Organ-on-chip platforms for modeling drug–drug interactions in multi-organ platforms
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Organ-on-Chip Technology Evolution and Objectives
Organ-on-chip (OoC) technology has evolved significantly over the past decade, transforming from simple microfluidic cell culture systems to sophisticated multi-organ platforms capable of mimicking complex physiological interactions. The evolution began in the early 2000s with rudimentary microfluidic devices that could maintain viable cells in controlled environments. By 2010, researchers had developed single-organ models that could replicate basic organ functions, such as the lung-on-chip developed by the Wyss Institute, which simulated the alveolar-capillary interface.
The mid-2010s marked a critical turning point with the emergence of more complex organ models incorporating multiple cell types and dynamic mechanical forces. These second-generation devices demonstrated improved physiological relevance by replicating organ-specific microenvironments, including fluid flow, mechanical strain, and electrical stimulation. This period also saw the first successful attempts at connecting different organ models to create rudimentary multi-organ systems.
Recent advancements have focused on developing integrated multi-organ platforms specifically designed to model drug-drug interactions (DDIs). These third-generation systems incorporate physiologically-based pharmacokinetic principles to accurately represent drug absorption, distribution, metabolism, and excretion across multiple organ systems. The integration of sensors for real-time monitoring of cellular responses and metabolite concentrations has further enhanced their analytical capabilities.
The primary objective of current OoC technology development is to create validated platforms that can reliably predict complex DDIs that occur when multiple drugs interact within different organs simultaneously. This includes modeling first-pass metabolism in liver-based systems, blood-brain barrier penetration, and nephrotoxicity assessment. Researchers aim to develop platforms that can capture both pharmacokinetic interactions (how one drug affects another's concentration) and pharmacodynamic interactions (how drugs affect each other's mechanisms of action).
Another critical objective is standardization and scalability. For OoC technology to transition from research tools to industry-standard platforms for drug development, reproducible manufacturing processes and validation protocols must be established. This includes developing standardized cell sources, materials, and operational parameters that ensure consistent performance across different laboratories and applications.
Looking forward, the field is moving toward personalized medicine applications, with objectives to incorporate patient-derived cells for individualized DDI testing. Additionally, researchers are working to extend the viability of these systems from days to weeks or months, enabling the study of chronic drug exposures and long-term toxicity effects that are often missed in traditional testing paradigms.
The mid-2010s marked a critical turning point with the emergence of more complex organ models incorporating multiple cell types and dynamic mechanical forces. These second-generation devices demonstrated improved physiological relevance by replicating organ-specific microenvironments, including fluid flow, mechanical strain, and electrical stimulation. This period also saw the first successful attempts at connecting different organ models to create rudimentary multi-organ systems.
Recent advancements have focused on developing integrated multi-organ platforms specifically designed to model drug-drug interactions (DDIs). These third-generation systems incorporate physiologically-based pharmacokinetic principles to accurately represent drug absorption, distribution, metabolism, and excretion across multiple organ systems. The integration of sensors for real-time monitoring of cellular responses and metabolite concentrations has further enhanced their analytical capabilities.
The primary objective of current OoC technology development is to create validated platforms that can reliably predict complex DDIs that occur when multiple drugs interact within different organs simultaneously. This includes modeling first-pass metabolism in liver-based systems, blood-brain barrier penetration, and nephrotoxicity assessment. Researchers aim to develop platforms that can capture both pharmacokinetic interactions (how one drug affects another's concentration) and pharmacodynamic interactions (how drugs affect each other's mechanisms of action).
Another critical objective is standardization and scalability. For OoC technology to transition from research tools to industry-standard platforms for drug development, reproducible manufacturing processes and validation protocols must be established. This includes developing standardized cell sources, materials, and operational parameters that ensure consistent performance across different laboratories and applications.
Looking forward, the field is moving toward personalized medicine applications, with objectives to incorporate patient-derived cells for individualized DDI testing. Additionally, researchers are working to extend the viability of these systems from days to weeks or months, enabling the study of chronic drug exposures and long-term toxicity effects that are often missed in traditional testing paradigms.
Market Analysis for Multi-Organ Drug Interaction Platforms
The global market for organ-on-chip (OOC) platforms focusing on multi-organ drug interaction modeling is experiencing significant growth, driven by increasing demand for more accurate preclinical drug testing methods. Current market valuations place the overall OOC sector at approximately $45 million in 2022, with projections indicating growth to reach $600 million by 2030, representing a compound annual growth rate (CAGR) of 38.7%.
Multi-organ platforms specifically represent an emerging segment within this market, currently accounting for about 15% of the total OOC market but expected to grow at an accelerated rate compared to single-organ systems. This growth is primarily fueled by pharmaceutical companies seeking to reduce the high failure rates of drug candidates in clinical trials due to unforeseen drug-drug interactions (DDIs) and toxicity issues.
The pharmaceutical industry remains the largest end-user segment, contributing nearly 65% of the market revenue for multi-organ platforms. Academic research institutions account for approximately 25%, while contract research organizations (CROs) make up the remaining 10%. Geographically, North America dominates with 45% market share, followed by Europe (30%), Asia-Pacific (20%), and rest of the world (5%).
Key market drivers include the rising costs of drug development, estimated at $2.6 billion per approved drug, with late-stage failures due to toxicity issues accounting for nearly 30% of these costs. Multi-organ platforms offer potential savings of $300-500 million per drug development cycle by identifying problematic drug interactions earlier.
Regulatory support is also bolstering market growth, with the FDA's Predictive Toxicology Roadmap and similar initiatives in Europe encouraging the adoption of alternative testing methods. Additionally, the push to reduce animal testing under various ethical guidelines has created market opportunities for in vitro alternatives.
Market challenges include the high cost of multi-organ platforms, with current systems priced between $50,000-$200,000, limiting adoption particularly among smaller research institutions. Technical challenges in maintaining multiple tissue types simultaneously and achieving physiologically relevant organ-to-organ interactions also present barriers to widespread implementation.
Consumer demand trends indicate growing interest in personalized medicine applications, with patient-derived cells on multi-organ platforms potentially enabling individualized drug interaction predictions. This segment is expected to grow at 45% CAGR over the next five years, outpacing the overall market growth rate.
Multi-organ platforms specifically represent an emerging segment within this market, currently accounting for about 15% of the total OOC market but expected to grow at an accelerated rate compared to single-organ systems. This growth is primarily fueled by pharmaceutical companies seeking to reduce the high failure rates of drug candidates in clinical trials due to unforeseen drug-drug interactions (DDIs) and toxicity issues.
The pharmaceutical industry remains the largest end-user segment, contributing nearly 65% of the market revenue for multi-organ platforms. Academic research institutions account for approximately 25%, while contract research organizations (CROs) make up the remaining 10%. Geographically, North America dominates with 45% market share, followed by Europe (30%), Asia-Pacific (20%), and rest of the world (5%).
Key market drivers include the rising costs of drug development, estimated at $2.6 billion per approved drug, with late-stage failures due to toxicity issues accounting for nearly 30% of these costs. Multi-organ platforms offer potential savings of $300-500 million per drug development cycle by identifying problematic drug interactions earlier.
Regulatory support is also bolstering market growth, with the FDA's Predictive Toxicology Roadmap and similar initiatives in Europe encouraging the adoption of alternative testing methods. Additionally, the push to reduce animal testing under various ethical guidelines has created market opportunities for in vitro alternatives.
Market challenges include the high cost of multi-organ platforms, with current systems priced between $50,000-$200,000, limiting adoption particularly among smaller research institutions. Technical challenges in maintaining multiple tissue types simultaneously and achieving physiologically relevant organ-to-organ interactions also present barriers to widespread implementation.
Consumer demand trends indicate growing interest in personalized medicine applications, with patient-derived cells on multi-organ platforms potentially enabling individualized drug interaction predictions. This segment is expected to grow at 45% CAGR over the next five years, outpacing the overall market growth rate.
Current Challenges in Multi-Organ-on-Chip Systems
Despite significant advancements in multi-organ-on-chip (MOC) technology, several critical challenges continue to impede its widespread adoption for modeling drug-drug interactions (DDIs). One fundamental obstacle remains the physiological relevance of these systems. Current MOC platforms struggle to accurately recapitulate the complex microenvironments of multiple organs simultaneously, including tissue-specific extracellular matrices, mechanical forces, and biochemical gradients that influence drug metabolism and transport.
Scaling and standardization present persistent technical hurdles. The miniaturization of multiple organ systems while maintaining appropriate physiological scaling ratios (allometric scaling) between different organ compartments remains difficult. This challenge is compounded by the lack of standardized fabrication protocols and materials, resulting in significant variability between platforms developed by different research groups and limiting cross-study comparability.
The integration of functional vascular networks represents another significant challenge. Current MOC systems often rely on simplified fluidic channels rather than true vasculature, limiting their ability to model complex pharmacokinetic processes such as first-pass metabolism and drug distribution across multiple organs. The absence of functional endothelial barriers further compromises the physiological relevance of drug transport studies.
Long-term viability and stability of cellular components across different organ modules present ongoing difficulties. Various cell types have different optimal culture conditions, making it challenging to maintain all organ modules at peak functionality simultaneously. This becomes particularly problematic when studying delayed drug-drug interactions that may manifest over extended periods.
Analytical integration poses substantial technical challenges. Real-time, non-invasive monitoring of cellular responses and drug concentrations across multiple organ compartments requires sophisticated sensing technologies that are not yet fully developed. Current analytical methods often necessitate endpoint measurements, limiting the dynamic assessment of drug interactions.
Computational modeling integration remains underdeveloped. While MOC systems generate rich datasets, there is a significant gap in computational frameworks that can effectively integrate this experimental data with physiologically-based pharmacokinetic (PBPK) models to predict complex DDIs across multiple organs.
Regulatory acceptance represents a final major hurdle. Despite their potential, MOC platforms lack validated protocols and quality control standards necessary for regulatory acceptance. The absence of clear guidelines for translating MOC findings to human outcomes limits their application in formal drug development processes and clinical decision-making.
Scaling and standardization present persistent technical hurdles. The miniaturization of multiple organ systems while maintaining appropriate physiological scaling ratios (allometric scaling) between different organ compartments remains difficult. This challenge is compounded by the lack of standardized fabrication protocols and materials, resulting in significant variability between platforms developed by different research groups and limiting cross-study comparability.
The integration of functional vascular networks represents another significant challenge. Current MOC systems often rely on simplified fluidic channels rather than true vasculature, limiting their ability to model complex pharmacokinetic processes such as first-pass metabolism and drug distribution across multiple organs. The absence of functional endothelial barriers further compromises the physiological relevance of drug transport studies.
Long-term viability and stability of cellular components across different organ modules present ongoing difficulties. Various cell types have different optimal culture conditions, making it challenging to maintain all organ modules at peak functionality simultaneously. This becomes particularly problematic when studying delayed drug-drug interactions that may manifest over extended periods.
Analytical integration poses substantial technical challenges. Real-time, non-invasive monitoring of cellular responses and drug concentrations across multiple organ compartments requires sophisticated sensing technologies that are not yet fully developed. Current analytical methods often necessitate endpoint measurements, limiting the dynamic assessment of drug interactions.
Computational modeling integration remains underdeveloped. While MOC systems generate rich datasets, there is a significant gap in computational frameworks that can effectively integrate this experimental data with physiologically-based pharmacokinetic (PBPK) models to predict complex DDIs across multiple organs.
Regulatory acceptance represents a final major hurdle. Despite their potential, MOC platforms lack validated protocols and quality control standards necessary for regulatory acceptance. The absence of clear guidelines for translating MOC findings to human outcomes limits their application in formal drug development processes and clinical decision-making.
Current Multi-Organ Platform Architectures and Approaches
01 Microfluidic organ-on-chip platforms for drug interaction studies
Microfluidic organ-on-chip platforms provide controlled environments that mimic the physiological conditions of human organs, allowing for the study of drug-drug interactions in a more realistic setting than traditional cell cultures. These platforms incorporate multiple cell types and fluid flow systems to replicate organ functionality, enabling researchers to observe how different drugs interact when administered simultaneously or sequentially. The microfluidic nature of these systems allows for precise control of drug concentrations and exposure times.- Microfluidic organ-on-chip platforms for drug interaction studies: Microfluidic organ-on-chip platforms provide controlled environments for studying drug-drug interactions by mimicking physiological conditions of human organs. These platforms enable real-time monitoring of cellular responses to multiple drugs, allowing researchers to observe synergistic or antagonistic effects. The microfluidic systems can incorporate multiple cell types and tissues to better represent the complexity of human organs, providing more accurate predictions of drug interactions than traditional in vitro methods.
- Multi-organ integration systems for comprehensive drug interaction assessment: Advanced organ-on-chip platforms that integrate multiple organ systems allow for the assessment of complex drug-drug interactions across different tissues. These integrated systems can simulate the metabolic pathways of drugs through various organs, providing insights into how one drug might affect the metabolism or efficacy of another. By connecting liver, kidney, intestine, and other organ chips, researchers can track drug absorption, distribution, metabolism, and excretion processes to better predict potential interactions in the human body.
- Sensor integration for real-time monitoring of drug interactions: Organ-on-chip platforms equipped with integrated sensors enable real-time monitoring of cellular responses to drug combinations. These sensors can detect changes in cellular metabolism, membrane potential, secreted biomarkers, and other parameters that indicate drug-drug interactions. The continuous monitoring capabilities allow researchers to observe the temporal dynamics of drug interactions, providing insights into both immediate and delayed effects that might occur when multiple drugs are administered simultaneously or sequentially.
- AI and computational modeling for predicting drug-drug interactions: Artificial intelligence and computational modeling approaches are being integrated with organ-on-chip platforms to enhance the prediction of drug-drug interactions. These systems analyze data generated from the organ chips to identify patterns and mechanisms of interaction between different compounds. Machine learning algorithms can process large datasets from multiple experiments to develop predictive models that help researchers understand potential interactions before clinical trials, reducing the risk of adverse events and improving drug development efficiency.
- Personalized medicine applications using patient-derived cells: Organ-on-chip platforms using patient-derived cells enable personalized assessment of drug-drug interactions. By incorporating cells with specific genetic backgrounds or disease states, these systems can predict how individual patients might respond to drug combinations. This approach allows for the identification of potential adverse interactions based on a patient's unique physiology, supporting the development of personalized treatment regimens that minimize harmful drug interactions while maximizing therapeutic efficacy.
02 Multi-organ chip systems for comprehensive drug interaction analysis
Multi-organ chip systems connect different organ models through a circulatory system, allowing for the study of complex drug-drug interactions that involve multiple organs. These integrated systems can simulate how drugs are metabolized in one organ and affect another, providing insights into systemic drug interactions that cannot be observed in single-organ models. By incorporating liver, kidney, intestine, and other organ models, researchers can evaluate ADME (absorption, distribution, metabolism, excretion) properties and potential cross-organ toxicity resulting from drug combinations.Expand Specific Solutions03 Sensor integration in organ-on-chip for real-time monitoring of drug interactions
Advanced organ-on-chip platforms incorporate various sensors that enable real-time monitoring of cellular responses to drug combinations. These integrated sensing technologies can detect changes in cellular metabolism, membrane potential, pH, oxygen consumption, and other parameters that indicate drug-drug interactions. The continuous monitoring capability allows researchers to observe the temporal dynamics of drug interactions, including delayed effects that might be missed in endpoint assays.Expand Specific Solutions04 AI and computational modeling for predicting drug-drug interactions
Artificial intelligence and computational modeling are being integrated with organ-on-chip platforms to enhance the prediction of drug-drug interactions. These computational approaches analyze data generated from the organ-on-chip experiments to identify patterns and mechanisms of drug interactions. Machine learning algorithms can be trained on experimental data to predict potential interactions between drugs that have not been tested together, accelerating the drug development process and reducing the need for extensive experimental testing.Expand Specific Solutions05 Personalized medicine applications using patient-derived cells on organ-on-chip
Organ-on-chip platforms using patient-derived cells enable personalized evaluation of drug-drug interactions. By incorporating cells from individual patients into these platforms, researchers can study how specific genetic backgrounds influence drug interactions, allowing for personalized medication regimens that avoid harmful interactions. This approach is particularly valuable for patients with complex medical conditions requiring multiple medications, as it can identify potential adverse interactions before drugs are administered to the patient.Expand Specific Solutions
Leading Companies and Research Institutions in Organ-on-Chip Field
Organ-on-chip technology for multi-organ drug-drug interaction modeling is in an early growth phase, with market size expanding rapidly due to increasing demand for more predictive preclinical models. The competitive landscape features academic institutions leading fundamental research (MIT, Harvard, Vanderbilt) alongside specialized commercial entities like Emulate, Inc. and Swiss Medical Union SA. Technical maturity varies significantly across players, with established companies offering standardized platforms while research institutions focus on advancing microfluidic integration, physiological relevance, and multi-organ connectivity. The field is transitioning from proof-of-concept to commercial application, with growing emphasis on validation against clinical data and regulatory acceptance for pharmaceutical testing applications.
Massachusetts Institute of Technology
Technical Solution: MIT has developed an advanced multi-organ-on-chip platform specifically designed for drug-drug interaction studies called PhysioMimix. This system integrates multiple miniaturized organ models within a single microfluidic network to recreate the complex interplay between different organs during drug metabolism and distribution. MIT's approach features a modular design where individual organ units (particularly liver, kidney, intestine, and cardiac tissues) are connected through a recirculating endothelial-lined vascular channel that mimics physiological blood flow. Their platform incorporates 3D bioprinting technology to create more anatomically accurate tissue structures with precise spatial organization of different cell types. For drug-drug interaction studies, MIT researchers have implemented real-time monitoring capabilities using integrated microsensors that can detect changes in cellular metabolism, barrier integrity, and electrophysiological activity across the connected organs. The system also includes computational modeling components that integrate experimental data to predict pharmacokinetic profiles and potential interaction mechanisms. MIT has validated their platform by successfully recapitulating clinically observed drug-drug interactions involving cytochrome P450 enzyme inhibition and induction, drug transporter interactions, and complex multi-mechanism interactions that traditional models fail to capture. Their technology enables the simultaneous testing of multiple drug combinations at physiologically relevant concentrations and exposure times, providing insights into both acute and chronic interaction effects.
Strengths: Highly sophisticated integration of multiple technologies (microfluidics, tissue engineering, sensing, computational modeling); excellent reproducibility and physiological relevance; strong validation against clinical data. Weaknesses: Complex system requiring significant expertise to operate; higher cost compared to conventional methods; still evolving standardization protocols for regulatory acceptance.
President & Fellows of Harvard College
Technical Solution: Harvard's Wyss Institute has pioneered organ-on-chip technology for multi-organ drug interaction studies through their Human Organs-on-Chips platform. Their approach features microengineered devices containing hollow microfluidic channels lined with living human cells that recreate tissue-tissue interfaces, mechanical forces, and physiological functions of human organs. For multi-organ drug-drug interaction modeling, Harvard researchers have developed a "body-on-a-chip" system that interconnects multiple organ chips (including liver, kidney, intestine, and blood-brain barrier chips) via an engineered vascular system. This integrated platform enables the study of ADME (absorption, distribution, metabolism, excretion) processes across different organs and allows for the assessment of how drug metabolism in one organ affects drug efficacy or toxicity in others. Their technology incorporates microfluidic pumps that precisely control fluid flow between organ compartments, mimicking physiological blood circulation rates. The platform also features integrated biosensors for continuous monitoring of cellular responses and automated sampling ports for pharmacokinetic analysis. Harvard researchers have validated this system by successfully predicting clinically observed drug-drug interactions that were not detected in conventional cell culture models or animal studies, demonstrating its potential for improving drug development and reducing reliance on animal testing.
Strengths: Cutting-edge research with strong scientific validation; highly sophisticated engineering allowing precise control of physiological parameters; extensive publications demonstrating predictive capabilities for complex drug interactions. Weaknesses: Complex technology with significant barriers to widespread adoption; still evolving from research tool to standardized platform; requires interdisciplinary expertise spanning engineering, biology, and pharmacology.
Key Patents and Publications in Drug-Drug Interaction Modeling
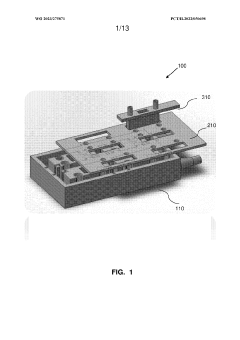
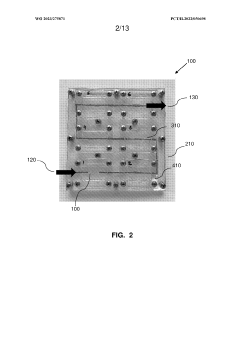
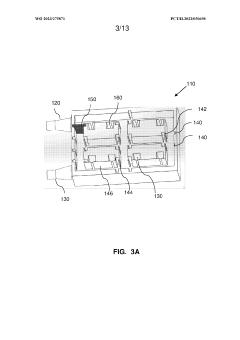
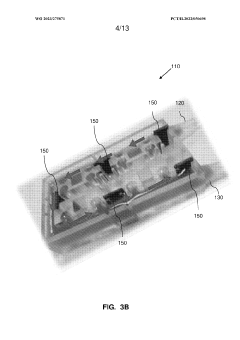
Multi-layered modular organ-on-a-chip platform
PatentWO2023275871A1
Innovation
- A modular organ-on-a-chip platform with a cartridge configuration that includes organ chips, a receptacle for blood or blood equivalent medium, and a cover layer, allowing direct fluid supply to all chips without excess conduits, enabling easy assembly and disassembly, and providing a clear view of cultured cells for monitoring.
Multi-organ-on-chip and application thereof in drug evaluation
PatentWO2023168857A1
Innovation
- Microalgae are used to generate oxygen through photosynthesis when there is light, and oxygen is consumed through respiration when there is light. The light intensity of the microalgae is used to adjust the oxygen concentration to achieve independent control of different cell culture chambers.
Regulatory Considerations for Organ-on-Chip Validation
The regulatory landscape for organ-on-chip (OOC) technologies presents unique challenges that must be addressed before these platforms can be widely adopted for drug-drug interaction (DDI) studies. Current regulatory frameworks were primarily designed for traditional in vitro and animal testing models, creating a significant gap in guidance specifically tailored to OOC validation. This regulatory uncertainty represents a major hurdle for pharmaceutical companies considering investment in these technologies.
The FDA and EMA have begun acknowledging OOC platforms through various initiatives, including the FDA's Advancing Alternative Methods program and the EMA's Innovation Task Force. These programs aim to establish preliminary frameworks for evaluating the reliability and reproducibility of OOC systems. However, comprehensive validation protocols specific to multi-organ platforms remain underdeveloped, particularly for complex DDI applications that involve multiple interconnected organ systems.
Key validation parameters requiring regulatory consideration include physiological relevance, reproducibility across laboratories, standardization of cell sources, and correlation with clinical outcomes. The qualification of OOC platforms for regulatory decision-making will likely require extensive cross-validation with established models and clinical data. This process is particularly challenging for multi-organ platforms where the complexity increases exponentially with each additional organ system incorporated.
Standardization represents another critical regulatory consideration. Currently, there is significant variability in chip design, cell sourcing, culture conditions, and analytical endpoints across different research groups and commercial platforms. Regulatory bodies will need to establish minimum performance standards and reference materials to enable meaningful comparisons between different OOC systems and traditional testing methods.
Data interpretation frameworks present additional challenges, as multi-organ platforms generate complex, multidimensional datasets that may not align with existing regulatory paradigms. Regulatory agencies and industry stakeholders must collaborate to develop appropriate biomarkers and endpoints that accurately reflect the predictive value of these systems for human DDI outcomes.
The path toward regulatory acceptance will likely involve a phased approach, beginning with the use of OOC platforms as complementary tools alongside traditional methods, followed by gradual implementation as replacement methods once sufficient validation data accumulates. Industry-academic-regulatory partnerships, such as the EU-ToxRisk program and the FDA's Organs-on-Chips Project, are working to establish these validation frameworks through collaborative research initiatives.
For pharmaceutical companies investing in OOC technology, early engagement with regulatory agencies through formal consultation processes is advisable to align development strategies with evolving regulatory expectations. This proactive approach can help shape validation protocols that will ultimately support regulatory acceptance of OOC platforms for DDI assessment in drug development programs.
The FDA and EMA have begun acknowledging OOC platforms through various initiatives, including the FDA's Advancing Alternative Methods program and the EMA's Innovation Task Force. These programs aim to establish preliminary frameworks for evaluating the reliability and reproducibility of OOC systems. However, comprehensive validation protocols specific to multi-organ platforms remain underdeveloped, particularly for complex DDI applications that involve multiple interconnected organ systems.
Key validation parameters requiring regulatory consideration include physiological relevance, reproducibility across laboratories, standardization of cell sources, and correlation with clinical outcomes. The qualification of OOC platforms for regulatory decision-making will likely require extensive cross-validation with established models and clinical data. This process is particularly challenging for multi-organ platforms where the complexity increases exponentially with each additional organ system incorporated.
Standardization represents another critical regulatory consideration. Currently, there is significant variability in chip design, cell sourcing, culture conditions, and analytical endpoints across different research groups and commercial platforms. Regulatory bodies will need to establish minimum performance standards and reference materials to enable meaningful comparisons between different OOC systems and traditional testing methods.
Data interpretation frameworks present additional challenges, as multi-organ platforms generate complex, multidimensional datasets that may not align with existing regulatory paradigms. Regulatory agencies and industry stakeholders must collaborate to develop appropriate biomarkers and endpoints that accurately reflect the predictive value of these systems for human DDI outcomes.
The path toward regulatory acceptance will likely involve a phased approach, beginning with the use of OOC platforms as complementary tools alongside traditional methods, followed by gradual implementation as replacement methods once sufficient validation data accumulates. Industry-academic-regulatory partnerships, such as the EU-ToxRisk program and the FDA's Organs-on-Chips Project, are working to establish these validation frameworks through collaborative research initiatives.
For pharmaceutical companies investing in OOC technology, early engagement with regulatory agencies through formal consultation processes is advisable to align development strategies with evolving regulatory expectations. This proactive approach can help shape validation protocols that will ultimately support regulatory acceptance of OOC platforms for DDI assessment in drug development programs.
Scaling Challenges and Manufacturing Solutions
The scaling of organ-on-chip (OOC) technology from laboratory prototypes to commercial manufacturing presents significant challenges that must be addressed to realize the full potential of these platforms for drug-drug interaction studies. Current manufacturing approaches often rely on labor-intensive, manual processes that limit throughput and introduce variability between devices. This inconsistency undermines the reliability of multi-organ platforms when modeling complex drug-drug interactions across different tissue types.
Material selection represents a critical scaling challenge, as many laboratory-grade materials used in prototype development are not suitable for mass production. Polydimethylsiloxane (PDMS), commonly used in academic research due to its optical clarity and gas permeability, presents manufacturing limitations including batch-to-batch variability and drug absorption issues that can confound interaction studies. Alternative thermoplastics offer better manufacturing scalability but may compromise certain biological functionalities.
Microfluidic channel fabrication at scale requires transitioning from soft lithography techniques to injection molding or hot embossing processes. While these methods enable higher throughput, they often sacrifice the fine feature resolution necessary for recreating physiologically relevant microenvironments where drug interactions occur. The integration of sensors and electrodes for real-time monitoring of drug effects presents additional manufacturing complexities that impact scalability.
Cell sourcing and integration represent another significant barrier to scaling multi-organ platforms. Maintaining consistent cell quality across manufacturing batches is challenging, particularly when primary human cells are required. Automated cell seeding technologies are emerging but require further refinement to ensure uniform cell distribution across multiple organ compartments while maintaining sterility throughout the manufacturing process.
Quality control methodologies must evolve alongside manufacturing processes. Current approaches often rely on visual inspection and functional testing that cannot be easily automated. Advanced imaging techniques and computational modeling are being developed to predict platform performance, but standardized testing protocols specific to drug-drug interaction applications remain underdeveloped.
Several companies are addressing these challenges through innovative approaches. Emulate, TissUse, and CN Bio have developed semi-automated manufacturing processes that improve reproducibility while maintaining biological relevance. Academic-industrial partnerships are exploring novel materials and fabrication techniques specifically designed for scalable production of multi-organ platforms. Regulatory agencies, including the FDA, are working with stakeholders to establish manufacturing standards that will facilitate broader adoption of these technologies for drug interaction studies.
Material selection represents a critical scaling challenge, as many laboratory-grade materials used in prototype development are not suitable for mass production. Polydimethylsiloxane (PDMS), commonly used in academic research due to its optical clarity and gas permeability, presents manufacturing limitations including batch-to-batch variability and drug absorption issues that can confound interaction studies. Alternative thermoplastics offer better manufacturing scalability but may compromise certain biological functionalities.
Microfluidic channel fabrication at scale requires transitioning from soft lithography techniques to injection molding or hot embossing processes. While these methods enable higher throughput, they often sacrifice the fine feature resolution necessary for recreating physiologically relevant microenvironments where drug interactions occur. The integration of sensors and electrodes for real-time monitoring of drug effects presents additional manufacturing complexities that impact scalability.
Cell sourcing and integration represent another significant barrier to scaling multi-organ platforms. Maintaining consistent cell quality across manufacturing batches is challenging, particularly when primary human cells are required. Automated cell seeding technologies are emerging but require further refinement to ensure uniform cell distribution across multiple organ compartments while maintaining sterility throughout the manufacturing process.
Quality control methodologies must evolve alongside manufacturing processes. Current approaches often rely on visual inspection and functional testing that cannot be easily automated. Advanced imaging techniques and computational modeling are being developed to predict platform performance, but standardized testing protocols specific to drug-drug interaction applications remain underdeveloped.
Several companies are addressing these challenges through innovative approaches. Emulate, TissUse, and CN Bio have developed semi-automated manufacturing processes that improve reproducibility while maintaining biological relevance. Academic-industrial partnerships are exploring novel materials and fabrication techniques specifically designed for scalable production of multi-organ platforms. Regulatory agencies, including the FDA, are working with stakeholders to establish manufacturing standards that will facilitate broader adoption of these technologies for drug interaction studies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!