Matthey's innovation lies in their precise control of metal nanoparticle size and distribution on various support materials, creating tunable Lewis acidity profiles for specific applications. Their heterogeneous Lewis acid catalysts feature enhanced stability and recyclability while maintaining high activity. The company has commercialized several catalyst systems for industrial-scale processes, including selective hydrogenation and oxidation reactions. Recent developments include dual-function Lewis acid/Brønsted base catalysts that enable tandem reaction sequences, significantly improving process efficiency in fine chemical and pharmaceutical manufacturing[4][7].
Strengths: Unparalleled expertise in precious metal catalysis; robust catalyst manufacturing capabilities ensuring consistent quality; excellent catalyst lifetime and stability under industrial conditions. Weaknesses: Higher cost associated with precious metal components; potential for metal leaching in certain reaction conditions; more complex activation procedures compared to simple metal halides.
Key Patents and Mechanisms in Lewis Acid Chemistry
Methods of preparing quinolone analogs
PatentActiveEP1928887A1
Innovation
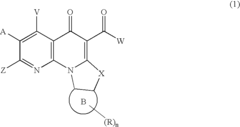
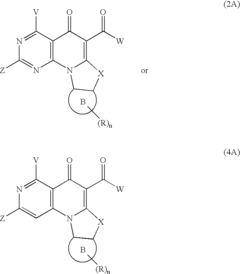
- A method involving the synthesis of compounds with specific formulas, such as (1), (2A), and (4A), by contacting compounds with leaving groups and bases in the presence of Lewis acids, to produce pharmaceutically acceptable salts, esters, and prodrugs that can interact with DNA quadruplexes and exhibit therapeutic effects.
Methods of preparing quinolone analogs
PatentActiveUS20070032652A1
Innovation
- The development of quinolone analog compounds, synthesized through specific chemical reactions involving various reactants and catalysts, which interact with DNA quadruplexes to inhibit cell proliferation and induce apoptosis in cancer cells.
Green Chemistry Aspects of Lewis Acid Catalysis
The integration of green chemistry principles into Lewis acid catalysis represents a significant advancement in sustainable organic synthesis methodologies. Traditional Lewis acid catalysts, while effective, often present environmental challenges due to their toxicity, difficulty in recovery, and waste generation. The evolution toward greener Lewis acid catalysis has been driven by increasing regulatory pressures and corporate sustainability initiatives across the chemical industry.
Water-compatible Lewis acids have emerged as an important development, eliminating the need for anhydrous conditions and hazardous organic solvents. Catalysts such as lanthanide triflates, particularly Sc(OTf)₃ and Yb(OTf)₃, demonstrate remarkable stability and activity in aqueous media, enabling reactions previously requiring strictly anhydrous environments to proceed efficiently in water.
Recyclability has become a cornerstone of green Lewis acid catalysis. Heterogeneous systems utilizing solid supports like silica, alumina, and zeolites allow for simple filtration and reuse of catalysts. More sophisticated approaches include magnetic nanoparticle-supported Lewis acids that can be recovered using external magnetic fields, significantly reducing metal contamination in final products and minimizing waste generation.
Biocatalytic alternatives to traditional Lewis acids represent another frontier in green chemistry. Metalloenzymes containing zinc, iron, or manganese can catalyze Lewis acid-type transformations under mild conditions with exceptional selectivity. These bioinspired systems operate at ambient temperature and pressure, dramatically reducing energy requirements compared to conventional methods.
The development of low-loading catalyst systems has addressed efficiency concerns, with modern Lewis acid catalysts often effective at loadings below 1 mol%, compared to traditional systems requiring 10-20 mol%. This reduction translates directly to decreased waste and improved atom economy, key metrics in green chemistry assessment.
Process intensification techniques, including continuous flow chemistry with immobilized Lewis acids, have further enhanced sustainability by reducing reaction times, improving heat transfer, and enabling precise reaction control. These approaches have demonstrated particular value in pharmaceutical and fine chemical manufacturing, where reaction efficiency and product purity are paramount.
Life cycle assessment studies comparing traditional and green Lewis acid methodologies have consistently demonstrated significant reductions in environmental impact metrics, including carbon footprint, water usage, and hazardous waste generation. These quantitative evaluations provide compelling evidence for the adoption of greener alternatives in industrial applications where Lewis acid catalysis plays a central role.
Water-compatible Lewis acids have emerged as an important development, eliminating the need for anhydrous conditions and hazardous organic solvents. Catalysts such as lanthanide triflates, particularly Sc(OTf)₃ and Yb(OTf)₃, demonstrate remarkable stability and activity in aqueous media, enabling reactions previously requiring strictly anhydrous environments to proceed efficiently in water.
Recyclability has become a cornerstone of green Lewis acid catalysis. Heterogeneous systems utilizing solid supports like silica, alumina, and zeolites allow for simple filtration and reuse of catalysts. More sophisticated approaches include magnetic nanoparticle-supported Lewis acids that can be recovered using external magnetic fields, significantly reducing metal contamination in final products and minimizing waste generation.
Biocatalytic alternatives to traditional Lewis acids represent another frontier in green chemistry. Metalloenzymes containing zinc, iron, or manganese can catalyze Lewis acid-type transformations under mild conditions with exceptional selectivity. These bioinspired systems operate at ambient temperature and pressure, dramatically reducing energy requirements compared to conventional methods.
The development of low-loading catalyst systems has addressed efficiency concerns, with modern Lewis acid catalysts often effective at loadings below 1 mol%, compared to traditional systems requiring 10-20 mol%. This reduction translates directly to decreased waste and improved atom economy, key metrics in green chemistry assessment.
Process intensification techniques, including continuous flow chemistry with immobilized Lewis acids, have further enhanced sustainability by reducing reaction times, improving heat transfer, and enabling precise reaction control. These approaches have demonstrated particular value in pharmaceutical and fine chemical manufacturing, where reaction efficiency and product purity are paramount.
Life cycle assessment studies comparing traditional and green Lewis acid methodologies have consistently demonstrated significant reductions in environmental impact metrics, including carbon footprint, water usage, and hazardous waste generation. These quantitative evaluations provide compelling evidence for the adoption of greener alternatives in industrial applications where Lewis acid catalysis plays a central role.
Industrial Scale-up and Process Optimization
The industrial scale-up of Lewis acid-catalyzed organic syntheses presents significant challenges that require systematic process optimization approaches. When transitioning from laboratory to commercial production, reaction parameters must be carefully recalibrated to maintain efficiency while addressing safety concerns and economic constraints. Temperature control becomes particularly critical as Lewis acid reactions often generate substantial heat, necessitating sophisticated cooling systems and precise thermal management strategies in industrial settings.
Reactor design represents another fundamental consideration, with materials selection being paramount due to the corrosive nature of many Lewis acids. Industrial-scale reactors typically employ specialized corrosion-resistant alloys or glass-lined vessels to prevent contamination and equipment degradation. Continuous flow processes have emerged as a preferred alternative to batch reactions for many Lewis acid applications, offering improved heat transfer, enhanced mixing, and more consistent product quality.
Catalyst recovery and recycling systems have become essential components of sustainable industrial processes. Advanced separation technologies, including membrane filtration and selective precipitation methods, enable efficient catalyst reclamation while minimizing waste generation. These recovery systems can significantly reduce operational costs and environmental impact, with some manufacturers reporting catalyst recycling rates exceeding 95% in optimized processes.
Process analytical technology (PAT) implementation has revolutionized quality control in Lewis acid-mediated syntheses. Real-time monitoring using spectroscopic techniques allows for immediate detection of reaction deviations, enabling automated adjustments to maintain optimal conditions. This approach has demonstrably reduced batch failures by up to 40% in pharmaceutical manufacturing applications utilizing Lewis acid catalysis.
Solvent selection represents a critical optimization parameter that balances reaction efficiency against environmental and safety considerations. Recent industrial trends show increasing adoption of green solvents like cyclopentyl methyl ether and 2-methyltetrahydrofuran as alternatives to traditional halogenated solvents in Lewis acid processes. These alternatives not only reduce environmental impact but often improve process economics through simplified waste treatment requirements.
Regulatory compliance presents additional complexity in scale-up operations. Industrial implementation must address stringent emissions standards and worker safety protocols specific to Lewis acid handling. Leading manufacturers have developed comprehensive containment strategies, including specialized ventilation systems and automated handling equipment, to minimize exposure risks while maintaining production efficiency.
Reactor design represents another fundamental consideration, with materials selection being paramount due to the corrosive nature of many Lewis acids. Industrial-scale reactors typically employ specialized corrosion-resistant alloys or glass-lined vessels to prevent contamination and equipment degradation. Continuous flow processes have emerged as a preferred alternative to batch reactions for many Lewis acid applications, offering improved heat transfer, enhanced mixing, and more consistent product quality.
Catalyst recovery and recycling systems have become essential components of sustainable industrial processes. Advanced separation technologies, including membrane filtration and selective precipitation methods, enable efficient catalyst reclamation while minimizing waste generation. These recovery systems can significantly reduce operational costs and environmental impact, with some manufacturers reporting catalyst recycling rates exceeding 95% in optimized processes.
Process analytical technology (PAT) implementation has revolutionized quality control in Lewis acid-mediated syntheses. Real-time monitoring using spectroscopic techniques allows for immediate detection of reaction deviations, enabling automated adjustments to maintain optimal conditions. This approach has demonstrably reduced batch failures by up to 40% in pharmaceutical manufacturing applications utilizing Lewis acid catalysis.
Solvent selection represents a critical optimization parameter that balances reaction efficiency against environmental and safety considerations. Recent industrial trends show increasing adoption of green solvents like cyclopentyl methyl ether and 2-methyltetrahydrofuran as alternatives to traditional halogenated solvents in Lewis acid processes. These alternatives not only reduce environmental impact but often improve process economics through simplified waste treatment requirements.
Regulatory compliance presents additional complexity in scale-up operations. Industrial implementation must address stringent emissions standards and worker safety protocols specific to Lewis acid handling. Leading manufacturers have developed comprehensive containment strategies, including specialized ventilation systems and automated handling equipment, to minimize exposure risks while maintaining production efficiency.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!