Material Selection for Electrode Design in High-Temperature Electrolytic Cells
AUG 1, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electrode Materials Evolution and Objectives
The evolution of electrode materials for high-temperature electrolytic cells has been a critical area of research and development in the field of electrochemistry. Over the past decades, significant advancements have been made in material selection, driven by the need for improved performance, durability, and cost-effectiveness in various industrial applications.
Initially, electrode materials were primarily focused on traditional metals and alloys, such as platinum, nickel, and stainless steel. These materials offered good electrical conductivity and reasonable resistance to high-temperature corrosion. However, they often suffered from degradation issues and limited efficiency in harsh electrolytic environments.
As the demand for more efficient and durable electrodes grew, researchers began exploring advanced ceramic materials and composites. The introduction of perovskite-type oxides, such as lanthanum strontium manganite (LSM) and lanthanum strontium cobalt ferrite (LSCF), marked a significant milestone in electrode material development. These materials exhibited excellent catalytic activity and stability at high temperatures, making them suitable for applications in solid oxide fuel cells (SOFCs) and electrolyzers.
Parallel to ceramic developments, carbon-based materials, including graphite and carbon nanotubes, gained attention for their unique properties. These materials offered high surface area, good electrical conductivity, and potential for functionalization, opening new avenues for electrode design in specific applications.
The advent of nanotechnology further revolutionized electrode material selection. Nanostructured materials and nanocomposites provided enhanced surface area, improved catalytic activity, and better charge transfer properties. This led to the development of novel electrode architectures, such as nanoparticle-decorated surfaces and hierarchical porous structures, which significantly boosted the performance of high-temperature electrolytic cells.
Recent years have seen a shift towards more sustainable and cost-effective materials. Researchers are now focusing on abundant and environmentally friendly elements to replace rare and expensive materials. This trend has led to the exploration of transition metal oxides, nitrides, and carbides as potential electrode materials, offering a balance between performance and economic viability.
The primary objectives in electrode material selection for high-temperature electrolytic cells continue to evolve. Current research aims to develop materials with exceptional electrochemical performance, long-term stability under extreme conditions, and resistance to poisoning and degradation. Additionally, there is a growing emphasis on materials that can operate efficiently at lower temperatures, reducing overall system costs and expanding the range of potential applications.
Future objectives include the development of multifunctional electrode materials that can adapt to varying operational conditions, self-healing properties to extend electrode lifespan, and materials compatible with next-generation electrolytes. The integration of computational modeling and high-throughput screening techniques is expected to accelerate the discovery and optimization of novel electrode materials, paving the way for more efficient and sustainable high-temperature electrolytic processes.
Initially, electrode materials were primarily focused on traditional metals and alloys, such as platinum, nickel, and stainless steel. These materials offered good electrical conductivity and reasonable resistance to high-temperature corrosion. However, they often suffered from degradation issues and limited efficiency in harsh electrolytic environments.
As the demand for more efficient and durable electrodes grew, researchers began exploring advanced ceramic materials and composites. The introduction of perovskite-type oxides, such as lanthanum strontium manganite (LSM) and lanthanum strontium cobalt ferrite (LSCF), marked a significant milestone in electrode material development. These materials exhibited excellent catalytic activity and stability at high temperatures, making them suitable for applications in solid oxide fuel cells (SOFCs) and electrolyzers.
Parallel to ceramic developments, carbon-based materials, including graphite and carbon nanotubes, gained attention for their unique properties. These materials offered high surface area, good electrical conductivity, and potential for functionalization, opening new avenues for electrode design in specific applications.
The advent of nanotechnology further revolutionized electrode material selection. Nanostructured materials and nanocomposites provided enhanced surface area, improved catalytic activity, and better charge transfer properties. This led to the development of novel electrode architectures, such as nanoparticle-decorated surfaces and hierarchical porous structures, which significantly boosted the performance of high-temperature electrolytic cells.
Recent years have seen a shift towards more sustainable and cost-effective materials. Researchers are now focusing on abundant and environmentally friendly elements to replace rare and expensive materials. This trend has led to the exploration of transition metal oxides, nitrides, and carbides as potential electrode materials, offering a balance between performance and economic viability.
The primary objectives in electrode material selection for high-temperature electrolytic cells continue to evolve. Current research aims to develop materials with exceptional electrochemical performance, long-term stability under extreme conditions, and resistance to poisoning and degradation. Additionally, there is a growing emphasis on materials that can operate efficiently at lower temperatures, reducing overall system costs and expanding the range of potential applications.
Future objectives include the development of multifunctional electrode materials that can adapt to varying operational conditions, self-healing properties to extend electrode lifespan, and materials compatible with next-generation electrolytes. The integration of computational modeling and high-throughput screening techniques is expected to accelerate the discovery and optimization of novel electrode materials, paving the way for more efficient and sustainable high-temperature electrolytic processes.
Market Demand Analysis for High-Temperature Electrolysis
The market demand for high-temperature electrolysis technology has been steadily growing, driven by the increasing need for clean energy solutions and sustainable industrial processes. This technology plays a crucial role in various sectors, including hydrogen production, carbon capture and utilization, and the synthesis of valuable chemicals.
In the hydrogen production sector, high-temperature electrolysis offers significant advantages over traditional low-temperature methods. The global hydrogen market is projected to expand rapidly, with a compound annual growth rate (CAGR) of over 9% from 2021 to 2028. This growth is primarily fueled by the rising demand for clean energy sources and the transition towards a hydrogen-based economy.
The industrial sector, particularly in steel manufacturing and chemical production, is another key driver for high-temperature electrolysis technology. As industries seek to reduce their carbon footprint and improve energy efficiency, the demand for high-temperature electrolysis solutions is expected to surge. The steel industry alone accounts for a substantial portion of global CO2 emissions, creating a significant market opportunity for electrolysis-based decarbonization technologies.
In the field of carbon capture and utilization, high-temperature electrolysis is gaining traction as a promising method for converting CO2 into valuable products. This market segment is anticipated to grow substantially in the coming years, driven by stringent environmental regulations and the increasing focus on circular economy principles.
The energy storage sector also presents a significant market opportunity for high-temperature electrolysis. As renewable energy sources become more prevalent, the need for efficient energy storage solutions grows. High-temperature electrolysis can be used to produce hydrogen as a storage medium, addressing the intermittency issues associated with renewable energy sources.
Geographically, Europe and North America are currently leading the market for high-temperature electrolysis technologies, with Asia-Pacific expected to witness rapid growth in the coming years. Government initiatives, such as the European Green Deal and various national hydrogen strategies, are providing strong support for the adoption of these technologies.
Despite the promising market outlook, several challenges need to be addressed to fully realize the potential of high-temperature electrolysis. These include reducing the overall system costs, improving the durability and efficiency of electrodes, and scaling up the technology for large-scale industrial applications. Overcoming these challenges will be crucial in meeting the growing market demand and accelerating the widespread adoption of high-temperature electrolysis across various industries.
In the hydrogen production sector, high-temperature electrolysis offers significant advantages over traditional low-temperature methods. The global hydrogen market is projected to expand rapidly, with a compound annual growth rate (CAGR) of over 9% from 2021 to 2028. This growth is primarily fueled by the rising demand for clean energy sources and the transition towards a hydrogen-based economy.
The industrial sector, particularly in steel manufacturing and chemical production, is another key driver for high-temperature electrolysis technology. As industries seek to reduce their carbon footprint and improve energy efficiency, the demand for high-temperature electrolysis solutions is expected to surge. The steel industry alone accounts for a substantial portion of global CO2 emissions, creating a significant market opportunity for electrolysis-based decarbonization technologies.
In the field of carbon capture and utilization, high-temperature electrolysis is gaining traction as a promising method for converting CO2 into valuable products. This market segment is anticipated to grow substantially in the coming years, driven by stringent environmental regulations and the increasing focus on circular economy principles.
The energy storage sector also presents a significant market opportunity for high-temperature electrolysis. As renewable energy sources become more prevalent, the need for efficient energy storage solutions grows. High-temperature electrolysis can be used to produce hydrogen as a storage medium, addressing the intermittency issues associated with renewable energy sources.
Geographically, Europe and North America are currently leading the market for high-temperature electrolysis technologies, with Asia-Pacific expected to witness rapid growth in the coming years. Government initiatives, such as the European Green Deal and various national hydrogen strategies, are providing strong support for the adoption of these technologies.
Despite the promising market outlook, several challenges need to be addressed to fully realize the potential of high-temperature electrolysis. These include reducing the overall system costs, improving the durability and efficiency of electrodes, and scaling up the technology for large-scale industrial applications. Overcoming these challenges will be crucial in meeting the growing market demand and accelerating the widespread adoption of high-temperature electrolysis across various industries.
Current Challenges in Electrode Material Selection
The selection of electrode materials for high-temperature electrolytic cells presents several significant challenges. One of the primary issues is the extreme operating conditions these materials must withstand. Electrodes are exposed to highly corrosive environments, including molten salts and aggressive gases, at temperatures often exceeding 800°C. This combination of high temperature and corrosive media severely limits the range of suitable materials.
Thermal stability is a critical factor in material selection. Electrodes must maintain their structural integrity and electrochemical performance at elevated temperatures for extended periods. Many materials that exhibit excellent electrochemical properties at room temperature degrade rapidly under high-temperature conditions, leading to reduced efficiency and shortened cell lifetimes.
Electrical conductivity is another crucial consideration. The electrode material must possess high electrical conductivity to minimize ohmic losses and improve overall cell efficiency. However, many materials that exhibit good conductivity at room temperature may experience significant changes in their electrical properties at high temperatures, potentially compromising cell performance.
Chemical compatibility with the electrolyte and other cell components is essential. The electrode material must not react with the electrolyte or other cell materials in ways that could lead to contamination, degradation, or the formation of unwanted byproducts. This requirement further narrows the pool of suitable materials.
Mechanical strength and dimensional stability are also important factors. Electrodes must withstand thermal cycling and maintain their shape and structure over time. Thermal expansion mismatches between the electrode and other cell components can lead to stress, cracking, and ultimately, cell failure.
The cost and availability of materials pose additional challenges. Many materials that meet the technical requirements for high-temperature electrolytic cells are rare or expensive, making large-scale implementation economically unfeasible. Balancing performance with cost-effectiveness is a constant struggle in material selection.
Manufacturability and scalability present further obstacles. Some materials that perform well in laboratory settings may be difficult to produce or shape into the required electrode geometries at an industrial scale. This limitation can hinder the transition from research to practical application.
Lastly, the long-term stability and durability of electrode materials remain significant challenges. The harsh operating conditions of high-temperature electrolytic cells can lead to gradual degradation of electrode materials over time, affecting cell performance and lifespan. Developing materials that can maintain their properties and performance over extended periods is crucial for the commercial viability of these systems.
Thermal stability is a critical factor in material selection. Electrodes must maintain their structural integrity and electrochemical performance at elevated temperatures for extended periods. Many materials that exhibit excellent electrochemical properties at room temperature degrade rapidly under high-temperature conditions, leading to reduced efficiency and shortened cell lifetimes.
Electrical conductivity is another crucial consideration. The electrode material must possess high electrical conductivity to minimize ohmic losses and improve overall cell efficiency. However, many materials that exhibit good conductivity at room temperature may experience significant changes in their electrical properties at high temperatures, potentially compromising cell performance.
Chemical compatibility with the electrolyte and other cell components is essential. The electrode material must not react with the electrolyte or other cell materials in ways that could lead to contamination, degradation, or the formation of unwanted byproducts. This requirement further narrows the pool of suitable materials.
Mechanical strength and dimensional stability are also important factors. Electrodes must withstand thermal cycling and maintain their shape and structure over time. Thermal expansion mismatches between the electrode and other cell components can lead to stress, cracking, and ultimately, cell failure.
The cost and availability of materials pose additional challenges. Many materials that meet the technical requirements for high-temperature electrolytic cells are rare or expensive, making large-scale implementation economically unfeasible. Balancing performance with cost-effectiveness is a constant struggle in material selection.
Manufacturability and scalability present further obstacles. Some materials that perform well in laboratory settings may be difficult to produce or shape into the required electrode geometries at an industrial scale. This limitation can hinder the transition from research to practical application.
Lastly, the long-term stability and durability of electrode materials remain significant challenges. The harsh operating conditions of high-temperature electrolytic cells can lead to gradual degradation of electrode materials over time, affecting cell performance and lifespan. Developing materials that can maintain their properties and performance over extended periods is crucial for the commercial viability of these systems.
Existing Electrode Material Solutions
01 High-temperature resistant electrode materials
Development of electrode materials capable of withstanding high temperatures is crucial for various applications. These materials often include refractory metals, ceramics, or composite structures that maintain their electrical properties and structural integrity under extreme heat conditions. Such electrodes are essential in high-temperature sensors, furnaces, and advanced energy systems.- High-temperature resistant electrode materials: Development of electrode materials capable of withstanding high temperatures is crucial for various applications. These materials often include refractory metals, ceramics, or composite structures that maintain their electrical properties and structural integrity at elevated temperatures. Such electrodes are essential in high-temperature sensors, furnaces, and energy conversion devices.
- Coatings for improved temperature resistance: Application of specialized coatings on electrode surfaces can significantly enhance their temperature resistance. These coatings may include ceramic layers, metal alloys, or nanostructured materials that provide thermal insulation and protection against oxidation at high temperatures. Such coatings extend the operational range and lifespan of electrodes in extreme environments.
- Novel electrode designs for thermal management: Innovative electrode designs incorporating heat dissipation features or thermal management systems can improve temperature resistance. These designs may include internal cooling channels, heat sinks, or thermally conductive pathways that help maintain electrode temperature within operational limits. Such designs are particularly important in high-power applications where heat generation is significant.
- Composite electrode materials for enhanced thermal properties: Development of composite electrode materials combining different components with complementary thermal and electrical properties can enhance overall temperature resistance. These composites may include combinations of metals, ceramics, and carbon-based materials, offering a balance between electrical conductivity and thermal stability. Such materials are particularly useful in applications requiring both high temperature resistance and specific electrical characteristics.
- Temperature-resistant electrode fabrication techniques: Advanced manufacturing techniques play a crucial role in producing temperature-resistant electrodes. These may include specialized sintering processes, additive manufacturing, or novel deposition methods that create electrodes with improved microstructures and thermal properties. Such fabrication techniques enable the production of electrodes with enhanced temperature resistance and tailored characteristics for specific applications.
02 Coatings for temperature resistance enhancement
Application of specialized coatings on electrode surfaces can significantly improve their temperature resistance. These coatings may include ceramic layers, metal alloys, or nanostructured materials that act as thermal barriers while maintaining electrical conductivity. Such coatings protect the underlying electrode material from degradation and extend the operational temperature range.Expand Specific Solutions03 Novel electrode designs for heat dissipation
Innovative electrode designs incorporating heat dissipation features can enhance temperature resistance. These designs may include internal cooling channels, fins, or porous structures that facilitate heat transfer away from critical areas. Such designs allow electrodes to operate at higher temperatures without compromising performance or lifespan.Expand Specific Solutions04 Composite electrode materials for thermal stability
Development of composite electrode materials combines the benefits of multiple components to achieve superior temperature resistance. These composites may integrate high-conductivity materials with thermally stable compounds, resulting in electrodes that maintain performance under extreme conditions. Such materials find applications in fuel cells, batteries, and high-temperature electrolysis.Expand Specific Solutions05 Nanotechnology-enhanced temperature-resistant electrodes
Incorporation of nanotechnology in electrode design and fabrication can significantly improve temperature resistance. Nanostructured materials, such as carbon nanotubes or metal nanoparticles, can enhance thermal stability and electrical properties. These advanced electrodes offer improved performance in high-temperature environments for various industrial and research applications.Expand Specific Solutions
Key Players in Electrolytic Cell Industry
The market for high-temperature electrolytic cell electrode materials is in a growth phase, driven by increasing demand for advanced energy storage and conversion technologies. The global market size is expanding, with significant potential in renewable energy and industrial applications. Technologically, the field is advancing rapidly, with companies like NGK Insulators and Corning leading in ceramic and glass-based solutions. Research institutions such as Dalian Institute of Chemical Physics and Kyushu University are pushing boundaries in material science. Established players like Sumitomo Electric Industries and Noritake Co. are leveraging their expertise in industrial ceramics to develop innovative electrode materials. Emerging companies and academic collaborations are also contributing to the competitive landscape, fostering a dynamic environment for technological breakthroughs in this critical area.
Dalian Institute of Chemical Physics Chinese Academy of Sci
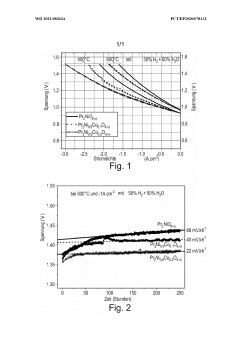
Technical Solution: The Dalian Institute of Chemical Physics (DICP) has developed advanced electrode materials for high-temperature electrolytic cells, focusing on perovskite-type oxides. Their research has shown that La0.6Sr0.4Co0.2Fe0.8O3-δ (LSCF) exhibits excellent electrochemical performance and stability at high temperatures[1]. DICP has also explored the use of nanostructured electrodes to enhance the catalytic activity and durability of the materials. Their innovative approach involves the incorporation of nanoparticles into the electrode structure, which has been shown to significantly improve the electrode's performance in solid oxide electrolysis cells (SOECs)[2]. Additionally, DICP has developed novel fabrication techniques, such as infiltration methods, to create highly porous electrode structures that facilitate gas diffusion and increase the active surface area for electrochemical reactions[3].
Strengths: Expertise in perovskite materials, nanostructured electrodes, and advanced fabrication techniques. Weaknesses: Potential scalability issues for large-scale production and long-term stability concerns in industrial applications.
NGK Insulators, Ltd.
Technical Solution: NGK Insulators has made significant advancements in electrode materials for high-temperature electrolytic cells, particularly in the field of solid oxide fuel cells (SOFCs) and solid oxide electrolysis cells (SOECs). Their research focuses on developing ceramic-based electrodes with enhanced durability and performance at elevated temperatures. NGK has pioneered the use of scandia-stabilized zirconia (ScSZ) as an electrolyte material, which exhibits higher ionic conductivity compared to traditional yttria-stabilized zirconia (YSZ)[4]. For the cathode, NGK has developed composite materials combining lanthanum strontium cobalt ferrite (LSCF) with gadolinium-doped ceria (GDC) to improve oxygen reduction kinetics and long-term stability[5]. On the anode side, they have explored nickel-based cermet materials with enhanced sulfur tolerance and coking resistance for fuel electrode applications[6].
Strengths: Extensive experience in ceramic materials and mass production capabilities. Weaknesses: Potential limitations in cost-effectiveness for large-scale implementation and challenges in achieving high power densities.
Core Innovations in High-Temperature Electrodes
Electrode material, method for the production thereof, and use of same
PatentWO2021083624A1
Innovation
- A compound material with the formula M2NiO1-xCoxO4+δ or La1-yMNO1-xCoxO4+δ, where M is Pr or Nd, and x and δ are within specific ranges, is used to create electrodes with enhanced performance and longevity, particularly suitable for high-temperature electrolysis, and produced through a method involving mixing oxides, drying, and annealing at high temperatures.
Electrode material, fuel cell including the same, and method of manufacturing the same
PatentActiveUS20110236789A1
Innovation
- Development of an electrode material with the composition La1-sAsNi1-x-y-zCuxFeyBzO3-δ, where A and B are selected from alkaline earth metals, transition metals excluding Fe and Cu, and rare earths excluding La, with specific molar ratios and firing conditions to achieve high conductivity and low thermal expansion, eliminating the need for costly materials like Pt.
Environmental Impact of Electrode Materials
The selection of electrode materials for high-temperature electrolytic cells has significant environmental implications that must be carefully considered. These materials, while crucial for the efficiency and durability of the cells, can have both direct and indirect environmental impacts throughout their lifecycle.
Firstly, the extraction and processing of raw materials for electrode production often involve energy-intensive processes and can lead to habitat disruption, soil erosion, and water pollution. For instance, the mining of rare earth elements, which are sometimes used in advanced electrode designs, can result in the release of radioactive materials and toxic chemicals into the environment if not properly managed.
During the manufacturing phase, the production of electrode materials may involve the use of hazardous chemicals and generate toxic waste. The energy consumption in this stage also contributes to greenhouse gas emissions, particularly if the energy source is not renewable. Additionally, the transportation of raw materials and finished electrodes adds to the carbon footprint of the entire process.
In the operational phase, the environmental impact of electrode materials is closely tied to their performance and longevity. More durable electrodes that can withstand high temperatures and corrosive environments for extended periods reduce the frequency of replacement, thereby minimizing waste generation and the need for additional resource extraction. However, some high-performance materials may contain toxic elements that could potentially leach into the electrolyte or be released as particulates during operation.
The end-of-life stage of electrode materials presents another set of environmental challenges. Proper recycling and disposal methods are essential to prevent the release of harmful substances into the environment. Some electrode materials, particularly those containing precious metals or rare earth elements, offer opportunities for recycling and resource recovery, which can help mitigate the environmental impact of primary material extraction.
Furthermore, the choice of electrode materials can indirectly affect the environment through its influence on the overall efficiency of the electrolytic process. More efficient electrodes can reduce energy consumption, leading to lower greenhouse gas emissions if the energy source is fossil fuel-based. They can also improve the yield and purity of the products, potentially reducing waste and the need for further processing steps.
In conclusion, the environmental impact of electrode materials in high-temperature electrolytic cells is multifaceted and extends beyond their immediate use. A comprehensive life cycle assessment is necessary to fully understand and mitigate these impacts. Researchers and engineers must strive to develop electrode materials that not only meet the technical requirements of high-temperature electrolysis but also minimize environmental harm throughout their lifecycle.
Firstly, the extraction and processing of raw materials for electrode production often involve energy-intensive processes and can lead to habitat disruption, soil erosion, and water pollution. For instance, the mining of rare earth elements, which are sometimes used in advanced electrode designs, can result in the release of radioactive materials and toxic chemicals into the environment if not properly managed.
During the manufacturing phase, the production of electrode materials may involve the use of hazardous chemicals and generate toxic waste. The energy consumption in this stage also contributes to greenhouse gas emissions, particularly if the energy source is not renewable. Additionally, the transportation of raw materials and finished electrodes adds to the carbon footprint of the entire process.
In the operational phase, the environmental impact of electrode materials is closely tied to their performance and longevity. More durable electrodes that can withstand high temperatures and corrosive environments for extended periods reduce the frequency of replacement, thereby minimizing waste generation and the need for additional resource extraction. However, some high-performance materials may contain toxic elements that could potentially leach into the electrolyte or be released as particulates during operation.
The end-of-life stage of electrode materials presents another set of environmental challenges. Proper recycling and disposal methods are essential to prevent the release of harmful substances into the environment. Some electrode materials, particularly those containing precious metals or rare earth elements, offer opportunities for recycling and resource recovery, which can help mitigate the environmental impact of primary material extraction.
Furthermore, the choice of electrode materials can indirectly affect the environment through its influence on the overall efficiency of the electrolytic process. More efficient electrodes can reduce energy consumption, leading to lower greenhouse gas emissions if the energy source is fossil fuel-based. They can also improve the yield and purity of the products, potentially reducing waste and the need for further processing steps.
In conclusion, the environmental impact of electrode materials in high-temperature electrolytic cells is multifaceted and extends beyond their immediate use. A comprehensive life cycle assessment is necessary to fully understand and mitigate these impacts. Researchers and engineers must strive to develop electrode materials that not only meet the technical requirements of high-temperature electrolysis but also minimize environmental harm throughout their lifecycle.
Cost-Benefit Analysis of Advanced Electrode Materials
The cost-benefit analysis of advanced electrode materials for high-temperature electrolytic cells is a critical consideration in the development and implementation of these technologies. Advanced materials, such as ceramics, noble metals, and advanced alloys, offer significant performance improvements over traditional electrode materials. However, their adoption must be carefully evaluated against the associated costs.
Ceramic electrodes, particularly those based on perovskite structures, demonstrate excellent stability and conductivity at high temperatures. These materials can significantly extend the operational lifespan of electrolytic cells, reducing long-term replacement costs. However, the initial investment in ceramic electrodes is substantially higher than conventional materials, necessitating a thorough lifecycle cost analysis to justify their implementation.
Noble metal electrodes, such as platinum or iridium, exhibit exceptional resistance to corrosion and maintain high catalytic activity in harsh electrolytic environments. While these properties can lead to improved efficiency and reduced maintenance requirements, the scarcity and high cost of noble metals present a significant barrier to widespread adoption. The cost-benefit ratio for noble metal electrodes is highly dependent on the specific application and the potential for material recovery and recycling at the end of the electrode's life.
Advanced alloys, including nickel-based superalloys and intermetallics, offer a balance between performance and cost. These materials can provide enhanced durability and electrical properties compared to standard alloys, potentially increasing cell efficiency and lifespan. The cost premium for advanced alloys is generally lower than for ceramics or noble metals, making them an attractive option for many applications. However, their long-term performance in extreme electrolytic conditions must be carefully evaluated to ensure that the benefits outweigh the increased material costs.
The economic viability of advanced electrode materials also depends on factors beyond direct material costs. Improved electrode performance can lead to significant energy savings over the operational life of the electrolytic cell. For energy-intensive processes, such as aluminum production or hydrogen generation, even small efficiency gains can translate into substantial cost savings. Additionally, advanced materials that reduce the frequency of cell shutdowns for maintenance or electrode replacement can increase overall productivity, providing indirect economic benefits.
Environmental considerations play an increasingly important role in the cost-benefit analysis of electrode materials. Advanced materials that enable more efficient processes or reduce the environmental impact of electrolytic operations may offer long-term economic advantages through reduced regulatory compliance costs or improved corporate sustainability profiles. However, the environmental impact of producing and disposing of advanced electrode materials must also be factored into the overall assessment.
In conclusion, the cost-benefit analysis of advanced electrode materials for high-temperature electrolytic cells is a complex, multifaceted evaluation. While the upfront costs of these materials are often higher, their potential to improve efficiency, extend operational lifespans, and reduce maintenance requirements can offer significant long-term economic benefits. A comprehensive analysis must consider not only direct material costs but also energy savings, productivity improvements, and environmental impacts to accurately assess the true value proposition of advanced electrode materials in electrolytic applications.
Ceramic electrodes, particularly those based on perovskite structures, demonstrate excellent stability and conductivity at high temperatures. These materials can significantly extend the operational lifespan of electrolytic cells, reducing long-term replacement costs. However, the initial investment in ceramic electrodes is substantially higher than conventional materials, necessitating a thorough lifecycle cost analysis to justify their implementation.
Noble metal electrodes, such as platinum or iridium, exhibit exceptional resistance to corrosion and maintain high catalytic activity in harsh electrolytic environments. While these properties can lead to improved efficiency and reduced maintenance requirements, the scarcity and high cost of noble metals present a significant barrier to widespread adoption. The cost-benefit ratio for noble metal electrodes is highly dependent on the specific application and the potential for material recovery and recycling at the end of the electrode's life.
Advanced alloys, including nickel-based superalloys and intermetallics, offer a balance between performance and cost. These materials can provide enhanced durability and electrical properties compared to standard alloys, potentially increasing cell efficiency and lifespan. The cost premium for advanced alloys is generally lower than for ceramics or noble metals, making them an attractive option for many applications. However, their long-term performance in extreme electrolytic conditions must be carefully evaluated to ensure that the benefits outweigh the increased material costs.
The economic viability of advanced electrode materials also depends on factors beyond direct material costs. Improved electrode performance can lead to significant energy savings over the operational life of the electrolytic cell. For energy-intensive processes, such as aluminum production or hydrogen generation, even small efficiency gains can translate into substantial cost savings. Additionally, advanced materials that reduce the frequency of cell shutdowns for maintenance or electrode replacement can increase overall productivity, providing indirect economic benefits.
Environmental considerations play an increasingly important role in the cost-benefit analysis of electrode materials. Advanced materials that enable more efficient processes or reduce the environmental impact of electrolytic operations may offer long-term economic advantages through reduced regulatory compliance costs or improved corporate sustainability profiles. However, the environmental impact of producing and disposing of advanced electrode materials must also be factored into the overall assessment.
In conclusion, the cost-benefit analysis of advanced electrode materials for high-temperature electrolytic cells is a complex, multifaceted evaluation. While the upfront costs of these materials are often higher, their potential to improve efficiency, extend operational lifespans, and reduce maintenance requirements can offer significant long-term economic benefits. A comprehensive analysis must consider not only direct material costs but also energy savings, productivity improvements, and environmental impacts to accurately assess the true value proposition of advanced electrode materials in electrolytic applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!