PA11 vs PA12 Chemical Resistance: Solvent Exposure and Compatibility Guide
AUG 20, 20258 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
PA11 and PA12 Overview
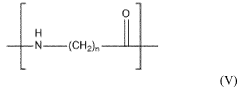
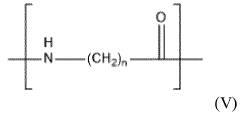
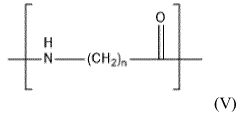
Polyamide 11 (PA11) and Polyamide 12 (PA12) are high-performance engineering thermoplastics belonging to the nylon family. These materials have gained significant attention in various industries due to their exceptional mechanical properties, chemical resistance, and thermal stability.
PA11, also known as Nylon 11, is derived from renewable castor oil, making it a bio-based polymer. It offers excellent impact resistance, low moisture absorption, and good dimensional stability. PA11 is widely used in automotive, oil and gas, and consumer goods industries.
PA12, or Nylon 12, is a synthetic polymer known for its low density, high flexibility, and superior chemical resistance. It exhibits excellent fatigue resistance and low friction properties, making it suitable for applications in automotive, aerospace, and medical industries.
Both PA11 and PA12 demonstrate remarkable chemical resistance, particularly against hydrocarbons, oils, and many solvents. However, their resistance profiles differ slightly, which can be crucial in specific applications.
The chemical resistance of PA11 and PA12 is influenced by various factors, including the specific grade of the material, temperature, exposure time, and the concentration of the chemical agent. Generally, PA12 shows slightly better resistance to acids and bases compared to PA11, while PA11 may have an edge in resistance to certain hydrocarbons.
Understanding the chemical resistance of these materials is essential for engineers and designers when selecting the appropriate polymer for specific applications. Factors such as the operating environment, exposure to chemicals, and required mechanical properties must be considered to ensure optimal performance and longevity of the final product.
In recent years, advancements in polymer science have led to the development of modified grades of PA11 and PA12 with enhanced chemical resistance properties. These innovations have expanded the application range of these materials, allowing them to be used in more demanding environments and specialized industries.
As environmental concerns grow, the bio-based nature of PA11 has attracted increased attention. Its renewable source and potential for biodegradability offer advantages in sustainability-focused applications, although this must be balanced against performance requirements.
PA11, also known as Nylon 11, is derived from renewable castor oil, making it a bio-based polymer. It offers excellent impact resistance, low moisture absorption, and good dimensional stability. PA11 is widely used in automotive, oil and gas, and consumer goods industries.
PA12, or Nylon 12, is a synthetic polymer known for its low density, high flexibility, and superior chemical resistance. It exhibits excellent fatigue resistance and low friction properties, making it suitable for applications in automotive, aerospace, and medical industries.
Both PA11 and PA12 demonstrate remarkable chemical resistance, particularly against hydrocarbons, oils, and many solvents. However, their resistance profiles differ slightly, which can be crucial in specific applications.
The chemical resistance of PA11 and PA12 is influenced by various factors, including the specific grade of the material, temperature, exposure time, and the concentration of the chemical agent. Generally, PA12 shows slightly better resistance to acids and bases compared to PA11, while PA11 may have an edge in resistance to certain hydrocarbons.
Understanding the chemical resistance of these materials is essential for engineers and designers when selecting the appropriate polymer for specific applications. Factors such as the operating environment, exposure to chemicals, and required mechanical properties must be considered to ensure optimal performance and longevity of the final product.
In recent years, advancements in polymer science have led to the development of modified grades of PA11 and PA12 with enhanced chemical resistance properties. These innovations have expanded the application range of these materials, allowing them to be used in more demanding environments and specialized industries.
As environmental concerns grow, the bio-based nature of PA11 has attracted increased attention. Its renewable source and potential for biodegradability offer advantages in sustainability-focused applications, although this must be balanced against performance requirements.
Market Demand Analysis
The market demand for chemical-resistant polymers, particularly PA11 and PA12, has been steadily increasing across various industries. This growth is primarily driven by the expanding applications in automotive, electronics, industrial machinery, and consumer goods sectors. The global polyamide market, which includes PA11 and PA12, is projected to reach significant market value in the coming years, with a compound annual growth rate (CAGR) outpacing many other polymer segments.
In the automotive industry, the demand for PA11 and PA12 is rising due to their excellent chemical resistance properties, which are crucial for fuel system components, hydraulic lines, and under-the-hood applications. The shift towards electric vehicles has further boosted the demand for these materials in battery casings and cooling systems, where chemical resistance is paramount.
The electronics sector is another major consumer of PA11 and PA12, particularly in the production of connectors, cable insulation, and protective housings. The increasing miniaturization of electronic devices and the need for components that can withstand harsh environmental conditions have led to a surge in demand for these chemically resistant polyamides.
Industrial machinery and equipment manufacturers are also contributing to the market growth. PA11 and PA12 are increasingly used in pneumatic systems, hydraulic hoses, and other components exposed to various chemicals and solvents. The oil and gas industry, in particular, has shown a strong preference for these materials due to their resistance to hydrocarbons and other aggressive fluids.
Consumer goods, especially in the sports and leisure sector, represent another growing market for PA11 and PA12. These materials are used in the production of high-performance sporting equipment, where resistance to sweat, oils, and cleaning agents is essential.
The market trend indicates a growing preference for bio-based alternatives, which has led to increased interest in PA11, derived from renewable castor oil. This shift is driven by sustainability concerns and regulatory pressures to reduce reliance on fossil-fuel-based materials.
Geographically, Asia-Pacific is expected to dominate the market growth, followed by North America and Europe. The rapid industrialization and increasing automotive production in countries like China and India are major factors contributing to this regional demand.
As industries continue to seek materials with superior chemical resistance and performance characteristics, the demand for PA11 and PA12 is expected to maintain its upward trajectory. The market is likely to see further innovations in material formulations and processing techniques to meet the evolving needs of various end-use industries.
In the automotive industry, the demand for PA11 and PA12 is rising due to their excellent chemical resistance properties, which are crucial for fuel system components, hydraulic lines, and under-the-hood applications. The shift towards electric vehicles has further boosted the demand for these materials in battery casings and cooling systems, where chemical resistance is paramount.
The electronics sector is another major consumer of PA11 and PA12, particularly in the production of connectors, cable insulation, and protective housings. The increasing miniaturization of electronic devices and the need for components that can withstand harsh environmental conditions have led to a surge in demand for these chemically resistant polyamides.
Industrial machinery and equipment manufacturers are also contributing to the market growth. PA11 and PA12 are increasingly used in pneumatic systems, hydraulic hoses, and other components exposed to various chemicals and solvents. The oil and gas industry, in particular, has shown a strong preference for these materials due to their resistance to hydrocarbons and other aggressive fluids.
Consumer goods, especially in the sports and leisure sector, represent another growing market for PA11 and PA12. These materials are used in the production of high-performance sporting equipment, where resistance to sweat, oils, and cleaning agents is essential.
The market trend indicates a growing preference for bio-based alternatives, which has led to increased interest in PA11, derived from renewable castor oil. This shift is driven by sustainability concerns and regulatory pressures to reduce reliance on fossil-fuel-based materials.
Geographically, Asia-Pacific is expected to dominate the market growth, followed by North America and Europe. The rapid industrialization and increasing automotive production in countries like China and India are major factors contributing to this regional demand.
As industries continue to seek materials with superior chemical resistance and performance characteristics, the demand for PA11 and PA12 is expected to maintain its upward trajectory. The market is likely to see further innovations in material formulations and processing techniques to meet the evolving needs of various end-use industries.
Chemical Resistance Challenges
The chemical resistance of polyamides, particularly PA11 and PA12, presents significant challenges in various industrial applications. These materials, while known for their excellent mechanical properties and thermal stability, can be susceptible to degradation when exposed to certain chemicals and solvents. The primary challenge lies in maintaining the structural integrity and performance of these polymers in diverse chemical environments.
One of the key issues is the potential for hydrolysis, especially in the presence of water or acidic substances. Both PA11 and PA12 can absorb moisture, which may lead to dimensional changes and a reduction in mechanical properties. This absorption can be particularly problematic in applications where precise dimensions are critical, such as in automotive or aerospace components.
Exposure to organic solvents poses another significant challenge. Certain solvents can cause swelling or partial dissolution of the polymer chains, leading to a loss of mechanical strength and potential failure of the material. The extent of this effect can vary depending on the specific solvent, concentration, and exposure time, making it crucial to understand the exact conditions in which these materials will be used.
Oxidative degradation is another concern, particularly in environments with high oxygen content or exposure to strong oxidizing agents. This can lead to chain scission and crosslinking, altering the polymer's properties and potentially compromising its performance over time. The rate and extent of oxidation can be influenced by factors such as temperature, UV exposure, and the presence of catalytic impurities.
The difference in chemical resistance between PA11 and PA12 adds another layer of complexity. While both are polyamides, their slightly different chemical structures result in varying resistance to specific chemicals. PA12, for instance, generally exhibits better resistance to hydrolysis compared to PA11, but may be more susceptible to certain organic solvents.
Temperature also plays a crucial role in chemical resistance challenges. Higher temperatures can accelerate chemical reactions and increase the rate of degradation, making it essential to consider the operating temperature range in conjunction with chemical exposure. This is particularly important in applications where thermal cycling or prolonged exposure to elevated temperatures is expected.
Addressing these challenges requires a comprehensive understanding of the specific chemical environment, operating conditions, and performance requirements of the application. It often necessitates careful material selection, potentially involving the use of additives or surface treatments to enhance chemical resistance. In some cases, it may be necessary to redesign components or systems to minimize exposure to problematic chemicals or to incorporate protective measures.
One of the key issues is the potential for hydrolysis, especially in the presence of water or acidic substances. Both PA11 and PA12 can absorb moisture, which may lead to dimensional changes and a reduction in mechanical properties. This absorption can be particularly problematic in applications where precise dimensions are critical, such as in automotive or aerospace components.
Exposure to organic solvents poses another significant challenge. Certain solvents can cause swelling or partial dissolution of the polymer chains, leading to a loss of mechanical strength and potential failure of the material. The extent of this effect can vary depending on the specific solvent, concentration, and exposure time, making it crucial to understand the exact conditions in which these materials will be used.
Oxidative degradation is another concern, particularly in environments with high oxygen content or exposure to strong oxidizing agents. This can lead to chain scission and crosslinking, altering the polymer's properties and potentially compromising its performance over time. The rate and extent of oxidation can be influenced by factors such as temperature, UV exposure, and the presence of catalytic impurities.
The difference in chemical resistance between PA11 and PA12 adds another layer of complexity. While both are polyamides, their slightly different chemical structures result in varying resistance to specific chemicals. PA12, for instance, generally exhibits better resistance to hydrolysis compared to PA11, but may be more susceptible to certain organic solvents.
Temperature also plays a crucial role in chemical resistance challenges. Higher temperatures can accelerate chemical reactions and increase the rate of degradation, making it essential to consider the operating temperature range in conjunction with chemical exposure. This is particularly important in applications where thermal cycling or prolonged exposure to elevated temperatures is expected.
Addressing these challenges requires a comprehensive understanding of the specific chemical environment, operating conditions, and performance requirements of the application. It often necessitates careful material selection, potentially involving the use of additives or surface treatments to enhance chemical resistance. In some cases, it may be necessary to redesign components or systems to minimize exposure to problematic chemicals or to incorporate protective measures.
Solvent Compatibility Solutions
01 Chemical resistance of PA11 and PA12
PA11 and PA12 exhibit excellent chemical resistance to various substances, including oils, fuels, and solvents. This property makes them suitable for applications in harsh chemical environments, such as automotive fuel systems and industrial equipment.- Chemical resistance of PA11 and PA12: PA11 and PA12 exhibit excellent chemical resistance to various substances, including oils, fuels, and solvents. This property makes them suitable for applications in harsh chemical environments, such as automotive and industrial sectors. The long-chain aliphatic structure of these polyamides contributes to their superior chemical resistance compared to other nylon types.
- Improving chemical resistance through blending: The chemical resistance of PA11 and PA12 can be further enhanced by blending them with other polymers or additives. This approach allows for tailoring the material properties to specific application requirements. Blending can improve resistance to specific chemicals or broaden the range of chemicals the material can withstand.
- Surface treatment for enhanced chemical resistance: Surface treatments can be applied to PA11 and PA12 to improve their chemical resistance. These treatments may include plasma treatment, chemical modification, or coating with protective layers. Such modifications can enhance the material's resistance to specific chemicals or harsh environments without altering the bulk properties of the polyamide.
- Chemical resistance in high-temperature applications: PA11 and PA12 maintain their chemical resistance at elevated temperatures, making them suitable for high-temperature applications in chemically aggressive environments. This property is particularly valuable in automotive, aerospace, and industrial applications where materials are exposed to both heat and chemicals simultaneously.
- Comparative chemical resistance of PA11 and PA12: While both PA11 and PA12 exhibit excellent chemical resistance, there are subtle differences between the two. PA12 generally shows slightly better resistance to hydrocarbons and some organic solvents, while PA11 may have advantages in certain other chemical environments. The choice between PA11 and PA12 often depends on the specific chemical exposure in the intended application.
02 Improving chemical resistance through blending
The chemical resistance of PA11 and PA12 can be further enhanced by blending them with other polymers or additives. This approach allows for tailoring the material properties to specific application requirements while maintaining or improving chemical resistance.Expand Specific Solutions03 Surface treatment for enhanced chemical resistance
Surface treatments can be applied to PA11 and PA12 components to improve their chemical resistance. These treatments may include coatings, plasma treatments, or chemical modifications that create a protective barrier against aggressive chemicals.Expand Specific Solutions04 Chemical resistance in high-temperature applications
PA11 and PA12 maintain their chemical resistance at elevated temperatures, making them suitable for use in high-temperature environments where exposure to chemicals is also a concern. This property is particularly valuable in automotive and industrial applications.Expand Specific Solutions05 Comparative chemical resistance of PA11 and PA12
While both PA11 and PA12 offer good chemical resistance, there are subtle differences in their performance against specific chemicals. Understanding these differences is crucial for selecting the most appropriate material for a given application, considering factors such as exposure time, concentration, and temperature.Expand Specific Solutions
Key Manufacturers
The competitive landscape for PA11 vs PA12 chemical resistance and solvent compatibility is characterized by a mature market with established players. The global polyamide market, which includes PA11 and PA12, is projected to reach $36.9 billion by 2027, growing at a CAGR of 5.7%. Key players like Arkema, BASF, and EMS-CHEMIE are at the forefront of innovation in this space. These companies have developed advanced formulations to enhance chemical resistance and solvent compatibility, catering to diverse industrial applications. The technology is well-established, with ongoing research focused on improving performance characteristics and expanding application areas in automotive, electronics, and industrial sectors.
Arkema France SA
Technical Solution: Arkema has developed advanced PA11 and PA12 formulations with enhanced chemical resistance. Their Rilsan® PA11 and Rilsamid® PA12 grades offer superior resistance to a wide range of solvents, including hydrocarbons, alcohols, and ketones. Arkema's PA11, derived from renewable castor oil, demonstrates excellent resistance to fuels, oils, and hydraulic fluids[1]. Their PA12 grades show improved resistance to zinc chloride and calcium chloride solutions compared to standard polyamides[2]. Arkema has also introduced Pebax® Rnew® elastomers, which combine PA11 or PA12 with polyether blocks, offering a balance of flexibility and chemical resistance for applications requiring both properties[3].
Strengths: Renewable source for PA11, excellent resistance to fuels and oils, improved resistance to specific chemical solutions. Weaknesses: May have higher cost compared to traditional polyamides, potential limitations in extreme chemical environments.
Solvay Specialty Polymers USA LLC
Technical Solution: Solvay has developed high-performance PA11 and PA12 grades under their Amodel® and Ixef® brands. Their Amodel® PPA (polyphthalamide) grades offer exceptional chemical resistance, particularly against automotive fluids, oils, and greases. Solvay's PA11 and PA12 formulations demonstrate improved resistance to hydrocarbons, alcohols, and ketones compared to standard polyamides[6]. The company has also introduced Technyl® 4earth®, a sustainable PA6.6 with enhanced chemical resistance properties derived from recycled materials[7]. Solvay's ongoing research focuses on developing polyamide grades that maintain their mechanical properties even after prolonged exposure to aggressive chemicals and solvents, addressing the needs of industries such as automotive, oil and gas, and industrial manufacturing.
Strengths: High-performance grades for extreme chemical environments, sustainable options available. Weaknesses: Premium pricing for specialized grades, potential limitations in certain highly aggressive chemical environments.
Innovative Resistance Techniques
Additive manufacturing method with biobased polyamide composition having high thermal stability
PatentWO2023203213A1
Innovation
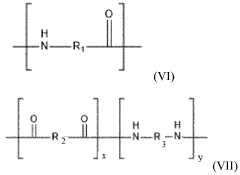
- An additive manufacturing method utilizing a polyamide composition with at least 50% by weight of polyamide comprising 90% recurring units of formula -NH-(CH2)s-C(O)- and/or -NH-(CH2)9-C(O)-, combined with reinforcing agents and additives, which is processed using Fused Filament Fabrication (FFF) to produce 3D objects with enhanced thermal stability and hydrophobic properties.
Surface coating of electrical enclosures
PatentInactiveEP3851211A1
Innovation
- Applying a high-performance polyamides composition to aluminum electrical enclosures through abrasive blasting to create a rough surface, followed by heating and drying the polyamides composition to form a protective layer, which enhances corrosion resistance and durability.
Regulatory Compliance
Regulatory compliance plays a crucial role in the use of PA11 and PA12 in various industries, particularly when considering their chemical resistance and solvent compatibility. Both materials are subject to stringent regulations due to their widespread applications in automotive, aerospace, medical, and consumer goods sectors.
In the United States, the Food and Drug Administration (FDA) has approved both PA11 and PA12 for food contact applications, provided they meet specific composition and manufacturing requirements. This approval extends to their use in food packaging, processing equipment, and utensils. However, manufacturers must ensure that the specific grade of polyamide used complies with the FDA's regulations outlined in 21 CFR 177.1500.
The European Union's Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation also impacts the use of PA11 and PA12. Both materials must be registered with the European Chemicals Agency (ECHA) if manufactured or imported in quantities exceeding one tonne per year. Additionally, any substances of very high concern (SVHCs) used in their production must be disclosed if present above 0.1% by weight.
In the automotive industry, PA11 and PA12 must comply with various standards set by organizations such as the Society of Automotive Engineers (SAE) and the International Organization for Standardization (ISO). These standards often include requirements for chemical resistance and compatibility with automotive fluids, such as fuel, oil, and coolants.
For medical applications, both materials must meet the biocompatibility requirements set forth in ISO 10993 and USP Class VI standards. This includes testing for cytotoxicity, sensitization, and irritation. Furthermore, medical devices incorporating PA11 or PA12 may require additional regulatory approvals, such as 510(k) clearance from the FDA in the United States or CE marking in the European Union.
Environmental regulations also impact the use of these materials. The European Union's End-of-Life Vehicles (ELV) Directive and Restriction of Hazardous Substances (RoHS) Directive place restrictions on certain substances in automotive and electronic applications, respectively. Manufacturers using PA11 or PA12 in these sectors must ensure compliance with these directives.
When considering chemical resistance and solvent compatibility, it is essential to note that regulatory compliance may vary depending on the specific application and exposure conditions. Manufacturers must conduct thorough testing and documentation to demonstrate compliance with relevant regulations and standards. This may include chemical resistance testing, leaching studies, and long-term exposure assessments to ensure the materials maintain their integrity and safety throughout their intended use.
In the United States, the Food and Drug Administration (FDA) has approved both PA11 and PA12 for food contact applications, provided they meet specific composition and manufacturing requirements. This approval extends to their use in food packaging, processing equipment, and utensils. However, manufacturers must ensure that the specific grade of polyamide used complies with the FDA's regulations outlined in 21 CFR 177.1500.
The European Union's Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation also impacts the use of PA11 and PA12. Both materials must be registered with the European Chemicals Agency (ECHA) if manufactured or imported in quantities exceeding one tonne per year. Additionally, any substances of very high concern (SVHCs) used in their production must be disclosed if present above 0.1% by weight.
In the automotive industry, PA11 and PA12 must comply with various standards set by organizations such as the Society of Automotive Engineers (SAE) and the International Organization for Standardization (ISO). These standards often include requirements for chemical resistance and compatibility with automotive fluids, such as fuel, oil, and coolants.
For medical applications, both materials must meet the biocompatibility requirements set forth in ISO 10993 and USP Class VI standards. This includes testing for cytotoxicity, sensitization, and irritation. Furthermore, medical devices incorporating PA11 or PA12 may require additional regulatory approvals, such as 510(k) clearance from the FDA in the United States or CE marking in the European Union.
Environmental regulations also impact the use of these materials. The European Union's End-of-Life Vehicles (ELV) Directive and Restriction of Hazardous Substances (RoHS) Directive place restrictions on certain substances in automotive and electronic applications, respectively. Manufacturers using PA11 or PA12 in these sectors must ensure compliance with these directives.
When considering chemical resistance and solvent compatibility, it is essential to note that regulatory compliance may vary depending on the specific application and exposure conditions. Manufacturers must conduct thorough testing and documentation to demonstrate compliance with relevant regulations and standards. This may include chemical resistance testing, leaching studies, and long-term exposure assessments to ensure the materials maintain their integrity and safety throughout their intended use.
Environmental Impact Assessment
The environmental impact assessment of PA11 and PA12 in terms of chemical resistance and solvent exposure is a critical consideration for their industrial applications. Both polyamides exhibit varying degrees of resistance to different solvents, which directly influences their environmental footprint throughout their lifecycle.
PA11, derived from renewable castor oil, generally demonstrates superior chemical resistance compared to PA12. This enhanced resistance translates to longer product lifespans and reduced frequency of replacement, potentially minimizing waste generation and resource consumption. PA11's bio-based origin also contributes to a lower carbon footprint during production, aligning with sustainability goals.
In contrast, PA12, typically derived from petroleum sources, may have a higher environmental impact during production. However, its specific chemical resistance properties can lead to extended service life in certain applications, potentially offsetting initial environmental costs through reduced replacement rates.
When exposed to solvents, both materials may experience changes in their physical and chemical properties. The extent of these changes varies depending on the specific solvent and exposure conditions. In some cases, solvent exposure can lead to material degradation, potentially releasing microplastics or chemical byproducts into the environment. The environmental persistence and potential toxicity of these byproducts must be carefully evaluated.
The compatibility of PA11 and PA12 with different solvents also affects their recyclability and end-of-life management. Materials with higher chemical resistance are often easier to clean and recycle, reducing the overall environmental impact. However, the presence of residual solvents in recycled materials can complicate the recycling process and potentially introduce contaminants into new products.
In industrial settings, the choice between PA11 and PA12 based on their chemical resistance properties can have significant implications for workplace safety and environmental protection. Materials with higher resistance to aggressive solvents reduce the risk of leaks, spills, and associated environmental contamination. This aspect is particularly important in industries handling hazardous chemicals or operating in sensitive ecosystems.
The long-term environmental effects of PA11 and PA12 exposure to various solvents require ongoing research and monitoring. Factors such as biodegradability, potential for bioaccumulation, and impacts on aquatic and terrestrial ecosystems need thorough assessment. Additionally, the environmental implications of manufacturing processes, including solvent use in production and potential emissions, should be considered in a comprehensive lifecycle analysis.
PA11, derived from renewable castor oil, generally demonstrates superior chemical resistance compared to PA12. This enhanced resistance translates to longer product lifespans and reduced frequency of replacement, potentially minimizing waste generation and resource consumption. PA11's bio-based origin also contributes to a lower carbon footprint during production, aligning with sustainability goals.
In contrast, PA12, typically derived from petroleum sources, may have a higher environmental impact during production. However, its specific chemical resistance properties can lead to extended service life in certain applications, potentially offsetting initial environmental costs through reduced replacement rates.
When exposed to solvents, both materials may experience changes in their physical and chemical properties. The extent of these changes varies depending on the specific solvent and exposure conditions. In some cases, solvent exposure can lead to material degradation, potentially releasing microplastics or chemical byproducts into the environment. The environmental persistence and potential toxicity of these byproducts must be carefully evaluated.
The compatibility of PA11 and PA12 with different solvents also affects their recyclability and end-of-life management. Materials with higher chemical resistance are often easier to clean and recycle, reducing the overall environmental impact. However, the presence of residual solvents in recycled materials can complicate the recycling process and potentially introduce contaminants into new products.
In industrial settings, the choice between PA11 and PA12 based on their chemical resistance properties can have significant implications for workplace safety and environmental protection. Materials with higher resistance to aggressive solvents reduce the risk of leaks, spills, and associated environmental contamination. This aspect is particularly important in industries handling hazardous chemicals or operating in sensitive ecosystems.
The long-term environmental effects of PA11 and PA12 exposure to various solvents require ongoing research and monitoring. Factors such as biodegradability, potential for bioaccumulation, and impacts on aquatic and terrestrial ecosystems need thorough assessment. Additionally, the environmental implications of manufacturing processes, including solvent use in production and potential emissions, should be considered in a comprehensive lifecycle analysis.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!