Lewis Acid Mechanism in Organic Transformations
AUG 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lewis Acid Catalysis Background and Objectives
Lewis acid catalysis has evolved significantly since the pioneering work of Gilbert N. Lewis, who first conceptualized the electron pair theory in 1923. This fundamental concept defined Lewis acids as electron pair acceptors, establishing a theoretical framework that has guided organic synthesis for nearly a century. The evolution of Lewis acid catalysis has been marked by progressive refinements in understanding reaction mechanisms, catalyst design, and application scope, transforming from simple metal halides to sophisticated chiral complexes capable of highly selective transformations.
The field experienced its first major expansion during the 1950s-1970s with the application of aluminum-based Lewis acids in Friedel-Crafts reactions. Subsequently, the 1980s-1990s witnessed the emergence of lanthanide-based Lewis acids, particularly for asymmetric synthesis. The 21st century has brought unprecedented growth with the development of designer Lewis acids featuring tailored electronic and steric properties for specific transformations.
Current technological trends in Lewis acid catalysis include the development of water-tolerant Lewis acids, bifunctional catalytic systems combining Lewis acidity with other activation modes, and heterogeneous Lewis acid catalysts for sustainable chemistry applications. The integration of computational methods for catalyst design and mechanistic studies represents another significant advancement, enabling rational catalyst development with predictable reactivity patterns.
The primary objective of this technical research is to comprehensively analyze the mechanistic aspects of Lewis acid-mediated organic transformations, with particular emphasis on understanding the electronic and structural factors that govern reactivity and selectivity. This includes elucidating coordination geometries, orbital interactions, and transition state arrangements that determine reaction outcomes.
Secondary objectives include identifying emerging Lewis acid catalysts with enhanced performance characteristics, evaluating sustainable approaches to Lewis acid catalysis, and exploring novel reaction pathways enabled by Lewis acid activation. The research aims to bridge fundamental mechanistic understanding with practical applications, providing insights that can guide the development of more efficient and selective catalytic systems.
The ultimate goal is to establish predictive models for Lewis acid-substrate interactions that can facilitate the rational design of catalysts for challenging transformations, particularly in the areas of C-C bond formation, asymmetric synthesis, and late-stage functionalization of complex molecules. This research has significant implications for pharmaceutical development, materials science, and fine chemical production, where selective transformations under mild conditions remain highly desirable.
The field experienced its first major expansion during the 1950s-1970s with the application of aluminum-based Lewis acids in Friedel-Crafts reactions. Subsequently, the 1980s-1990s witnessed the emergence of lanthanide-based Lewis acids, particularly for asymmetric synthesis. The 21st century has brought unprecedented growth with the development of designer Lewis acids featuring tailored electronic and steric properties for specific transformations.
Current technological trends in Lewis acid catalysis include the development of water-tolerant Lewis acids, bifunctional catalytic systems combining Lewis acidity with other activation modes, and heterogeneous Lewis acid catalysts for sustainable chemistry applications. The integration of computational methods for catalyst design and mechanistic studies represents another significant advancement, enabling rational catalyst development with predictable reactivity patterns.
The primary objective of this technical research is to comprehensively analyze the mechanistic aspects of Lewis acid-mediated organic transformations, with particular emphasis on understanding the electronic and structural factors that govern reactivity and selectivity. This includes elucidating coordination geometries, orbital interactions, and transition state arrangements that determine reaction outcomes.
Secondary objectives include identifying emerging Lewis acid catalysts with enhanced performance characteristics, evaluating sustainable approaches to Lewis acid catalysis, and exploring novel reaction pathways enabled by Lewis acid activation. The research aims to bridge fundamental mechanistic understanding with practical applications, providing insights that can guide the development of more efficient and selective catalytic systems.
The ultimate goal is to establish predictive models for Lewis acid-substrate interactions that can facilitate the rational design of catalysts for challenging transformations, particularly in the areas of C-C bond formation, asymmetric synthesis, and late-stage functionalization of complex molecules. This research has significant implications for pharmaceutical development, materials science, and fine chemical production, where selective transformations under mild conditions remain highly desirable.
Market Applications of Lewis Acid Chemistry
Lewis acid chemistry has established itself as a cornerstone technology across multiple industrial sectors, with market applications continuing to expand due to its versatility in facilitating organic transformations. The pharmaceutical industry represents the largest market segment, where Lewis acid catalysts enable critical reactions in the synthesis of active pharmaceutical ingredients (APIs). This sector values Lewis acid chemistry for its ability to achieve high stereoselectivity and regioselectivity in complex molecule synthesis, directly impacting drug efficacy and safety profiles.
The fine chemicals industry constitutes another significant market, utilizing Lewis acid catalysis in the production of specialty chemicals, fragrances, and flavors. Companies like BASF, Dow Chemical, and Solvay have integrated Lewis acid technologies into their manufacturing processes to improve yield and reduce waste, aligning with green chemistry principles that are increasingly demanded by consumers and regulators.
Polymer chemistry represents a rapidly growing application area, where Lewis acids facilitate polymerization reactions for materials used in electronics, automotive components, and consumer goods. The market for biodegradable polymers, in particular, has seen substantial growth, with Lewis acid catalysts playing a crucial role in developing sustainable alternatives to traditional plastics.
The agrochemical sector employs Lewis acid chemistry in the synthesis of pesticides, herbicides, and fertilizers, contributing to improved agricultural productivity. This market segment is projected to expand as global food security concerns intensify and sustainable farming practices gain prominence.
In the energy sector, Lewis acids are finding applications in catalytic processes for biofuel production and in the development of more efficient energy storage materials. The push toward renewable energy sources has created new opportunities for Lewis acid chemistry in catalyst design for conversion of biomass to usable fuels.
The electronics industry utilizes Lewis acid chemistry in semiconductor manufacturing and in the production of advanced materials for displays and sensors. As devices continue to miniaturize and performance demands increase, the precision offered by Lewis acid-mediated reactions becomes increasingly valuable.
Cosmetics and personal care products represent an emerging market for Lewis acid applications, particularly in the synthesis of novel ingredients with enhanced properties. Consumer demand for natural yet effective products has driven innovation in this space, with Lewis acid chemistry enabling the modification of natural compounds to improve stability and efficacy.
The global market value for Lewis acid catalysts was estimated at approximately $2.3 billion in 2022, with projected annual growth rates of 5-7% through 2028, underscoring the expanding commercial significance of this chemistry across diverse industrial applications.
The fine chemicals industry constitutes another significant market, utilizing Lewis acid catalysis in the production of specialty chemicals, fragrances, and flavors. Companies like BASF, Dow Chemical, and Solvay have integrated Lewis acid technologies into their manufacturing processes to improve yield and reduce waste, aligning with green chemistry principles that are increasingly demanded by consumers and regulators.
Polymer chemistry represents a rapidly growing application area, where Lewis acids facilitate polymerization reactions for materials used in electronics, automotive components, and consumer goods. The market for biodegradable polymers, in particular, has seen substantial growth, with Lewis acid catalysts playing a crucial role in developing sustainable alternatives to traditional plastics.
The agrochemical sector employs Lewis acid chemistry in the synthesis of pesticides, herbicides, and fertilizers, contributing to improved agricultural productivity. This market segment is projected to expand as global food security concerns intensify and sustainable farming practices gain prominence.
In the energy sector, Lewis acids are finding applications in catalytic processes for biofuel production and in the development of more efficient energy storage materials. The push toward renewable energy sources has created new opportunities for Lewis acid chemistry in catalyst design for conversion of biomass to usable fuels.
The electronics industry utilizes Lewis acid chemistry in semiconductor manufacturing and in the production of advanced materials for displays and sensors. As devices continue to miniaturize and performance demands increase, the precision offered by Lewis acid-mediated reactions becomes increasingly valuable.
Cosmetics and personal care products represent an emerging market for Lewis acid applications, particularly in the synthesis of novel ingredients with enhanced properties. Consumer demand for natural yet effective products has driven innovation in this space, with Lewis acid chemistry enabling the modification of natural compounds to improve stability and efficacy.
The global market value for Lewis acid catalysts was estimated at approximately $2.3 billion in 2022, with projected annual growth rates of 5-7% through 2028, underscoring the expanding commercial significance of this chemistry across diverse industrial applications.
Current Challenges in Lewis Acid Catalysis
Despite significant advancements in Lewis acid catalysis over recent decades, several persistent challenges continue to impede broader industrial application and further scientific development. One of the most significant obstacles remains catalyst selectivity, particularly in complex molecular environments with multiple potential coordination sites. Current Lewis acid catalysts often exhibit poor discrimination between similar functional groups, leading to undesired side reactions and diminished yields in organic transformations.
Water and oxygen sensitivity presents another major hurdle for many Lewis acid systems. Traditional Lewis acids such as aluminum chloride, boron trifluoride, and titanium tetrachloride rapidly decompose upon exposure to moisture, necessitating strictly anhydrous conditions that complicate scale-up and increase operational costs in industrial settings. This sensitivity severely limits their practical utility in many commercial applications.
Catalyst loading requirements remain problematically high for numerous Lewis acid-catalyzed transformations. Many systems require stoichiometric or near-stoichiometric quantities of the Lewis acid, contradicting principles of atom economy and green chemistry. This inefficiency translates directly to increased waste generation and higher production costs, making many processes economically unviable at commercial scale.
The recovery and recycling of homogeneous Lewis acid catalysts continue to pose significant technical challenges. Most conventional systems suffer from difficult separation procedures, catalyst degradation during workup, and substantial activity loss upon recycling attempts. This limitation stands in stark contrast to the growing industrial demand for sustainable catalytic processes with minimal environmental impact.
Mechanistic understanding remains incomplete for many Lewis acid-catalyzed transformations. The precise nature of coordination events, transition states, and reaction intermediates is often poorly characterized, hampering rational catalyst design efforts. This knowledge gap is particularly pronounced for reactions involving complex substrates or multiple mechanistic pathways.
Computational prediction tools for Lewis acid catalyst performance still lack sufficient accuracy for reliable application in catalyst development. Current models struggle to account for solvent effects, entropic contributions, and dynamic structural changes during catalysis, limiting their predictive power for novel catalyst systems.
Lastly, the development of chiral Lewis acids for asymmetric transformations continues to face challenges in achieving high enantioselectivity across diverse substrate classes. While impressive results have been achieved for specific reactions, broadly applicable chiral Lewis acid platforms remain elusive, particularly for challenging bond formations beyond conventional carbon-carbon coupling reactions.
Water and oxygen sensitivity presents another major hurdle for many Lewis acid systems. Traditional Lewis acids such as aluminum chloride, boron trifluoride, and titanium tetrachloride rapidly decompose upon exposure to moisture, necessitating strictly anhydrous conditions that complicate scale-up and increase operational costs in industrial settings. This sensitivity severely limits their practical utility in many commercial applications.
Catalyst loading requirements remain problematically high for numerous Lewis acid-catalyzed transformations. Many systems require stoichiometric or near-stoichiometric quantities of the Lewis acid, contradicting principles of atom economy and green chemistry. This inefficiency translates directly to increased waste generation and higher production costs, making many processes economically unviable at commercial scale.
The recovery and recycling of homogeneous Lewis acid catalysts continue to pose significant technical challenges. Most conventional systems suffer from difficult separation procedures, catalyst degradation during workup, and substantial activity loss upon recycling attempts. This limitation stands in stark contrast to the growing industrial demand for sustainable catalytic processes with minimal environmental impact.
Mechanistic understanding remains incomplete for many Lewis acid-catalyzed transformations. The precise nature of coordination events, transition states, and reaction intermediates is often poorly characterized, hampering rational catalyst design efforts. This knowledge gap is particularly pronounced for reactions involving complex substrates or multiple mechanistic pathways.
Computational prediction tools for Lewis acid catalyst performance still lack sufficient accuracy for reliable application in catalyst development. Current models struggle to account for solvent effects, entropic contributions, and dynamic structural changes during catalysis, limiting their predictive power for novel catalyst systems.
Lastly, the development of chiral Lewis acids for asymmetric transformations continues to face challenges in achieving high enantioselectivity across diverse substrate classes. While impressive results have been achieved for specific reactions, broadly applicable chiral Lewis acid platforms remain elusive, particularly for challenging bond formations beyond conventional carbon-carbon coupling reactions.
Contemporary Lewis Acid Catalytic Systems
01 Lewis acid catalysis in organic synthesis
Lewis acids serve as powerful catalysts in various organic synthesis reactions by accepting electron pairs from substrates. These catalysts facilitate reactions such as alkylation, acylation, and polymerization by activating functional groups through coordination. The electron-deficient nature of Lewis acids enables them to form complexes with electron-rich substrates, lowering activation energies and promoting selective transformations. Common Lewis acids used include aluminum, boron, and titanium compounds, which can be tailored for specific reaction requirements.- Lewis acid catalysis in organic synthesis: Lewis acids serve as catalysts in various organic synthesis reactions by accepting electron pairs from substrates. These catalysts facilitate reactions such as alkylation, acylation, and cyclization by activating functional groups through coordination. The mechanism typically involves the Lewis acid forming a complex with electron-rich sites of reactants, making them more susceptible to nucleophilic attack. This catalytic approach enables more efficient and selective transformations under milder conditions than traditional methods.
- Lewis acid-mediated polymerization mechanisms: Lewis acids play a crucial role in polymerization processes by initiating and controlling chain growth reactions. In cationic polymerization, Lewis acids generate carbocations that propagate polymer chain formation. These catalysts can influence polymer properties including molecular weight distribution, stereochemistry, and branching patterns. The mechanism typically involves coordination with monomers, followed by electron rearrangement that facilitates bond formation. This approach is particularly valuable for polymerizing vinyl monomers and ring-opening polymerization of cyclic compounds.
- Lewis acid catalysts in petroleum and hydrocarbon processing: Lewis acids are employed in petroleum refining and hydrocarbon transformation processes. These catalysts facilitate isomerization, alkylation, cracking, and reforming reactions by interacting with hydrocarbon substrates. The mechanism involves the Lewis acid abstracting hydride ions or coordinating with π-bonds to generate reactive intermediates. This catalytic approach enables the conversion of low-value hydrocarbons into higher-value products under controlled conditions. The selectivity and activity of these processes can be tuned by modifying the Lewis acid strength and reaction parameters.
- Metal-based Lewis acid catalyst systems: Metal-based compounds function as Lewis acids in various chemical transformations. Transition metals, lanthanides, and main group metals with vacant orbitals can accept electron pairs from substrates. These catalysts often feature ligands that modify their electronic and steric properties, influencing reactivity and selectivity. The mechanism typically involves coordination of the substrate to the metal center, followed by activation for subsequent reaction steps. These catalyst systems can be homogeneous or heterogeneous, with the latter offering advantages in separation and recyclability.
- Lewis acid-base interactions in material science: Lewis acid-base interactions are fundamental in developing advanced materials with specific properties. These interactions govern surface chemistry, adhesion, and interfacial phenomena in composite materials. The mechanism involves electron pair donation from Lewis bases to Lewis acid sites, creating bonds that influence material characteristics. This principle is applied in designing catalytic supports, adsorbents, sensors, and functional coatings. Understanding these interactions enables the creation of materials with tailored surface properties, controlled porosity, and enhanced stability for various applications.
02 Lewis acid-mediated polymerization mechanisms
Lewis acids play a crucial role in polymerization processes by initiating and controlling chain growth reactions. These catalysts activate monomers through coordination, facilitating the formation of reactive intermediates that propagate polymer chains. The mechanism typically involves the Lewis acid coordinating with functional groups in the monomer, creating a polarized complex that becomes susceptible to nucleophilic attack. This mechanism is particularly important in the production of various polymers including polyolefins, polyesters, and specialty polymers with controlled molecular weight and stereochemistry.Expand Specific Solutions03 Lewis acid-base interactions in metal-organic frameworks
Metal-organic frameworks (MOFs) utilize Lewis acid-base interactions as fundamental principles in their structure and function. The metal centers in MOFs act as Lewis acids, coordinating with organic linkers that serve as Lewis bases. These interactions create three-dimensional networks with unique properties for applications in catalysis, gas storage, and separation. The strength and selectivity of these Lewis acid sites can be tuned by modifying the metal centers or the coordination environment, allowing for customized materials with specific catalytic activities or adsorption properties.Expand Specific Solutions04 Lewis acid mechanisms in petroleum refining processes
Lewis acids are extensively employed in petroleum refining processes, particularly in catalytic cracking, isomerization, and alkylation reactions. These catalysts interact with hydrocarbons by accepting electron pairs from C-C or C-H bonds, facilitating bond cleavage and rearrangement. The mechanism typically involves the formation of carbocation intermediates through Lewis acid coordination, followed by subsequent reactions that lead to desired petroleum products. Various metal halides and supported metal catalysts function as Lewis acids in these processes, enabling more efficient conversion of crude oil fractions into valuable fuels and petrochemicals.Expand Specific Solutions05 Novel Lewis acid catalysts and synthetic applications
Recent developments in Lewis acid chemistry have led to the creation of novel catalysts with enhanced selectivity, activity, and environmental compatibility. These include chiral Lewis acids for asymmetric synthesis, supported Lewis acids for heterogeneous catalysis, and Lewis acid-surfactant combined catalysts for aqueous reactions. The mechanisms involve precise coordination geometries that enable stereoselective transformations and regioselective functionalization of complex molecules. These advanced Lewis acid systems have found applications in pharmaceutical synthesis, fine chemical production, and green chemistry processes, offering improved efficiency and reduced environmental impact compared to traditional catalysts.Expand Specific Solutions
Leading Research Groups and Industrial Players
The Lewis Acid Mechanism in Organic Transformations field is currently in a mature development stage with established fundamental principles, yet continues to evolve with new catalytic applications. The global market for Lewis acid catalysts is estimated at $1.2-1.5 billion, growing steadily at 4-5% annually, driven by pharmaceutical and fine chemical industries. Leading companies demonstrate varying levels of technical sophistication: Johnson Matthey, Merck Patent GmbH, and Wanhua Chemical have developed proprietary Lewis acid catalysts with high selectivity; while academic institutions like University of California and Zhejiang University of Technology focus on novel mechanistic insights. Japan Science & Technology Agency and Sumitomo Chemical are advancing green chemistry applications, while China Petroleum & Chemical Corp leverages Lewis acid catalysis for large-scale petrochemical processes.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed advanced Lewis acid catalyst systems primarily focused on petrochemical transformations and hydrocarbon upgrading processes. Their technology centers on modified zeolite-based solid Lewis acids that combine strong acidity with shape-selective properties for enhanced reaction control[1]. Sinopec has pioneered the use of metal-organic framework (MOF) derived Lewis acid catalysts containing zirconium, aluminum, and tin centers that demonstrate exceptional activity in alkylation, isomerization, and oligomerization reactions critical to petroleum refining[3]. Their proprietary catalyst preparation methods include controlled dealumination techniques that create isolated Lewis acid sites with tunable strength and density. Sinopec has successfully implemented these catalysts in industrial-scale alkylation units, achieving higher octane products with reduced catalyst consumption compared to conventional processes[6]. Recent innovations include bifunctional Lewis acid catalysts that combine acidic and redox properties for one-pot cascade reactions, enabling more efficient production routes for high-value petrochemical intermediates with reduced energy consumption and improved carbon efficiency.
Strengths: Exceptional thermal and hydrothermal stability suitable for harsh industrial conditions; lower catalyst replacement frequency in continuous operations; ability to process complex feedstocks with impurities. Weaknesses: Lower selectivity in fine chemical applications compared to homogeneous systems; higher mass transfer limitations due to heterogeneous nature; more energy-intensive regeneration processes required.
Johnson Matthey Plc
Technical Solution: Johnson Matthey has developed sophisticated Lewis acid catalyst technologies focusing on precious metal-based systems for selective organic transformations. Their approach centers on platinum group metal (PGM) Lewis acids, particularly those based on platinum, palladium, and rhodium, which offer unique reactivity profiles compared to traditional main group Lewis acids[1]. Johnson Matthey's catalysts feature carefully designed ligand environments that modulate Lewis acidity while introducing secondary coordination spheres that enhance substrate orientation and reaction selectivity. Their technology includes heterogeneous PGM Lewis acid catalysts supported on functionalized carbon materials that maintain high activity while enabling simple catalyst separation and recycling in pharmaceutical and fine chemical applications[3]. Johnson Matthey has pioneered the development of flow chemistry platforms utilizing their Lewis acid catalysts, achieving significant process intensification for challenging transformations including asymmetric hydrogenations and C-H activations[6]. Recent innovations include bimetallic Lewis acid systems that combine traditional Lewis acidic centers with PGM sites, enabling tandem catalytic processes that reduce synthetic steps and improve overall efficiency in complex molecule synthesis.
Strengths: Exceptional chemoselectivity in multifunctional molecule transformations; lower catalyst loadings than traditional Lewis acids; compatibility with continuous manufacturing technologies. Weaknesses: Higher raw material costs due to precious metal content; more complex catalyst preparation and quality control requirements; potential for metal contamination in pharmaceutical products requiring additional purification steps.
Key Mechanistic Insights and Breakthroughs
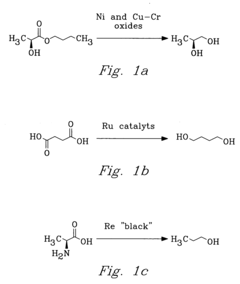
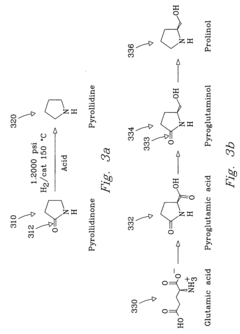
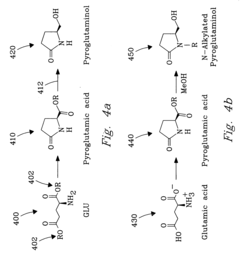
Process for chemical reaction of amino acids and amides yielding selective conversion products
PatentInactiveUS20040225133A1
Innovation
- The process involves selecting starting materials such as L-glutamic acid and L-pyroglutamic acid, reacting them under controlled conditions with precious-metal catalysts at elevated temperatures and specific pH levels, and using a reduction catalyst to achieve high yields and selectivity in the formation of desirable products.
Green Chemistry Aspects of Lewis Acid Catalysis
The integration of Lewis acid catalysis with green chemistry principles represents a significant advancement in sustainable organic synthesis. Traditional Lewis acid catalysts, often based on metal halides like AlCl3, BF3, and TiCl4, have been fundamental in numerous organic transformations but typically present environmental challenges including toxicity, difficulty in recovery, and waste generation.
Recent developments have focused on designing environmentally benign Lewis acid catalysts that maintain high catalytic efficiency while reducing ecological impact. Water-compatible Lewis acids, such as lanthanide triflates and scandium-based catalysts, have emerged as promising alternatives that enable reactions in aqueous media, eliminating the need for hazardous organic solvents and improving reaction safety profiles.
Recyclable heterogeneous Lewis acid catalysts represent another significant advancement in green chemistry. These include clay-supported catalysts, zeolites, and metal-organic frameworks (MOFs) that offer the advantage of easy separation from reaction mixtures and potential reuse across multiple reaction cycles, substantially reducing waste generation and resource consumption in industrial processes.
Biocatalytic alternatives to traditional Lewis acids have also gained attention, with enzymes capable of catalyzing Lewis acid-type reactions under mild conditions. These biological catalysts operate at ambient temperatures and pressures, often in aqueous environments, dramatically reducing energy requirements and environmental impact compared to conventional methods.
The development of solvent-free Lewis acid catalysis techniques further aligns with green chemistry objectives by eliminating reaction media altogether. Mechanochemical approaches and neat reaction conditions have demonstrated success in various transformations, offering pathways to reduced waste and energy consumption while maintaining synthetic utility.
Life cycle assessment studies of modern Lewis acid catalytic processes reveal significant improvements in environmental metrics compared to traditional methods. Reduced E-factors (environmental impact factors), lower carbon footprints, and decreased energy consumption highlight the ecological benefits of green Lewis acid catalysis approaches in industrial applications.
Future directions in this field include the development of bio-derived Lewis acids from renewable resources, further optimization of catalyst recovery systems, and the integration of continuous flow technologies to enhance efficiency and minimize waste in large-scale applications. These advancements continue to bridge the gap between synthetic utility and environmental responsibility in organic chemistry.
Recent developments have focused on designing environmentally benign Lewis acid catalysts that maintain high catalytic efficiency while reducing ecological impact. Water-compatible Lewis acids, such as lanthanide triflates and scandium-based catalysts, have emerged as promising alternatives that enable reactions in aqueous media, eliminating the need for hazardous organic solvents and improving reaction safety profiles.
Recyclable heterogeneous Lewis acid catalysts represent another significant advancement in green chemistry. These include clay-supported catalysts, zeolites, and metal-organic frameworks (MOFs) that offer the advantage of easy separation from reaction mixtures and potential reuse across multiple reaction cycles, substantially reducing waste generation and resource consumption in industrial processes.
Biocatalytic alternatives to traditional Lewis acids have also gained attention, with enzymes capable of catalyzing Lewis acid-type reactions under mild conditions. These biological catalysts operate at ambient temperatures and pressures, often in aqueous environments, dramatically reducing energy requirements and environmental impact compared to conventional methods.
The development of solvent-free Lewis acid catalysis techniques further aligns with green chemistry objectives by eliminating reaction media altogether. Mechanochemical approaches and neat reaction conditions have demonstrated success in various transformations, offering pathways to reduced waste and energy consumption while maintaining synthetic utility.
Life cycle assessment studies of modern Lewis acid catalytic processes reveal significant improvements in environmental metrics compared to traditional methods. Reduced E-factors (environmental impact factors), lower carbon footprints, and decreased energy consumption highlight the ecological benefits of green Lewis acid catalysis approaches in industrial applications.
Future directions in this field include the development of bio-derived Lewis acids from renewable resources, further optimization of catalyst recovery systems, and the integration of continuous flow technologies to enhance efficiency and minimize waste in large-scale applications. These advancements continue to bridge the gap between synthetic utility and environmental responsibility in organic chemistry.
Computational Approaches to Lewis Acid Mechanisms
Computational approaches have revolutionized the study of Lewis acid mechanisms in organic transformations, providing unprecedented insights into reaction pathways and energetics. Density Functional Theory (DFT) calculations have emerged as the predominant computational method, offering an optimal balance between accuracy and computational cost. These calculations enable researchers to determine activation energies, transition state geometries, and reaction coordinates for Lewis acid-catalyzed processes, thereby elucidating mechanistic details that would be challenging to observe experimentally.
Molecular dynamics (MD) simulations complement static DFT calculations by incorporating temperature effects and conformational flexibility. This approach is particularly valuable for understanding Lewis acid interactions in solution, where solvent effects and dynamic rearrangements significantly influence reaction outcomes. Advanced MD techniques such as metadynamics and umbrella sampling allow for the exploration of rare events and free energy landscapes associated with Lewis acid-substrate interactions.
Machine learning algorithms have recently been integrated with computational chemistry to accelerate the discovery and optimization of Lewis acid catalysts. These algorithms can identify patterns in reaction data, predict catalytic activity, and suggest novel Lewis acid structures with enhanced selectivity or reactivity. Notable examples include graph neural networks that can learn from molecular structures and predict Lewis acidity based on electronic and steric parameters.
Quantum mechanical/molecular mechanical (QM/MM) hybrid methods have proven invaluable for modeling Lewis acid mechanisms in complex environments, such as enzymatic systems or heterogeneous catalysts. By treating the reactive center quantum mechanically while representing the surrounding environment with classical force fields, these approaches capture both electronic effects at the reaction site and long-range interactions that modulate Lewis acid behavior.
Computational screening has emerged as a powerful strategy for catalyst discovery, enabling the virtual evaluation of thousands of potential Lewis acids before experimental validation. High-throughput computational workflows can systematically vary Lewis acid structures, calculate relevant descriptors such as LUMO energies or charge distributions, and rank candidates based on predicted performance metrics.
Microkinetic modeling integrates computational data into comprehensive reaction networks, allowing researchers to predict how Lewis acid catalysts will perform under realistic reaction conditions. These models account for competing pathways, catalyst deactivation, and concentration effects, bridging the gap between fundamental mechanistic insights and practical catalyst design.
Molecular dynamics (MD) simulations complement static DFT calculations by incorporating temperature effects and conformational flexibility. This approach is particularly valuable for understanding Lewis acid interactions in solution, where solvent effects and dynamic rearrangements significantly influence reaction outcomes. Advanced MD techniques such as metadynamics and umbrella sampling allow for the exploration of rare events and free energy landscapes associated with Lewis acid-substrate interactions.
Machine learning algorithms have recently been integrated with computational chemistry to accelerate the discovery and optimization of Lewis acid catalysts. These algorithms can identify patterns in reaction data, predict catalytic activity, and suggest novel Lewis acid structures with enhanced selectivity or reactivity. Notable examples include graph neural networks that can learn from molecular structures and predict Lewis acidity based on electronic and steric parameters.
Quantum mechanical/molecular mechanical (QM/MM) hybrid methods have proven invaluable for modeling Lewis acid mechanisms in complex environments, such as enzymatic systems or heterogeneous catalysts. By treating the reactive center quantum mechanically while representing the surrounding environment with classical force fields, these approaches capture both electronic effects at the reaction site and long-range interactions that modulate Lewis acid behavior.
Computational screening has emerged as a powerful strategy for catalyst discovery, enabling the virtual evaluation of thousands of potential Lewis acids before experimental validation. High-throughput computational workflows can systematically vary Lewis acid structures, calculate relevant descriptors such as LUMO energies or charge distributions, and rank candidates based on predicted performance metrics.
Microkinetic modeling integrates computational data into comprehensive reaction networks, allowing researchers to predict how Lewis acid catalysts will perform under realistic reaction conditions. These models account for competing pathways, catalyst deactivation, and concentration effects, bridging the gap between fundamental mechanistic insights and practical catalyst design.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!