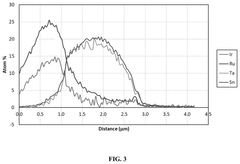
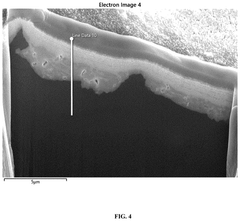
New Anode Developments for Electrolytic Cells in Industrial Processes
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Anode Evolution and Objectives
The evolution of anodes in electrolytic cells has been a crucial aspect of industrial process development over the past century. Initially, carbon-based anodes were the primary choice for many electrochemical applications due to their low cost and reasonable conductivity. However, these anodes suffered from rapid degradation and contamination issues, leading to the exploration of more advanced materials.
In the mid-20th century, the introduction of dimensionally stable anodes (DSAs) marked a significant milestone in anode technology. These anodes, typically consisting of a titanium substrate coated with precious metal oxides, offered superior stability and longevity compared to their carbon counterparts. The development of DSAs revolutionized chlor-alkali production and other electrochemical industries by dramatically reducing energy consumption and improving process efficiency.
Recent decades have seen a focus on enhancing the performance and sustainability of anodes. Research has been directed towards developing novel coating compositions that can withstand harsh operating conditions while maintaining high catalytic activity. The incorporation of mixed metal oxides and advanced nanostructured materials has shown promising results in improving anode durability and reducing overpotential.
The current objectives in anode development for electrolytic cells are multifaceted. One primary goal is to further increase the lifespan of anodes, reducing the frequency of replacements and associated downtime in industrial processes. This involves developing more corrosion-resistant materials and optimizing anode geometries to distribute current more evenly.
Another critical objective is to improve the energy efficiency of electrolytic processes. This entails designing anodes with lower overpotentials, which can significantly reduce the overall energy consumption of industrial electrochemical operations. Researchers are exploring advanced catalytic materials and surface modifications to achieve this goal.
Sustainability is also a key focus in modern anode development. There is a growing emphasis on reducing or eliminating the use of precious metals in anode fabrication, driven by both economic and environmental considerations. This has led to increased research into alternative materials such as transition metal oxides and carbon-based composites that can offer comparable performance to traditional precious metal-based anodes.
Furthermore, the development of anodes capable of facilitating new electrochemical processes is an emerging objective. This includes anodes designed for water electrolysis in hydrogen production, CO2 reduction for carbon capture and utilization, and advanced oxidation processes for wastewater treatment. These applications require anodes with specific catalytic properties and stability in diverse electrolyte environments.
In the mid-20th century, the introduction of dimensionally stable anodes (DSAs) marked a significant milestone in anode technology. These anodes, typically consisting of a titanium substrate coated with precious metal oxides, offered superior stability and longevity compared to their carbon counterparts. The development of DSAs revolutionized chlor-alkali production and other electrochemical industries by dramatically reducing energy consumption and improving process efficiency.
Recent decades have seen a focus on enhancing the performance and sustainability of anodes. Research has been directed towards developing novel coating compositions that can withstand harsh operating conditions while maintaining high catalytic activity. The incorporation of mixed metal oxides and advanced nanostructured materials has shown promising results in improving anode durability and reducing overpotential.
The current objectives in anode development for electrolytic cells are multifaceted. One primary goal is to further increase the lifespan of anodes, reducing the frequency of replacements and associated downtime in industrial processes. This involves developing more corrosion-resistant materials and optimizing anode geometries to distribute current more evenly.
Another critical objective is to improve the energy efficiency of electrolytic processes. This entails designing anodes with lower overpotentials, which can significantly reduce the overall energy consumption of industrial electrochemical operations. Researchers are exploring advanced catalytic materials and surface modifications to achieve this goal.
Sustainability is also a key focus in modern anode development. There is a growing emphasis on reducing or eliminating the use of precious metals in anode fabrication, driven by both economic and environmental considerations. This has led to increased research into alternative materials such as transition metal oxides and carbon-based composites that can offer comparable performance to traditional precious metal-based anodes.
Furthermore, the development of anodes capable of facilitating new electrochemical processes is an emerging objective. This includes anodes designed for water electrolysis in hydrogen production, CO2 reduction for carbon capture and utilization, and advanced oxidation processes for wastewater treatment. These applications require anodes with specific catalytic properties and stability in diverse electrolyte environments.
Market Demand Analysis
The market demand for new anode developments in electrolytic cells for industrial processes has been steadily increasing due to the growing emphasis on energy efficiency, environmental sustainability, and process optimization. Industries such as chlor-alkali production, aluminum smelting, and water treatment are actively seeking innovative anode technologies to enhance their operational performance and reduce environmental impact.
In the chlor-alkali sector, which produces essential chemicals like chlorine, caustic soda, and hydrogen, there is a significant push towards dimensionally stable anodes (DSAs) that offer longer lifespans and improved energy efficiency. The global chlor-alkali market, valued at over $30 billion, is expected to grow at a CAGR of 5% through 2025, driving the demand for advanced anode materials.
The aluminum industry, another major consumer of electrolytic cell technology, is experiencing a surge in demand for inert anodes. These anodes promise to eliminate carbon emissions from the smelting process, aligning with stringent environmental regulations and corporate sustainability goals. With the global aluminum market projected to reach $250 billion by 2026, the potential for new anode technologies is substantial.
Water treatment and wastewater management sectors are also contributing to the market demand for innovative anodes. As water scarcity becomes a global concern, there is an increasing need for efficient electrochemical water treatment systems. Advanced anodes capable of generating powerful oxidants for contaminant removal are highly sought after in this rapidly expanding market.
The push for circular economy practices and resource recovery is further fueling the demand for new anode developments. Industries are looking for anodes that can facilitate the recovery of valuable metals from waste streams and enable more efficient recycling processes. This trend is particularly evident in the electronics and battery recycling sectors, where the recovery of precious and rare earth metals is becoming increasingly important.
Energy storage and renewable energy integration are also driving factors in the market for new anode technologies. As the world transitions towards cleaner energy sources, there is a growing need for efficient electrolysis systems for hydrogen production. Anodes that can withstand high current densities and operate in harsh alkaline environments are in high demand for green hydrogen production.
The market is also seeing increased interest from emerging economies, particularly in Asia-Pacific and Latin America, where rapid industrialization is driving the adoption of advanced electrolytic processes. These regions represent significant growth opportunities for new anode technologies, as industries seek to leapfrog older technologies and implement state-of-the-art solutions.
In the chlor-alkali sector, which produces essential chemicals like chlorine, caustic soda, and hydrogen, there is a significant push towards dimensionally stable anodes (DSAs) that offer longer lifespans and improved energy efficiency. The global chlor-alkali market, valued at over $30 billion, is expected to grow at a CAGR of 5% through 2025, driving the demand for advanced anode materials.
The aluminum industry, another major consumer of electrolytic cell technology, is experiencing a surge in demand for inert anodes. These anodes promise to eliminate carbon emissions from the smelting process, aligning with stringent environmental regulations and corporate sustainability goals. With the global aluminum market projected to reach $250 billion by 2026, the potential for new anode technologies is substantial.
Water treatment and wastewater management sectors are also contributing to the market demand for innovative anodes. As water scarcity becomes a global concern, there is an increasing need for efficient electrochemical water treatment systems. Advanced anodes capable of generating powerful oxidants for contaminant removal are highly sought after in this rapidly expanding market.
The push for circular economy practices and resource recovery is further fueling the demand for new anode developments. Industries are looking for anodes that can facilitate the recovery of valuable metals from waste streams and enable more efficient recycling processes. This trend is particularly evident in the electronics and battery recycling sectors, where the recovery of precious and rare earth metals is becoming increasingly important.
Energy storage and renewable energy integration are also driving factors in the market for new anode technologies. As the world transitions towards cleaner energy sources, there is a growing need for efficient electrolysis systems for hydrogen production. Anodes that can withstand high current densities and operate in harsh alkaline environments are in high demand for green hydrogen production.
The market is also seeing increased interest from emerging economies, particularly in Asia-Pacific and Latin America, where rapid industrialization is driving the adoption of advanced electrolytic processes. These regions represent significant growth opportunities for new anode technologies, as industries seek to leapfrog older technologies and implement state-of-the-art solutions.
Current Anode Challenges
The current challenges faced by anodes in electrolytic cells for industrial processes are multifaceted and significant. One of the primary issues is the degradation of anode materials over time, which leads to reduced efficiency and increased operational costs. This degradation is often caused by the harsh chemical environment within the electrolytic cell, where anodes are exposed to corrosive electrolytes and high temperatures.
Another major challenge is the formation of unwanted byproducts during the electrolysis process. For instance, in aluminum production, carbon anodes produce carbon dioxide as a byproduct, contributing to greenhouse gas emissions. This environmental concern has led to increased pressure on industries to develop more sustainable anode technologies.
The energy efficiency of current anode materials is also a significant challenge. Many industrial electrolytic processes require substantial amounts of electricity, and improving the energy efficiency of anodes could lead to significant cost savings and reduced environmental impact. However, developing materials that can maintain high conductivity while withstanding the harsh conditions of the electrolytic cell remains a complex task.
Dimensional stability is another critical issue for anodes in industrial processes. As anodes wear down or expand during operation, it can lead to uneven current distribution and reduced process efficiency. This problem is particularly acute in large-scale industrial applications where even small variations can have significant impacts on overall performance.
The cost of anode materials and their replacement frequency also pose challenges for industrial operators. Many high-performance anode materials are expensive, and frequent replacements can significantly impact the economic viability of electrolytic processes. Balancing performance with cost-effectiveness remains a key challenge in anode development.
Furthermore, the scalability of new anode technologies presents a significant hurdle. While promising materials may be developed in laboratory settings, translating these innovations to large-scale industrial applications often encounters unforeseen challenges related to manufacturing, durability, and process integration.
Lastly, the increasing demand for high-purity products in industries such as electronics and pharmaceuticals places additional requirements on anode performance. Anodes must not only be efficient and durable but also avoid introducing impurities into the final product, which adds another layer of complexity to their design and material selection.
Another major challenge is the formation of unwanted byproducts during the electrolysis process. For instance, in aluminum production, carbon anodes produce carbon dioxide as a byproduct, contributing to greenhouse gas emissions. This environmental concern has led to increased pressure on industries to develop more sustainable anode technologies.
The energy efficiency of current anode materials is also a significant challenge. Many industrial electrolytic processes require substantial amounts of electricity, and improving the energy efficiency of anodes could lead to significant cost savings and reduced environmental impact. However, developing materials that can maintain high conductivity while withstanding the harsh conditions of the electrolytic cell remains a complex task.
Dimensional stability is another critical issue for anodes in industrial processes. As anodes wear down or expand during operation, it can lead to uneven current distribution and reduced process efficiency. This problem is particularly acute in large-scale industrial applications where even small variations can have significant impacts on overall performance.
The cost of anode materials and their replacement frequency also pose challenges for industrial operators. Many high-performance anode materials are expensive, and frequent replacements can significantly impact the economic viability of electrolytic processes. Balancing performance with cost-effectiveness remains a key challenge in anode development.
Furthermore, the scalability of new anode technologies presents a significant hurdle. While promising materials may be developed in laboratory settings, translating these innovations to large-scale industrial applications often encounters unforeseen challenges related to manufacturing, durability, and process integration.
Lastly, the increasing demand for high-purity products in industries such as electronics and pharmaceuticals places additional requirements on anode performance. Anodes must not only be efficient and durable but also avoid introducing impurities into the final product, which adds another layer of complexity to their design and material selection.
State-of-the-Art Anodes
01 Improved anode materials for enhanced performance
Advanced materials are used to create anodes with superior conductivity, durability, and efficiency. These materials may include metal alloys, composites, or specially treated surfaces that enhance the overall performance of electrolytic cells.- Improved anode materials for enhanced performance: Advanced materials are being developed for anodes in electrolytic cells to improve their performance. These materials often include novel alloys, composites, or coatings that enhance conductivity, durability, and efficiency. Such improvements can lead to increased current density, reduced energy consumption, and extended anode lifespan in various electrolytic processes.
- Optimized anode design and configuration: Innovative designs and configurations of anodes are being explored to enhance their performance in electrolytic cells. This includes optimizing the shape, size, and arrangement of anodes to improve current distribution, reduce voltage drop, and minimize anode wear. Such design improvements can lead to more efficient electrolysis processes and reduced operational costs.
- Advanced coating techniques for anode protection: Various coating techniques are being developed to protect anodes from corrosion and degradation in harsh electrolytic environments. These coatings can include ceramic, metallic, or composite materials that enhance the anode's resistance to chemical attack and erosion. Improved coating methods can significantly extend anode lifespan and maintain consistent performance over time.
- Electrochemical surface modification of anodes: Electrochemical techniques are being employed to modify the surface properties of anodes, enhancing their performance in electrolytic cells. These modifications can include surface activation, passivation, or the creation of specific surface structures that improve catalytic activity, reduce overpotential, and increase current efficiency. Such treatments can lead to more effective and economical electrolysis processes.
- Monitoring and control systems for anode performance: Advanced monitoring and control systems are being developed to optimize anode performance in real-time during electrolytic processes. These systems can include sensors, data analytics, and automated adjustment mechanisms to maintain optimal operating conditions, detect early signs of anode degradation, and ensure consistent performance. Such technologies can lead to improved process efficiency and reduced downtime in industrial electrolysis applications.
02 Optimized anode design and structure
Innovative anode designs and structures are developed to maximize surface area, improve current distribution, and minimize energy losses. This may involve specific geometries, perforations, or layered constructions that enhance the anode's effectiveness in electrolytic processes.Expand Specific Solutions03 Coating technologies for anode protection
Advanced coating techniques are employed to protect anodes from corrosion and extend their lifespan. These coatings may also improve the anode's catalytic properties, leading to increased efficiency and reduced energy consumption in electrolytic cells.Expand Specific Solutions04 Temperature control and management systems
Sophisticated temperature control and management systems are integrated into electrolytic cells to maintain optimal operating conditions for anodes. These systems help prevent overheating, reduce thermal stress, and ensure consistent performance throughout the electrolysis process.Expand Specific Solutions05 Monitoring and control mechanisms for anode performance
Advanced monitoring and control mechanisms are implemented to continuously assess and optimize anode performance in real-time. These systems may include sensors, data analytics, and automated adjustments to maintain peak efficiency and extend the operational life of anodes in electrolytic cells.Expand Specific Solutions
Key Industry Players
The development of new anodes for electrolytic cells in industrial processes is in a mature stage, with ongoing research and innovation. The market size is substantial, driven by the growing demand for efficient and sustainable industrial processes. Technologically, the field is advancing rapidly, with companies like Industrie De Nora SpA, Rio Tinto Alcan International Ltd., and Umicore Galvanotechnik GmbH leading the way. Academic institutions such as Tianjin University and Massachusetts Institute of Technology are contributing significant research. The competitive landscape is characterized by a mix of established industrial players and innovative startups, with a focus on improving efficiency, durability, and environmental performance of electrolytic cells.
Industrie De Nora SpA
Technical Solution: Industrie De Nora SpA has developed advanced DSA (Dimensionally Stable Anodes) technology for electrolytic cells in industrial processes. Their latest innovation involves the use of mixed metal oxide coatings on titanium substrates, which significantly enhances the anode's durability and efficiency[1]. The company has also introduced a novel manufacturing process that allows for precise control of the coating composition and microstructure, resulting in anodes with optimized catalytic activity and reduced overpotential[3]. Furthermore, De Nora has implemented a proprietary activation technique that increases the active surface area of the anodes, leading to improved current distribution and reduced energy consumption in electrolytic processes[5].
Strengths: High durability, improved efficiency, and reduced energy consumption. Weaknesses: Potentially higher initial costs and limited applicability in certain specialized electrochemical processes.
Rio Tinto Alcan International Ltd.
Technical Solution: Rio Tinto Alcan has developed a revolutionary anode technology for aluminum smelting called the inert anode. This technology replaces traditional carbon anodes with a ceramic or metal-based anode that is not consumed during the electrolysis process[2]. The inert anode produces oxygen instead of carbon dioxide, significantly reducing greenhouse gas emissions. The company has also implemented advanced control systems and predictive modeling to optimize anode performance and lifespan[4]. Additionally, Rio Tinto Alcan has introduced a novel anode design that improves current distribution and reduces energy consumption in aluminum production[6].
Strengths: Environmentally friendly, reduced operational costs, and improved process efficiency. Weaknesses: High initial investment and potential challenges in scaling up the technology for large-scale industrial applications.
Innovative Anode Materials
Anode for oxygen evolution
PatentWO2012175673A1
Innovation
- A valve metal substrate with a catalytic layer comprising a mixture of iridium, tin, and a doping element such as bismuth, antimony, or niobium, combined with a protective valve metal oxide layer, forming small crystallites for enhanced catalytic activity and corrosion resistance, achieved through thermal decomposition of precursor salts at reduced temperatures.
Electrode for electrolytic processes and method for producing the same
PatentPendingUS20240328010A1
Innovation
- A valve metal electrode with a catalytic layer composed of metal oxides (17-42% Ta, 40-57% Ru, 19.5-26 Ir) and a barrier layer (25-40% Ta, 0-16% Ru, 0-9% Ir, 44-55% Sn) is developed, featuring a diffusion area with metal oxides (17-41% Ta, 17-39% Ru, 10-26% Ir) to enhance durability and stability, using a method involving pre-treatment, thermal decomposition, and specific precursor solutions.
Environmental Impact
The development of new anodes for electrolytic cells in industrial processes has significant environmental implications. These advancements aim to reduce the ecological footprint of various industries, particularly in sectors such as metal production, water treatment, and chemical manufacturing.
One of the primary environmental benefits of new anode technologies is the potential for increased energy efficiency. By improving the conductivity and reducing the overpotential of anodes, less electricity is required to drive electrolytic reactions. This translates to lower energy consumption and, consequently, reduced greenhouse gas emissions associated with power generation. In some cases, these improvements can lead to energy savings of up to 15-20% compared to traditional anode materials.
New anode materials also often exhibit enhanced durability and resistance to corrosion. This increased lifespan reduces the frequency of anode replacement, minimizing waste generation and the environmental impact associated with the production and disposal of anode materials. For instance, dimensionally stable anodes (DSAs) used in chlor-alkali production can last several years longer than their predecessors, significantly reducing material consumption and waste.
Another crucial environmental aspect is the reduction of harmful by-products. Advanced anode designs can minimize the formation of unwanted compounds during electrolysis. In water treatment applications, for example, novel anode materials can reduce the formation of chlorinated organic compounds, which are potentially harmful to aquatic ecosystems and human health.
The use of more environmentally friendly materials in anode construction is also a key focus. Researchers are exploring alternatives to toxic or rare materials, such as lead or platinum group metals, in favor of more abundant and less harmful options. This shift not only reduces the environmental impact of anode production but also addresses concerns about resource depletion and sustainable material use.
In some industrial processes, new anode developments are enabling the replacement of traditional chemical-based methods with electrochemical alternatives. This transition can lead to a significant reduction in chemical waste and the associated environmental risks of storage, handling, and disposal of hazardous substances.
Lastly, the improved selectivity of new anodes can enhance the overall efficiency of industrial processes. By promoting desired reactions and suppressing side reactions, these anodes can reduce waste production and improve resource utilization. This optimization contributes to a more circular economy approach in industrial operations, aligning with broader sustainability goals.
One of the primary environmental benefits of new anode technologies is the potential for increased energy efficiency. By improving the conductivity and reducing the overpotential of anodes, less electricity is required to drive electrolytic reactions. This translates to lower energy consumption and, consequently, reduced greenhouse gas emissions associated with power generation. In some cases, these improvements can lead to energy savings of up to 15-20% compared to traditional anode materials.
New anode materials also often exhibit enhanced durability and resistance to corrosion. This increased lifespan reduces the frequency of anode replacement, minimizing waste generation and the environmental impact associated with the production and disposal of anode materials. For instance, dimensionally stable anodes (DSAs) used in chlor-alkali production can last several years longer than their predecessors, significantly reducing material consumption and waste.
Another crucial environmental aspect is the reduction of harmful by-products. Advanced anode designs can minimize the formation of unwanted compounds during electrolysis. In water treatment applications, for example, novel anode materials can reduce the formation of chlorinated organic compounds, which are potentially harmful to aquatic ecosystems and human health.
The use of more environmentally friendly materials in anode construction is also a key focus. Researchers are exploring alternatives to toxic or rare materials, such as lead or platinum group metals, in favor of more abundant and less harmful options. This shift not only reduces the environmental impact of anode production but also addresses concerns about resource depletion and sustainable material use.
In some industrial processes, new anode developments are enabling the replacement of traditional chemical-based methods with electrochemical alternatives. This transition can lead to a significant reduction in chemical waste and the associated environmental risks of storage, handling, and disposal of hazardous substances.
Lastly, the improved selectivity of new anodes can enhance the overall efficiency of industrial processes. By promoting desired reactions and suppressing side reactions, these anodes can reduce waste production and improve resource utilization. This optimization contributes to a more circular economy approach in industrial operations, aligning with broader sustainability goals.
Cost-Benefit Analysis
The cost-benefit analysis of new anode developments for electrolytic cells in industrial processes reveals a complex interplay of economic factors and technological advancements. Initial investment costs for implementing novel anode technologies can be substantial, often requiring significant capital expenditure for research, development, and infrastructure modifications. However, these upfront costs must be weighed against the potential long-term benefits and operational savings.
One of the primary advantages of new anode developments is the potential for increased energy efficiency. Advanced anode materials and designs can reduce the overpotential required for electrolysis, leading to lower energy consumption per unit of product. This energy saving translates directly into reduced operational costs, which can be substantial in energy-intensive industries such as aluminum production or chlor-alkali processes.
Improved anode durability is another key factor in the cost-benefit equation. Newer anode materials often exhibit enhanced resistance to corrosion and degradation, resulting in extended operational lifetimes. This longevity reduces the frequency of anode replacements, minimizing production downtime and maintenance costs. The reduced need for raw materials and labor associated with anode replacement further contributes to overall cost savings.
Environmental considerations also play a crucial role in the cost-benefit analysis. Many new anode developments aim to reduce harmful emissions and improve the overall environmental footprint of electrolytic processes. While the immediate financial benefits may be less tangible, the long-term advantages of compliance with stricter environmental regulations and improved corporate sustainability can be significant.
The potential for increased product quality and purity is another important aspect to consider. Advanced anode technologies can lead to more precise control over electrolytic reactions, resulting in higher-grade outputs. This improvement in product quality can command premium prices in the market, offsetting the initial investment costs and potentially increasing profit margins.
However, the cost-benefit analysis must also account for potential risks and challenges. The implementation of new anode technologies may require extensive process modifications and operator training, leading to temporary production disruptions. Additionally, the long-term performance and reliability of novel anode materials in industrial-scale operations may not be fully established, introducing an element of uncertainty into the cost projections.
In conclusion, while the initial costs of adopting new anode developments can be significant, the potential long-term benefits in terms of energy efficiency, operational costs, environmental performance, and product quality often justify the investment. A thorough cost-benefit analysis should consider both immediate financial implications and broader strategic advantages to make informed decisions about implementing these technological advancements in industrial electrolytic processes.
One of the primary advantages of new anode developments is the potential for increased energy efficiency. Advanced anode materials and designs can reduce the overpotential required for electrolysis, leading to lower energy consumption per unit of product. This energy saving translates directly into reduced operational costs, which can be substantial in energy-intensive industries such as aluminum production or chlor-alkali processes.
Improved anode durability is another key factor in the cost-benefit equation. Newer anode materials often exhibit enhanced resistance to corrosion and degradation, resulting in extended operational lifetimes. This longevity reduces the frequency of anode replacements, minimizing production downtime and maintenance costs. The reduced need for raw materials and labor associated with anode replacement further contributes to overall cost savings.
Environmental considerations also play a crucial role in the cost-benefit analysis. Many new anode developments aim to reduce harmful emissions and improve the overall environmental footprint of electrolytic processes. While the immediate financial benefits may be less tangible, the long-term advantages of compliance with stricter environmental regulations and improved corporate sustainability can be significant.
The potential for increased product quality and purity is another important aspect to consider. Advanced anode technologies can lead to more precise control over electrolytic reactions, resulting in higher-grade outputs. This improvement in product quality can command premium prices in the market, offsetting the initial investment costs and potentially increasing profit margins.
However, the cost-benefit analysis must also account for potential risks and challenges. The implementation of new anode technologies may require extensive process modifications and operator training, leading to temporary production disruptions. Additionally, the long-term performance and reliability of novel anode materials in industrial-scale operations may not be fully established, introducing an element of uncertainty into the cost projections.
In conclusion, while the initial costs of adopting new anode developments can be significant, the potential long-term benefits in terms of energy efficiency, operational costs, environmental performance, and product quality often justify the investment. A thorough cost-benefit analysis should consider both immediate financial implications and broader strategic advantages to make informed decisions about implementing these technological advancements in industrial electrolytic processes.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!