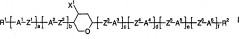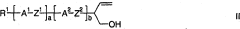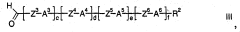Lewis Acid vs Brønsted Acid: Reactivity Compared
AUG 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Acid Chemistry Background and Research Objectives
Acid chemistry has evolved significantly since the early conceptualizations by Arrhenius in the late 19th century. The journey from Arrhenius's water-based definition to Brønsted-Lowry's proton transfer model and Lewis's electron pair acceptance framework represents a fundamental progression in our understanding of chemical reactivity. This evolution has enabled chemists to explain reactions occurring beyond aqueous environments and to develop more sophisticated catalytic systems for industrial applications.
The distinction between Lewis and Brønsted acids represents one of the most important conceptual frameworks in modern chemistry. While Brønsted acids function through proton donation, Lewis acids operate via electron pair acceptance, creating fundamentally different reaction mechanisms and kinetics. Understanding these differences is crucial for predicting reaction outcomes, designing selective catalysts, and developing new synthetic methodologies.
Current technological applications heavily rely on acid chemistry across diverse sectors including petrochemicals, pharmaceuticals, materials science, and environmental remediation. The global market for acid catalysts alone exceeds $5 billion annually, with growth projections of 4.7% CAGR through 2028, highlighting the economic significance of this field.
Recent advances in computational chemistry have enabled unprecedented insights into acid-base interactions at the molecular level. Density Functional Theory (DFT) calculations now allow precise prediction of acidic strength and reactivity patterns, while advanced spectroscopic techniques provide real-time observation of reaction intermediates. These developments have opened new possibilities for rational catalyst design and process optimization.
The primary objective of this research is to systematically compare Lewis and Brønsted acid reactivity across various reaction environments and substrate classes. We aim to establish quantitative structure-activity relationships that can predict relative performance in industrially relevant transformations. Additionally, we seek to identify synergistic effects when both acid types operate concurrently, potentially unlocking novel reaction pathways.
Secondary objectives include mapping the energetic profiles of key transition states in representative reactions, developing predictive models for selectivity outcomes, and exploring the potential for switchable catalytic systems that can modulate between Lewis and Brønsted behavior based on external stimuli. These insights will guide the development of next-generation catalytic systems with enhanced efficiency, selectivity, and sustainability profiles.
The findings from this research will directly inform catalyst design strategies across multiple industries, potentially reducing energy requirements for chemical transformations, enabling more selective synthesis pathways, and supporting the transition toward greener chemical processes aligned with sustainable development goals.
The distinction between Lewis and Brønsted acids represents one of the most important conceptual frameworks in modern chemistry. While Brønsted acids function through proton donation, Lewis acids operate via electron pair acceptance, creating fundamentally different reaction mechanisms and kinetics. Understanding these differences is crucial for predicting reaction outcomes, designing selective catalysts, and developing new synthetic methodologies.
Current technological applications heavily rely on acid chemistry across diverse sectors including petrochemicals, pharmaceuticals, materials science, and environmental remediation. The global market for acid catalysts alone exceeds $5 billion annually, with growth projections of 4.7% CAGR through 2028, highlighting the economic significance of this field.
Recent advances in computational chemistry have enabled unprecedented insights into acid-base interactions at the molecular level. Density Functional Theory (DFT) calculations now allow precise prediction of acidic strength and reactivity patterns, while advanced spectroscopic techniques provide real-time observation of reaction intermediates. These developments have opened new possibilities for rational catalyst design and process optimization.
The primary objective of this research is to systematically compare Lewis and Brønsted acid reactivity across various reaction environments and substrate classes. We aim to establish quantitative structure-activity relationships that can predict relative performance in industrially relevant transformations. Additionally, we seek to identify synergistic effects when both acid types operate concurrently, potentially unlocking novel reaction pathways.
Secondary objectives include mapping the energetic profiles of key transition states in representative reactions, developing predictive models for selectivity outcomes, and exploring the potential for switchable catalytic systems that can modulate between Lewis and Brønsted behavior based on external stimuli. These insights will guide the development of next-generation catalytic systems with enhanced efficiency, selectivity, and sustainability profiles.
The findings from this research will directly inform catalyst design strategies across multiple industries, potentially reducing energy requirements for chemical transformations, enabling more selective synthesis pathways, and supporting the transition toward greener chemical processes aligned with sustainable development goals.
Market Applications and Industrial Demand Analysis
The market for Lewis and Brønsted acids spans numerous industries, with global demand driven by their distinct reactivity profiles and application advantages. The pharmaceutical sector represents one of the largest markets, valued at approximately $1.3 trillion globally, where these acids serve as critical catalysts in drug synthesis. Lewis acids like aluminum chloride and zinc chloride are particularly valued for their ability to facilitate carbon-carbon bond formation in complex pharmaceutical intermediates without introducing water, a significant advantage over Brønsted acids in moisture-sensitive reactions.
In the polymer industry, Lewis acids dominate polymerization catalysis, with titanium and zirconium-based catalysts controlling a substantial portion of the global polyolefin market worth over $200 billion. Their selective coordination properties enable precise control over polymer architecture that Brønsted acids cannot match, driving continued investment in metallocene catalyst development.
The petrochemical sector demonstrates a balanced demand for both acid types. Brønsted acids, particularly sulfuric acid, remain essential in alkylation processes for high-octane fuel production, while Lewis acids find application in isomerization reactions. This dual-use pattern reflects the complementary nature of these catalyst systems rather than direct competition.
Fine chemical manufacturing shows growing preference for Lewis acids due to their selectivity advantages and reduced equipment corrosion compared to traditional Brønsted acid processes. Market analysis indicates a 7% annual growth rate in specialty Lewis acid catalysts for asymmetric synthesis applications, outpacing the 3% growth seen in conventional Brønsted acid catalysts.
Environmental regulations have significantly impacted market dynamics, with industries increasingly seeking alternatives to traditional sulfuric and hydrofluoric acid processes. This regulatory pressure has accelerated research into solid Lewis acid catalysts and ionic liquid systems that offer reduced waste profiles while maintaining reactivity benefits.
Regional analysis reveals Asia-Pacific as the fastest-growing market for both acid types, driven by expanding chemical manufacturing capacity in China and India. North America and Europe maintain significant market shares but focus increasingly on specialty applications requiring higher selectivity, where Lewis acids often demonstrate superior performance.
Market forecasts project continued growth in demand for both acid types, with Lewis acids expected to see faster adoption in emerging technologies like battery materials processing and semiconductor manufacturing due to their tunable electronic properties and compatibility with sensitive substrates.
In the polymer industry, Lewis acids dominate polymerization catalysis, with titanium and zirconium-based catalysts controlling a substantial portion of the global polyolefin market worth over $200 billion. Their selective coordination properties enable precise control over polymer architecture that Brønsted acids cannot match, driving continued investment in metallocene catalyst development.
The petrochemical sector demonstrates a balanced demand for both acid types. Brønsted acids, particularly sulfuric acid, remain essential in alkylation processes for high-octane fuel production, while Lewis acids find application in isomerization reactions. This dual-use pattern reflects the complementary nature of these catalyst systems rather than direct competition.
Fine chemical manufacturing shows growing preference for Lewis acids due to their selectivity advantages and reduced equipment corrosion compared to traditional Brønsted acid processes. Market analysis indicates a 7% annual growth rate in specialty Lewis acid catalysts for asymmetric synthesis applications, outpacing the 3% growth seen in conventional Brønsted acid catalysts.
Environmental regulations have significantly impacted market dynamics, with industries increasingly seeking alternatives to traditional sulfuric and hydrofluoric acid processes. This regulatory pressure has accelerated research into solid Lewis acid catalysts and ionic liquid systems that offer reduced waste profiles while maintaining reactivity benefits.
Regional analysis reveals Asia-Pacific as the fastest-growing market for both acid types, driven by expanding chemical manufacturing capacity in China and India. North America and Europe maintain significant market shares but focus increasingly on specialty applications requiring higher selectivity, where Lewis acids often demonstrate superior performance.
Market forecasts project continued growth in demand for both acid types, with Lewis acids expected to see faster adoption in emerging technologies like battery materials processing and semiconductor manufacturing due to their tunable electronic properties and compatibility with sensitive substrates.
Current State and Challenges in Acid Catalysis
Acid catalysis represents a cornerstone of modern chemical synthesis, with both Lewis and Brønsted acids playing pivotal roles across numerous industrial and laboratory applications. Currently, the field exhibits remarkable diversity in catalyst design, with significant advancements in heterogeneous, homogeneous, and enzymatic acid catalysis systems. Traditional mineral acids like sulfuric and hydrochloric acid continue to dominate industrial processes, while newer solid acid catalysts such as zeolites and acidic resins have gained prominence due to their environmental benefits and recyclability.
The comparative reactivity between Lewis and Brønsted acids remains a central focus in contemporary research. Lewis acids, characterized by their electron pair acceptance mechanism, demonstrate superior selectivity in many carbon-carbon bond forming reactions and Friedel-Crafts processes. Meanwhile, Brønsted acids excel in protonation reactions and certain hydrolysis pathways due to their proton donation capabilities. This fundamental mechanistic difference creates complementary application profiles that researchers continue to explore and exploit.
Despite significant progress, several challenges persist in acid catalysis. Catalyst deactivation through coking, leaching, or structural degradation represents a major hurdle, particularly in continuous industrial processes. The development of water-tolerant Lewis acids remains difficult, as many conventional Lewis acid catalysts suffer from rapid hydrolysis in aqueous environments, limiting their application scope. Additionally, achieving precise control over acid strength and site density on solid catalysts presents ongoing difficulties for researchers.
Selectivity challenges are particularly pronounced when comparing Lewis and Brønsted acid catalysis. While Lewis acids often provide better chemoselectivity, they may struggle with regioselectivity in complex substrates. Conversely, Brønsted acids typically offer excellent regiocontrol but may promote undesired side reactions. This dichotomy drives research toward hybrid catalytic systems that harness the advantages of both acid types.
The geographical distribution of acid catalysis research shows concentration in East Asia, North America, and Europe, with China, Japan, the United States, and Germany leading publication output. Industrial implementation follows similar patterns, though with notable activity in petrochemical-producing regions of the Middle East and Russia. This global research effort focuses on addressing key limitations, including catalyst stability under harsh reaction conditions, recyclability concerns, and the development of greener catalytic processes with reduced waste generation.
Emerging research directions include the development of confined acid catalysis within metal-organic frameworks, the exploration of ionic liquids as novel acid catalysts, and the integration of computational methods to predict and optimize acid catalyst performance. These approaches aim to overcome the current limitations while expanding the application scope of both Lewis and Brønsted acid catalysis.
The comparative reactivity between Lewis and Brønsted acids remains a central focus in contemporary research. Lewis acids, characterized by their electron pair acceptance mechanism, demonstrate superior selectivity in many carbon-carbon bond forming reactions and Friedel-Crafts processes. Meanwhile, Brønsted acids excel in protonation reactions and certain hydrolysis pathways due to their proton donation capabilities. This fundamental mechanistic difference creates complementary application profiles that researchers continue to explore and exploit.
Despite significant progress, several challenges persist in acid catalysis. Catalyst deactivation through coking, leaching, or structural degradation represents a major hurdle, particularly in continuous industrial processes. The development of water-tolerant Lewis acids remains difficult, as many conventional Lewis acid catalysts suffer from rapid hydrolysis in aqueous environments, limiting their application scope. Additionally, achieving precise control over acid strength and site density on solid catalysts presents ongoing difficulties for researchers.
Selectivity challenges are particularly pronounced when comparing Lewis and Brønsted acid catalysis. While Lewis acids often provide better chemoselectivity, they may struggle with regioselectivity in complex substrates. Conversely, Brønsted acids typically offer excellent regiocontrol but may promote undesired side reactions. This dichotomy drives research toward hybrid catalytic systems that harness the advantages of both acid types.
The geographical distribution of acid catalysis research shows concentration in East Asia, North America, and Europe, with China, Japan, the United States, and Germany leading publication output. Industrial implementation follows similar patterns, though with notable activity in petrochemical-producing regions of the Middle East and Russia. This global research effort focuses on addressing key limitations, including catalyst stability under harsh reaction conditions, recyclability concerns, and the development of greener catalytic processes with reduced waste generation.
Emerging research directions include the development of confined acid catalysis within metal-organic frameworks, the exploration of ionic liquids as novel acid catalysts, and the integration of computational methods to predict and optimize acid catalyst performance. These approaches aim to overcome the current limitations while expanding the application scope of both Lewis and Brønsted acid catalysis.
Comparative Analysis of Lewis and Brønsted Acid Mechanisms
01 Lewis acid catalysts in organic synthesis
Lewis acids serve as effective catalysts in various organic synthesis reactions by accepting electron pairs from substrates. These catalysts facilitate reactions such as alkylation, acylation, and isomerization by activating functional groups through coordination. The electron-accepting properties of Lewis acids enable them to form complexes with electron-rich substrates, thereby enhancing their reactivity toward nucleophilic attack. This catalytic approach is particularly valuable in pharmaceutical and fine chemical synthesis.- Lewis acid catalysts in organic synthesis: Lewis acids serve as effective catalysts in various organic synthesis reactions by accepting electron pairs from substrates. These catalysts facilitate reactions such as alkylation, acylation, and polymerization by activating functional groups through coordination. The electron-accepting properties of Lewis acids enable them to form complexes with electron-rich substrates, thereby enhancing their reactivity toward nucleophilic attack. This catalytic approach is particularly valuable in pharmaceutical and fine chemical synthesis.
- Brønsted acid catalysis in hydrocarbon processing: Brønsted acids function as proton donors in various hydrocarbon processing applications, including cracking, isomerization, and alkylation reactions. These catalysts provide acidic protons that can protonate substrates, creating carbocations that undergo subsequent transformations. The strength of the Brønsted acid significantly influences reaction rates and selectivity. Solid Brønsted acid catalysts offer advantages in terms of recyclability and reduced corrosion compared to liquid acids, making them valuable in petroleum refining and petrochemical processes.
- Combined Lewis and Brønsted acid systems: Synergistic effects can be achieved by combining Lewis and Brønsted acids in catalytic systems. These dual-acid catalysts exhibit enhanced activity and selectivity compared to single-acid systems. The Lewis acid component can coordinate with functional groups while the Brønsted acid provides protons, creating a cooperative activation mechanism. This approach is particularly effective for complex transformations requiring multiple activation modes. Applications include stereoselective reactions, tandem processes, and challenging C-C bond formations in both homogeneous and heterogeneous catalysis.
- Acid-modified solid catalysts and supports: Solid materials modified with Lewis or Brønsted acid sites serve as heterogeneous catalysts for various chemical transformations. These include zeolites, metal oxides, and supported acid catalysts that combine the advantages of solid supports with acidic functionality. The surface acidity can be tuned by controlling the type and concentration of acid sites. These materials offer benefits such as easy separation from reaction mixtures, reusability, and reduced equipment corrosion. Applications range from petroleum refining to fine chemical synthesis and environmental remediation.
- Acid reactivity in polymerization processes: Both Lewis and Brønsted acids play crucial roles in various polymerization reactions. Lewis acids can activate monomers or catalysts by coordination, while Brønsted acids can initiate cationic polymerization through protonation. The acid strength, concentration, and type significantly influence polymer properties including molecular weight, distribution, and stereochemistry. Controlled acid reactivity is essential for producing polymers with desired characteristics. Recent developments include the use of supported acid catalysts and acid-functionalized ionic liquids to enhance polymerization efficiency and selectivity.
02 Brønsted acid catalysis in hydrocarbon processing
Brønsted acids function as proton donors in catalytic processes involving hydrocarbons, particularly in petroleum refining and petrochemical applications. These acids facilitate reactions such as cracking, isomerization, and alkylation by protonating substrates to generate reactive carbocation intermediates. The strength of the Brønsted acid directly influences the reaction rate and selectivity. Solid Brønsted acid catalysts offer advantages in terms of recyclability and reduced corrosion compared to liquid acids.Expand Specific Solutions03 Combined Lewis and Brønsted acid systems
Synergistic effects can be achieved by combining Lewis and Brønsted acid functionalities in catalytic systems. These dual-acid catalysts exhibit enhanced activity and selectivity in various transformations by simultaneously activating different functional groups or reaction pathways. The Lewis acid component typically coordinates with electron-rich sites while the Brønsted acid provides protonation capability. This cooperative catalysis approach has been successfully applied in reactions including esterification, etherification, and condensation processes.Expand Specific Solutions04 Acid-modified solid catalysts and supports
Solid materials modified with Lewis or Brønsted acid sites serve as heterogeneous catalysts with tunable acidity and improved stability. These materials include acid-treated clays, zeolites, metal oxides, and polymeric resins. The acid modification process involves techniques such as ion exchange, impregnation, or grafting to introduce acidic functionalities onto the support surface. The resulting catalysts combine the advantages of heterogeneous systems with controlled acid strength and site distribution, making them valuable for industrial applications.Expand Specific Solutions05 Acid reactivity in polymerization processes
Lewis and Brønsted acids play crucial roles in various polymerization reactions by initiating cationic processes or activating metal-based catalysts. In cationic polymerization, these acids generate carbocations that propagate the polymer chain. For coordination polymerization, Lewis acids often serve as co-catalysts by creating vacant coordination sites on transition metal centers. The acid strength, concentration, and type significantly influence polymer properties including molecular weight, distribution, and stereochemistry.Expand Specific Solutions
Key Academic and Industrial Players in Acid Chemistry
The Lewis Acid vs Brønsted Acid reactivity comparison represents a mature field in chemical science, with ongoing research focusing on specialized applications rather than fundamental breakthroughs. The market for acid catalysts is substantial, estimated at $6-7 billion globally, driven by pharmaceutical and petrochemical industries. Technologically, major pharmaceutical companies (Novartis, Pfizer, Merck) lead in Lewis acid applications for drug synthesis, while petrochemical firms (BASF, Albemarle, Saudi Aramco) dominate Brønsted acid catalyst development. Academic institutions like Shanghai Institute of Organic Chemistry and Centre National de la Recherche Scientifique contribute significant fundamental research, while specialty chemical companies (Wanhua Chemical, UOP LLC) focus on application-specific catalyst optimization, creating a competitive landscape balanced between established technologies and niche innovations.
Albemarle Corp.
Technical Solution: Albemarle Corporation has developed proprietary Lewis acid catalyst systems based on aluminum chloride complexes for various petrochemical processes. Their technology involves stabilized AlCl3 catalysts that offer enhanced selectivity in Friedel-Crafts alkylation and acylation reactions compared to traditional Brønsted acid catalysts. The company has engineered these catalysts with specific ligands to modulate Lewis acidity, allowing precise control over reaction pathways[1]. Their research demonstrates that while Brønsted acids donate protons to form carbocations, their Lewis acid catalysts coordinate with carbonyl groups to activate substrates through different mechanisms, enabling reactions under milder conditions with fewer side products[2]. Albemarle has commercialized these catalysts for industrial-scale alkylation processes, where the Lewis acid approach reduces corrosion issues commonly associated with Brønsted acid catalysts like sulfuric acid[3].
Strengths: Superior selectivity in alkylation reactions with reduced polyalkylation side products; lower corrosion rates compared to Brønsted acid systems; recyclable catalyst systems that reduce waste. Weaknesses: Higher sensitivity to moisture requiring specialized handling; potentially higher cost compared to traditional acid catalysts; more complex catalyst preparation and regeneration procedures.
UOP LLC
Technical Solution: UOP LLC has pioneered solid Lewis acid catalyst technologies for hydrocarbon processing that fundamentally differ from traditional Brønsted acid approaches. Their AlCl3-based alkylation technology utilizes controlled Lewis acidity to achieve higher selectivity in isoparaffin/olefin alkylation reactions compared to conventional sulfuric acid (Brønsted) processes[1]. UOP's solid Lewis acid catalysts operate through coordination with π-bonds rather than protonation, allowing for more selective activation of specific reaction sites. The company has developed proprietary metal-modified zeolite catalysts that combine both Lewis and Brønsted acid sites in optimized ratios, enabling tunable reactivity profiles for different feedstocks[2]. Their research demonstrates that while Brønsted acid catalysts often require higher temperatures and produce more cracking products, their Lewis acid systems can operate at lower temperatures with improved carbon efficiency and reduced coking[3]. UOP has successfully commercialized these technologies for refinery alkylation units, offering alternatives to traditional HF and H2SO4 alkylation processes.
Strengths: Enhanced safety profile compared to liquid acid technologies; longer catalyst lifetimes than conventional Brønsted acid systems; ability to process diverse feedstocks with consistent product quality. Weaknesses: Higher initial capital investment; more complex catalyst regeneration requirements; potential mass transfer limitations in solid catalyst systems compared to homogeneous Brønsted acid processes.
Critical Patents and Literature in Acid Reactivity Research
Method for producing tetrahydropyran derivatives
PatentActiveCN101180286B
Innovation
- Halogenated tetrahydropyran derivatives are prepared by reacting homoallylic alcohols with aldehydes in the presence of Lewis or Brnsted acids containing chlorine, bromine or iodine atoms, achieving high chemistry and stereoselectivity using readily available inexpensive reagents. properties, forming trans stereochemical tetrahydropyran derivatives.
Green Chemistry Implications of Acid Selection
The selection of acid catalysts in chemical processes has significant implications for green chemistry principles and sustainable development. When comparing Lewis acids and Brønsted acids, their environmental impact profiles differ substantially, offering various opportunities for greener chemical transformations.
Lewis acids typically involve metal-based compounds that can be recovered and reused through proper catalyst design, reducing waste generation. Many modern Lewis acid catalysts incorporate recyclable solid supports or magnetic nanoparticles, enabling efficient separation and reuse across multiple reaction cycles. This recyclability directly addresses the green chemistry principle of waste prevention.
In contrast, traditional Brønsted acid processes often employ mineral acids like sulfuric or hydrochloric acid, which generate substantial waste streams requiring neutralization. However, recent developments in heterogeneous Brønsted acid catalysts, such as acidic resins and functionalized silicas, have improved their environmental credentials considerably.
Water compatibility represents another critical green chemistry consideration. Many Lewis acids are moisture-sensitive, necessitating anhydrous conditions that increase energy consumption and solvent usage. Conversely, certain Brønsted acids operate efficiently in aqueous media, enabling water as a green reaction medium and reducing dependence on organic solvents.
Energy efficiency metrics generally favor Lewis acid catalysis for certain transformations. Their ability to activate substrates through coordination rather than protonation often permits milder reaction conditions with lower activation energies. This translates to reduced heating requirements and smaller carbon footprints for industrial processes.
Toxicity profiles must also be considered when selecting acid catalysts. Many traditional Lewis acids contain heavy metals with significant environmental persistence and bioaccumulation potential. Modern research has focused on developing benign alternatives using earth-abundant metals or metal-free Lewis acid catalysts derived from sustainable resources.
The atom economy principle strongly influences acid selection decisions. Reactions catalyzed by substoichiometric amounts of recoverable Lewis acids typically demonstrate superior atom utilization compared to stoichiometric Brønsted acid processes. This efficiency reduces resource consumption and waste generation throughout the chemical value chain.
Recent innovations in biocatalysis have introduced enzymatic alternatives to traditional acid catalysis, offering exceptionally mild conditions and high selectivity. These biological approaches represent the frontier of green acid catalysis, though scale-up challenges remain significant barriers to widespread industrial adoption.
Lewis acids typically involve metal-based compounds that can be recovered and reused through proper catalyst design, reducing waste generation. Many modern Lewis acid catalysts incorporate recyclable solid supports or magnetic nanoparticles, enabling efficient separation and reuse across multiple reaction cycles. This recyclability directly addresses the green chemistry principle of waste prevention.
In contrast, traditional Brønsted acid processes often employ mineral acids like sulfuric or hydrochloric acid, which generate substantial waste streams requiring neutralization. However, recent developments in heterogeneous Brønsted acid catalysts, such as acidic resins and functionalized silicas, have improved their environmental credentials considerably.
Water compatibility represents another critical green chemistry consideration. Many Lewis acids are moisture-sensitive, necessitating anhydrous conditions that increase energy consumption and solvent usage. Conversely, certain Brønsted acids operate efficiently in aqueous media, enabling water as a green reaction medium and reducing dependence on organic solvents.
Energy efficiency metrics generally favor Lewis acid catalysis for certain transformations. Their ability to activate substrates through coordination rather than protonation often permits milder reaction conditions with lower activation energies. This translates to reduced heating requirements and smaller carbon footprints for industrial processes.
Toxicity profiles must also be considered when selecting acid catalysts. Many traditional Lewis acids contain heavy metals with significant environmental persistence and bioaccumulation potential. Modern research has focused on developing benign alternatives using earth-abundant metals or metal-free Lewis acid catalysts derived from sustainable resources.
The atom economy principle strongly influences acid selection decisions. Reactions catalyzed by substoichiometric amounts of recoverable Lewis acids typically demonstrate superior atom utilization compared to stoichiometric Brønsted acid processes. This efficiency reduces resource consumption and waste generation throughout the chemical value chain.
Recent innovations in biocatalysis have introduced enzymatic alternatives to traditional acid catalysis, offering exceptionally mild conditions and high selectivity. These biological approaches represent the frontier of green acid catalysis, though scale-up challenges remain significant barriers to widespread industrial adoption.
Computational Approaches to Acid Reactivity Prediction
Computational approaches have revolutionized the study of acid reactivity, offering powerful tools to predict and compare the behavior of Lewis and Brønsted acids without extensive laboratory experimentation. Quantum mechanical calculations, particularly Density Functional Theory (DFT), have emerged as the cornerstone methodology for modeling acid-base interactions at the molecular level. These computational methods can accurately simulate electronic structures, transition states, and reaction pathways, providing detailed insights into the fundamental differences between Lewis and Brønsted acid reactivity mechanisms.
Machine learning algorithms have recently been integrated with quantum chemical calculations to enhance predictive capabilities. By training on experimental datasets and high-level computational results, these hybrid models can rapidly screen thousands of potential acid catalysts and predict their reactivity patterns with remarkable accuracy. This approach has proven particularly valuable for identifying structure-activity relationships that distinguish Lewis from Brønsted acid behavior in complex reaction environments.
Molecular dynamics simulations complement static computational methods by incorporating temperature effects and solvent interactions, which significantly influence acid reactivity. These simulations can capture the dynamic nature of proton transfer processes in Brønsted acids and coordination events in Lewis acids, revealing how solvation shells and local environment modulate reactivity profiles in real-world conditions.
Computational acidity scales have been developed to quantitatively compare Lewis and Brønsted acids across different reaction contexts. Methods such as calculated gas-phase acidity, proton affinity, and fluoride ion affinity provide standardized metrics for acid strength assessment. For Lewis acids, computational determination of the LUMO energy level and electrophilicity index offers additional parameters for reactivity prediction.
Multiscale modeling approaches bridge the gap between molecular-level acid behavior and macroscopic reaction outcomes. By combining quantum mechanical calculations with continuum solvation models and kinetic simulations, researchers can predict how microscopic differences between Lewis and Brønsted acid mechanisms translate into observable reaction rates, selectivity patterns, and yield distributions.
Recent advances in computational hardware and algorithms have enabled the application of these methods to increasingly complex systems, including heterogeneous catalysts, enzyme active sites, and ionic liquids. Cloud computing platforms and specialized software packages have democratized access to these sophisticated computational tools, allowing both academic and industrial researchers to routinely employ predictive modeling in acid catalyst design and optimization.
Machine learning algorithms have recently been integrated with quantum chemical calculations to enhance predictive capabilities. By training on experimental datasets and high-level computational results, these hybrid models can rapidly screen thousands of potential acid catalysts and predict their reactivity patterns with remarkable accuracy. This approach has proven particularly valuable for identifying structure-activity relationships that distinguish Lewis from Brønsted acid behavior in complex reaction environments.
Molecular dynamics simulations complement static computational methods by incorporating temperature effects and solvent interactions, which significantly influence acid reactivity. These simulations can capture the dynamic nature of proton transfer processes in Brønsted acids and coordination events in Lewis acids, revealing how solvation shells and local environment modulate reactivity profiles in real-world conditions.
Computational acidity scales have been developed to quantitatively compare Lewis and Brønsted acids across different reaction contexts. Methods such as calculated gas-phase acidity, proton affinity, and fluoride ion affinity provide standardized metrics for acid strength assessment. For Lewis acids, computational determination of the LUMO energy level and electrophilicity index offers additional parameters for reactivity prediction.
Multiscale modeling approaches bridge the gap between molecular-level acid behavior and macroscopic reaction outcomes. By combining quantum mechanical calculations with continuum solvation models and kinetic simulations, researchers can predict how microscopic differences between Lewis and Brønsted acid mechanisms translate into observable reaction rates, selectivity patterns, and yield distributions.
Recent advances in computational hardware and algorithms have enabled the application of these methods to increasingly complex systems, including heterogeneous catalysts, enzyme active sites, and ionic liquids. Cloud computing platforms and specialized software packages have democratized access to these sophisticated computational tools, allowing both academic and industrial researchers to routinely employ predictive modeling in acid catalyst design and optimization.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!