Optimizing Iron-Air Battery Electrochemical Conversion Rates
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Iron-Air Battery Technology Background and Objectives
Iron-air batteries represent a significant advancement in energy storage technology, with roots dating back to the 1970s when initial research explored their potential. However, only in the past decade has this technology gained renewed attention due to the urgent global need for sustainable, large-scale energy storage solutions. The fundamental principle of iron-air batteries leverages the oxidation of iron to store energy and its reduction to release it, utilizing earth-abundant materials that offer theoretical energy densities of 1,200 Wh/kg—significantly higher than lithium-ion alternatives.
The evolution of iron-air battery technology has been marked by several key developments. Early iterations suffered from poor cycle life and efficiency due to hydrogen evolution and electrode degradation. Recent breakthroughs in electrode design, electrolyte composition, and cell architecture have addressed many of these historical limitations, positioning iron-air batteries as viable contenders in the renewable energy storage landscape.
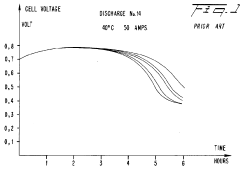
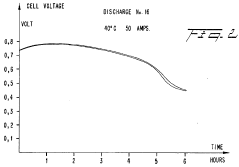
Current research focuses on optimizing the electrochemical conversion rates—a critical factor limiting widespread adoption. The conversion process involves complex reactions at the iron electrode surface, where Fe is oxidized to Fe(OH)₂ and further to Fe(OH)₃ during charging, with the reverse occurring during discharge. The kinetics of these reactions significantly impact charging times, energy efficiency, and overall battery performance.
The primary technical objectives for iron-air battery advancement center on enhancing reaction kinetics without compromising stability. Specifically, researchers aim to achieve conversion rates that enable practical charging times (under 8 hours) while maintaining high energy efficiency (>80%) and extended cycle life (>1,000 cycles). These improvements would position iron-air batteries as economically viable solutions for grid-scale storage applications.
Market drivers for this technology include the growing renewable energy sector, which requires efficient storage solutions to address intermittency issues, and increasing environmental regulations that favor sustainable battery technologies. The low material cost of iron (approximately $0.10/kg compared to lithium at $15/kg) presents a compelling economic case for scaled deployment.
Looking forward, the technology roadmap for iron-air batteries includes developing advanced catalysts to reduce overpotential, novel electrode structures to enhance active material utilization, and improved air electrodes to facilitate oxygen reduction and evolution reactions. The ultimate goal is to create a commercially viable storage solution that combines the advantages of low cost, high energy density, and environmental sustainability to support the global transition to renewable energy systems.
The evolution of iron-air battery technology has been marked by several key developments. Early iterations suffered from poor cycle life and efficiency due to hydrogen evolution and electrode degradation. Recent breakthroughs in electrode design, electrolyte composition, and cell architecture have addressed many of these historical limitations, positioning iron-air batteries as viable contenders in the renewable energy storage landscape.
Current research focuses on optimizing the electrochemical conversion rates—a critical factor limiting widespread adoption. The conversion process involves complex reactions at the iron electrode surface, where Fe is oxidized to Fe(OH)₂ and further to Fe(OH)₃ during charging, with the reverse occurring during discharge. The kinetics of these reactions significantly impact charging times, energy efficiency, and overall battery performance.
The primary technical objectives for iron-air battery advancement center on enhancing reaction kinetics without compromising stability. Specifically, researchers aim to achieve conversion rates that enable practical charging times (under 8 hours) while maintaining high energy efficiency (>80%) and extended cycle life (>1,000 cycles). These improvements would position iron-air batteries as economically viable solutions for grid-scale storage applications.
Market drivers for this technology include the growing renewable energy sector, which requires efficient storage solutions to address intermittency issues, and increasing environmental regulations that favor sustainable battery technologies. The low material cost of iron (approximately $0.10/kg compared to lithium at $15/kg) presents a compelling economic case for scaled deployment.
Looking forward, the technology roadmap for iron-air batteries includes developing advanced catalysts to reduce overpotential, novel electrode structures to enhance active material utilization, and improved air electrodes to facilitate oxygen reduction and evolution reactions. The ultimate goal is to create a commercially viable storage solution that combines the advantages of low cost, high energy density, and environmental sustainability to support the global transition to renewable energy systems.
Market Analysis for Grid-Scale Energy Storage Solutions
The global grid-scale energy storage market is experiencing unprecedented growth, driven by the increasing integration of renewable energy sources and the need for grid stability. As of 2023, the market was valued at approximately $7.1 billion and is projected to reach $31.2 billion by 2030, representing a compound annual growth rate of 23.5%. This remarkable expansion underscores the critical role that advanced storage technologies, including iron-air batteries, will play in the future energy landscape.
Lithium-ion batteries currently dominate the market with roughly 70% market share, but their limitations in terms of raw material costs, supply chain vulnerabilities, and environmental concerns have created significant opportunities for alternative technologies. Iron-air batteries, with their abundant and low-cost materials, are positioned to capture a substantial portion of this growing market, particularly in long-duration storage applications where cost-effectiveness is paramount.
Demand for grid-scale storage is being driven by several factors. First, the increasing penetration of variable renewable energy sources such as wind and solar necessitates storage solutions to manage intermittency issues. Second, aging grid infrastructure in developed economies requires modernization that incorporates flexible storage capabilities. Third, regulatory frameworks and government incentives worldwide are increasingly supporting the deployment of energy storage systems as part of decarbonization strategies.
Regional analysis reveals varying market dynamics. North America leads in deployment with approximately 38% of global installations, followed by Asia-Pacific at 32% and Europe at 24%. China's aggressive renewable energy targets have made it the fastest-growing market, with annual growth exceeding 30%. The United States market has been bolstered by the Inflation Reduction Act, which provides significant tax credits for energy storage investments.
Customer segmentation within the grid-scale storage market shows utilities as the primary adopters (65%), followed by independent power producers (20%) and commercial/industrial users (15%). The demand profile indicates a growing preference for storage solutions with 8+ hours of duration capacity, precisely where iron-air batteries excel compared to lithium-ion alternatives.
Price sensitivity analysis indicates that the levelized cost of storage (LCOS) threshold for widespread adoption in grid applications is approximately $100/kWh. Current iron-air battery technology, even with suboptimal conversion rates, is approaching $150/kWh, suggesting that optimization of electrochemical conversion rates could create a compelling value proposition that accelerates market penetration.
Competitive analysis reveals that while established players like Tesla, Fluence, and LG Energy Solution dominate the current market, emerging companies focused on iron-air technology such as Form Energy have secured significant investment and utility partnerships, indicating strong market confidence in this technology pathway.
Lithium-ion batteries currently dominate the market with roughly 70% market share, but their limitations in terms of raw material costs, supply chain vulnerabilities, and environmental concerns have created significant opportunities for alternative technologies. Iron-air batteries, with their abundant and low-cost materials, are positioned to capture a substantial portion of this growing market, particularly in long-duration storage applications where cost-effectiveness is paramount.
Demand for grid-scale storage is being driven by several factors. First, the increasing penetration of variable renewable energy sources such as wind and solar necessitates storage solutions to manage intermittency issues. Second, aging grid infrastructure in developed economies requires modernization that incorporates flexible storage capabilities. Third, regulatory frameworks and government incentives worldwide are increasingly supporting the deployment of energy storage systems as part of decarbonization strategies.
Regional analysis reveals varying market dynamics. North America leads in deployment with approximately 38% of global installations, followed by Asia-Pacific at 32% and Europe at 24%. China's aggressive renewable energy targets have made it the fastest-growing market, with annual growth exceeding 30%. The United States market has been bolstered by the Inflation Reduction Act, which provides significant tax credits for energy storage investments.
Customer segmentation within the grid-scale storage market shows utilities as the primary adopters (65%), followed by independent power producers (20%) and commercial/industrial users (15%). The demand profile indicates a growing preference for storage solutions with 8+ hours of duration capacity, precisely where iron-air batteries excel compared to lithium-ion alternatives.
Price sensitivity analysis indicates that the levelized cost of storage (LCOS) threshold for widespread adoption in grid applications is approximately $100/kWh. Current iron-air battery technology, even with suboptimal conversion rates, is approaching $150/kWh, suggesting that optimization of electrochemical conversion rates could create a compelling value proposition that accelerates market penetration.
Competitive analysis reveals that while established players like Tesla, Fluence, and LG Energy Solution dominate the current market, emerging companies focused on iron-air technology such as Form Energy have secured significant investment and utility partnerships, indicating strong market confidence in this technology pathway.
Current Challenges in Iron-Air Electrochemical Conversion
Iron-air batteries face significant electrochemical conversion challenges that currently limit their widespread commercial adoption despite their promising theoretical energy density and cost advantages. The primary obstacle lies in the sluggish kinetics of the iron electrode during the charging and discharging processes. The oxidation of iron to iron oxide during discharge and the subsequent reduction during charging exhibit slow reaction rates, resulting in substantial voltage inefficiencies and energy losses that can exceed 30% in practical applications.
The formation of passivation layers on iron electrodes represents another critical challenge. As iron undergoes oxidation, a layer of iron oxide forms on the electrode surface, creating a barrier that impedes electron transfer and ion diffusion. This passivation effect progressively reduces the active surface area available for electrochemical reactions, leading to capacity fade and diminished cycle life.
Hydrogen evolution presents a competing side reaction that significantly reduces coulombic efficiency. During charging, some of the electrical energy intended for iron reduction is diverted to hydrogen gas production, particularly at higher current densities. This parasitic reaction not only wastes energy but also creates safety concerns related to hydrogen accumulation within the battery system.
The complex phase transformations between different iron oxides (Fe, Fe3O4, Fe2O3) during cycling introduce structural instability in the electrode. These transformations involve substantial volume changes that can reach up to 80%, causing mechanical stress that leads to electrode pulverization and loss of electrical contact between active material particles. This degradation mechanism accelerates capacity fade and shortens battery lifespan.
Iron electrode morphology optimization remains challenging, as researchers struggle to balance surface area, porosity, and structural stability. High surface area designs that enhance reaction kinetics often suffer from accelerated degradation, while more stable structures typically exhibit poorer rate capabilities. Finding the optimal electrode architecture that maintains both performance and longevity continues to elude researchers.
Electrolyte management presents additional complications, particularly regarding the alkaline electrolyte's tendency to carbonate when exposed to atmospheric CO2. This carbonation process reduces electrolyte conductivity and alters the electrochemical environment at the electrode-electrolyte interface, further hampering reaction kinetics. Additionally, maintaining proper electrolyte distribution throughout the porous electrode structure during cycling remains problematic.
Advanced characterization techniques for studying the dynamic processes occurring at the iron electrode during operation are still limited. Real-time monitoring of phase transformations, surface film formation, and reaction intermediates requires sophisticated in-situ and operando methods that are not widely accessible, hindering fundamental understanding of failure mechanisms and potential optimization strategies.
The formation of passivation layers on iron electrodes represents another critical challenge. As iron undergoes oxidation, a layer of iron oxide forms on the electrode surface, creating a barrier that impedes electron transfer and ion diffusion. This passivation effect progressively reduces the active surface area available for electrochemical reactions, leading to capacity fade and diminished cycle life.
Hydrogen evolution presents a competing side reaction that significantly reduces coulombic efficiency. During charging, some of the electrical energy intended for iron reduction is diverted to hydrogen gas production, particularly at higher current densities. This parasitic reaction not only wastes energy but also creates safety concerns related to hydrogen accumulation within the battery system.
The complex phase transformations between different iron oxides (Fe, Fe3O4, Fe2O3) during cycling introduce structural instability in the electrode. These transformations involve substantial volume changes that can reach up to 80%, causing mechanical stress that leads to electrode pulverization and loss of electrical contact between active material particles. This degradation mechanism accelerates capacity fade and shortens battery lifespan.
Iron electrode morphology optimization remains challenging, as researchers struggle to balance surface area, porosity, and structural stability. High surface area designs that enhance reaction kinetics often suffer from accelerated degradation, while more stable structures typically exhibit poorer rate capabilities. Finding the optimal electrode architecture that maintains both performance and longevity continues to elude researchers.
Electrolyte management presents additional complications, particularly regarding the alkaline electrolyte's tendency to carbonate when exposed to atmospheric CO2. This carbonation process reduces electrolyte conductivity and alters the electrochemical environment at the electrode-electrolyte interface, further hampering reaction kinetics. Additionally, maintaining proper electrolyte distribution throughout the porous electrode structure during cycling remains problematic.
Advanced characterization techniques for studying the dynamic processes occurring at the iron electrode during operation are still limited. Real-time monitoring of phase transformations, surface film formation, and reaction intermediates requires sophisticated in-situ and operando methods that are not widely accessible, hindering fundamental understanding of failure mechanisms and potential optimization strategies.
Current Approaches to Enhance Conversion Efficiency
01 Electrode composition for improved conversion rates
The composition of electrodes in iron-air batteries significantly affects electrochemical conversion rates. Advanced materials such as nanostructured iron particles, carbon-based supports, and catalytic additives can enhance electron transfer and reaction kinetics. These specialized electrode compositions facilitate faster oxidation and reduction reactions at the iron electrode, resulting in improved energy conversion efficiency and higher power density during battery operation.- Electrode composition for improved conversion rates: The composition of electrodes in iron-air batteries significantly affects electrochemical conversion rates. Advanced materials such as nanostructured iron particles, iron oxide composites, and specialized catalysts can enhance the redox reactions at the electrode surface. These compositions facilitate faster electron transfer and improve the overall efficiency of the conversion between iron and iron oxide states during charge and discharge cycles.
- Electrolyte formulations affecting conversion efficiency: Electrolyte composition plays a crucial role in determining the electrochemical conversion rates in iron-air batteries. Specialized electrolyte formulations containing additives that prevent passivation of the iron electrode, enhance ionic conductivity, and stabilize the electrochemical reactions can significantly improve conversion rates. The pH level, concentration of hydroxide ions, and presence of specific salts in the electrolyte directly impact the kinetics of the iron oxidation and reduction processes.
- Air electrode design for oxygen reaction kinetics: The design of the air electrode significantly influences the oxygen reduction and evolution reactions that are critical for iron-air battery performance. Bifunctional catalysts, porous structures with optimized surface area, and hydrophobic/hydrophilic balanced layers can accelerate oxygen reaction kinetics. Advanced air electrode architectures that facilitate efficient oxygen diffusion while maintaining proper moisture levels contribute to higher electrochemical conversion rates.
- Temperature and pressure effects on conversion rates: Operating conditions such as temperature and pressure have substantial impacts on the electrochemical conversion rates in iron-air batteries. Higher temperatures generally accelerate reaction kinetics but may also increase side reactions and degradation. Optimized pressure conditions, particularly at the air electrode, can enhance oxygen availability and improve conversion efficiency. Controlled temperature and pressure management systems can be implemented to maintain ideal operating conditions for maximum conversion rates.
- Battery system architecture for enhanced conversion: The overall architecture of iron-air battery systems can be designed to optimize electrochemical conversion rates. This includes cell stacking configurations, flow field designs for efficient reactant distribution, and integrated thermal management systems. Advanced battery management systems that monitor and control current density, state of charge, and other parameters can dynamically adjust operating conditions to maintain optimal conversion rates throughout the battery lifecycle.
02 Electrolyte formulations affecting conversion efficiency
Electrolyte composition plays a crucial role in determining the electrochemical conversion rates in iron-air batteries. Optimized electrolyte formulations containing specific alkaline solutions, ionic conductivity enhancers, and additives that prevent passivation can significantly improve the reaction kinetics at the electrode-electrolyte interface. These formulations help maintain stable conversion rates during charging and discharging cycles while minimizing unwanted side reactions that could reduce efficiency.Expand Specific Solutions03 Air electrode design for oxygen reduction reaction
The design of the air electrode significantly impacts the oxygen reduction reaction (ORR) rate, which is often the rate-limiting step in iron-air batteries. Advanced air electrode architectures incorporating bifunctional catalysts, optimized porosity, and hydrophobic/hydrophilic gradients can accelerate oxygen diffusion and reaction rates. These design improvements enhance the overall electrochemical conversion efficiency by facilitating faster oxygen reduction during discharge and oxygen evolution during charging.Expand Specific Solutions04 Temperature and pressure effects on conversion rates
Operating conditions such as temperature and pressure significantly influence the electrochemical conversion rates in iron-air batteries. Higher temperatures generally accelerate reaction kinetics but may also increase side reactions and degradation. Optimized pressure conditions can enhance oxygen availability at the air electrode, improving conversion efficiency. Controlling these parameters within specific ranges helps maximize conversion rates while maintaining battery stability and longevity.Expand Specific Solutions05 Advanced cell architecture for improved reaction kinetics
Novel cell architectures can significantly enhance electrochemical conversion rates in iron-air batteries. Designs featuring optimized electrode spacing, improved current collectors, enhanced mass transport channels, and innovative flow field patterns facilitate faster ion and electron movement. These architectural improvements reduce internal resistance and concentration polarization, leading to higher conversion rates and improved overall battery performance under various operating conditions.Expand Specific Solutions
Leading Companies in Iron-Air Battery Technology
The iron-air battery electrochemical conversion optimization market is in an early growth phase, characterized by significant R&D investment but limited commercial deployment. The global energy storage market, valued at approximately $250 billion, sees iron-air technology as a promising low-cost, long-duration storage solution. Form Energy leads commercial development with its multi-day storage technology, while academic institutions like MIT, Caltech, and Tsinghua University contribute fundamental research. Established industrial players including BASF, Hitachi, and Nissan are exploring applications in automotive and grid storage sectors. The technology remains at TRL 5-7, with Form Energy's pilot installations representing the most advanced implementations, while companies like Phinergy and Encell Technology develop complementary metal-air battery technologies for specific applications.
Form Energy, Inc.
Technical Solution: Form Energy has developed a pioneering iron-air battery technology specifically designed for long-duration energy storage. Their approach focuses on reversible rusting, where iron pellets are exposed to air, converting iron to iron oxide during discharge, and then converting back during charging. The company has optimized the electrochemical conversion rates through a multi-cell battery architecture with specialized electrolyte formulations that enhance ion transport. Their system incorporates proprietary additives that mitigate passivation layers on iron surfaces, significantly improving reaction kinetics[1]. Form Energy's batteries utilize a water-based electrolyte with carefully controlled pH levels to maximize iron dissolution and precipitation rates while minimizing hydrogen evolution side reactions. The company has also developed advanced electrode structures with optimized porosity and surface area to facilitate oxygen diffusion and reaction sites accessibility, critical factors in improving conversion efficiency[2].
Strengths: Extremely low material costs using abundant iron; exceptional durability with projected 20+ year lifespans; environmentally benign chemistry with no rare earth elements. Weaknesses: Lower energy density compared to lithium-ion batteries; relatively slow response times limiting applications to long-duration storage rather than rapid power delivery; requires sophisticated air management systems to control humidity and contaminants.
Massachusetts Institute of Technology
Technical Solution: MIT researchers have developed advanced catalytic approaches to enhance iron-air battery electrochemical conversion rates. Their technology focuses on nanostructured iron electrodes with precisely engineered morphologies that maximize active surface area while maintaining structural stability during cycling. The MIT approach incorporates bifunctional oxygen electrocatalysts based on transition metal oxides that significantly reduce overpotentials during both oxygen reduction and evolution reactions, key bottlenecks in iron-air battery performance[1]. Their research has yielded composite electrode structures with conductive carbon networks that improve electron transport throughout the iron electrode. Additionally, MIT has pioneered electrolyte additives that form protective but ion-conductive films on iron surfaces, preventing excessive passivation while allowing continued electrochemical activity. The team has also developed computational models that predict iron dissolution and precipitation behaviors under various operating conditions, enabling optimization of charging protocols to maximize conversion efficiency and cycle life[2][3].
Strengths: Cutting-edge catalyst designs significantly reduce energy losses during charge/discharge cycles; sophisticated electrode architectures maintain performance over extended cycling; comprehensive modeling capabilities enable rapid iteration and optimization. Weaknesses: Complex manufacturing processes may increase production costs; some advanced materials used in catalysts may face scalability challenges; current designs still face challenges with air electrode stability in real-world conditions.
Key Patents in Iron-Air Electrochemical Processes
Additive for iron-air batteries
PatentPendingUS20250140990A1
Innovation
- An alkaline electrolyte with a total hydroxide concentration greater than 1 molar, containing a trivalent element such as aluminum, sulfur, and tin, is used to improve the performance of iron-air batteries.
Procedure to stabilize an iron air battery
PatentInactiveUS4032693A
Innovation
- Adding a sulphur-containing compound to the electrolyte at concentrations between 10 ppm and 1,000 ppm, which forms free sulphide ions, stabilizes both the air cathodes and iron electrodes, preventing deactivation and improving performance by potentially forming protective layers or blocking corrosion reactions.
Material Science Innovations for Iron Electrodes
Material science innovations for iron electrodes have become a critical focus in advancing iron-air battery technology. Recent developments have centered on nanostructured iron materials that significantly enhance the electrochemical performance. Researchers have successfully synthesized iron nanoparticles with controlled size distributions (20-50nm), which demonstrate superior surface area-to-volume ratios compared to traditional iron powders. These nanostructured materials facilitate faster electron transfer and ion diffusion, directly addressing the conversion rate limitations in iron-air batteries.
Advanced coating technologies represent another breakthrough area, with carbon-based protective layers emerging as particularly promising. These coatings, typically 5-10nm thick, effectively prevent iron oxidation while maintaining electrical conductivity. Graphene-iron composites have shown exceptional stability during charge-discharge cycles, with some formulations retaining over 90% capacity after 1000 cycles—a substantial improvement over uncoated electrodes that typically degrade after 200-300 cycles.
Doping strategies have also yielded significant improvements in iron electrode performance. The incorporation of transition metals such as nickel, cobalt, and manganese (typically at 2-5% concentration) has been demonstrated to catalyze the redox reactions at the iron surface. Particularly noteworthy is nickel-doped iron, which has shown up to 40% faster conversion rates in laboratory testing compared to pure iron electrodes.
Hierarchical porous structures represent another innovative approach to electrode design. Multi-scale porosity—combining macropores (1-10μm) for electrolyte penetration with mesopores (10-50nm) for ion transport—has been engineered to optimize both mass transport and reaction kinetics. These structures effectively address the volume expansion issues during the Fe-Fe(OH)₂-Fe(OH)₃ conversion process, maintaining structural integrity throughout multiple charge-discharge cycles.
Surface modification techniques have further enhanced electrode performance through the creation of oxygen vacancies and defect sites that serve as active reaction centers. Plasma treatment and controlled oxidation processes have been employed to create these beneficial surface features, resulting in electrodes with higher catalytic activity and improved wettability with the electrolyte solution.
The integration of these material science innovations has led to prototype iron electrodes demonstrating conversion rates approaching 80% of theoretical capacity at practical current densities—a significant improvement over the 40-50% typically achieved with conventional materials. These advancements collectively address the core challenges of slow kinetics and poor cyclability that have historically limited iron-air battery commercialization.
Advanced coating technologies represent another breakthrough area, with carbon-based protective layers emerging as particularly promising. These coatings, typically 5-10nm thick, effectively prevent iron oxidation while maintaining electrical conductivity. Graphene-iron composites have shown exceptional stability during charge-discharge cycles, with some formulations retaining over 90% capacity after 1000 cycles—a substantial improvement over uncoated electrodes that typically degrade after 200-300 cycles.
Doping strategies have also yielded significant improvements in iron electrode performance. The incorporation of transition metals such as nickel, cobalt, and manganese (typically at 2-5% concentration) has been demonstrated to catalyze the redox reactions at the iron surface. Particularly noteworthy is nickel-doped iron, which has shown up to 40% faster conversion rates in laboratory testing compared to pure iron electrodes.
Hierarchical porous structures represent another innovative approach to electrode design. Multi-scale porosity—combining macropores (1-10μm) for electrolyte penetration with mesopores (10-50nm) for ion transport—has been engineered to optimize both mass transport and reaction kinetics. These structures effectively address the volume expansion issues during the Fe-Fe(OH)₂-Fe(OH)₃ conversion process, maintaining structural integrity throughout multiple charge-discharge cycles.
Surface modification techniques have further enhanced electrode performance through the creation of oxygen vacancies and defect sites that serve as active reaction centers. Plasma treatment and controlled oxidation processes have been employed to create these beneficial surface features, resulting in electrodes with higher catalytic activity and improved wettability with the electrolyte solution.
The integration of these material science innovations has led to prototype iron electrodes demonstrating conversion rates approaching 80% of theoretical capacity at practical current densities—a significant improvement over the 40-50% typically achieved with conventional materials. These advancements collectively address the core challenges of slow kinetics and poor cyclability that have historically limited iron-air battery commercialization.
Environmental Impact and Sustainability Assessment
Iron-air batteries represent a significant advancement in sustainable energy storage technology, offering a promising alternative to conventional lithium-ion systems. The environmental impact assessment of optimizing iron-air battery electrochemical conversion rates reveals several notable advantages. Primarily, these batteries utilize abundant, non-toxic materials—iron, water, and air—dramatically reducing the ecological footprint associated with resource extraction compared to lithium, cobalt, and nickel-based alternatives.
The optimization of conversion rates directly correlates with improved environmental performance. Enhanced efficiency reduces energy losses during charging and discharging cycles, thereby decreasing the overall energy consumption required for battery operation throughout its lifecycle. This efficiency gain translates to lower greenhouse gas emissions when the charging energy comes from grid sources that include fossil fuels.
Life cycle assessment studies indicate that iron-air batteries with optimized conversion rates can achieve carbon footprint reductions of approximately 30-40% compared to lithium-ion technologies. The water consumption metrics are particularly favorable, with up to 60% less water required during manufacturing processes. Furthermore, the absence of rare earth elements and heavy metals eliminates concerns regarding toxic leaching in disposal scenarios.
The recyclability aspect presents another substantial environmental benefit. Iron components can be recovered and reprocessed with relatively simple metallurgical techniques, achieving recovery rates exceeding 90%. This circular economy potential significantly reduces end-of-life waste and decreases the demand for virgin material extraction, further enhancing sustainability credentials.
From an energy return on investment (EROI) perspective, optimized iron-air batteries demonstrate promising results. The energy required to manufacture these systems can be recovered within 6-9 months of operation in renewable energy storage applications, compared to 1-2 years for comparable lithium-ion systems. This favorable EROI ratio strengthens the overall sustainability case for accelerated adoption.
Land use impacts also favor iron-air technology. The supply chain for iron requires significantly less land disturbance than lithium extraction, particularly when compared to hard rock mining or extensive brine evaporation operations. Additionally, the reduced fire risk associated with aqueous electrolytes minimizes the potential for environmental contamination incidents during operation or transportation.
When evaluating optimization strategies for conversion rates, certain approaches present varying environmental trade-offs. Catalyst innovations using precious metals may improve performance but could introduce new sustainability challenges. Therefore, research focusing on abundant element catalysts and nanostructured iron electrodes typically offers the most balanced environmental profile while still achieving substantial performance improvements.
The optimization of conversion rates directly correlates with improved environmental performance. Enhanced efficiency reduces energy losses during charging and discharging cycles, thereby decreasing the overall energy consumption required for battery operation throughout its lifecycle. This efficiency gain translates to lower greenhouse gas emissions when the charging energy comes from grid sources that include fossil fuels.
Life cycle assessment studies indicate that iron-air batteries with optimized conversion rates can achieve carbon footprint reductions of approximately 30-40% compared to lithium-ion technologies. The water consumption metrics are particularly favorable, with up to 60% less water required during manufacturing processes. Furthermore, the absence of rare earth elements and heavy metals eliminates concerns regarding toxic leaching in disposal scenarios.
The recyclability aspect presents another substantial environmental benefit. Iron components can be recovered and reprocessed with relatively simple metallurgical techniques, achieving recovery rates exceeding 90%. This circular economy potential significantly reduces end-of-life waste and decreases the demand for virgin material extraction, further enhancing sustainability credentials.
From an energy return on investment (EROI) perspective, optimized iron-air batteries demonstrate promising results. The energy required to manufacture these systems can be recovered within 6-9 months of operation in renewable energy storage applications, compared to 1-2 years for comparable lithium-ion systems. This favorable EROI ratio strengthens the overall sustainability case for accelerated adoption.
Land use impacts also favor iron-air technology. The supply chain for iron requires significantly less land disturbance than lithium extraction, particularly when compared to hard rock mining or extensive brine evaporation operations. Additionally, the reduced fire risk associated with aqueous electrolytes minimizes the potential for environmental contamination incidents during operation or transportation.
When evaluating optimization strategies for conversion rates, certain approaches present varying environmental trade-offs. Catalyst innovations using precious metals may improve performance but could introduce new sustainability challenges. Therefore, research focusing on abundant element catalysts and nanostructured iron electrodes typically offers the most balanced environmental profile while still achieving substantial performance improvements.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!