Performance Benchmarking of Shape Memory Alloys in Biomedical Devices
SEP 19, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
SMA Biomedical Applications Background and Objectives
Shape Memory Alloys (SMAs) have evolved significantly since their discovery in the 1930s, with the most notable breakthrough occurring in 1962 when William J. Buehler at the Naval Ordnance Laboratory discovered the shape memory effect in nickel-titanium alloys, later named Nitinol. This revolutionary material demonstrated unique properties including superelasticity, shape memory effect, and biocompatibility, making it particularly valuable for biomedical applications.
The integration of SMAs into medical devices began in the 1980s, with early applications in orthodontics and orthopedics. The technological trajectory has since expanded exponentially, with SMAs now playing crucial roles in cardiovascular stents, surgical instruments, and implantable devices. This evolution represents a convergence of materials science, biomedical engineering, and clinical medicine, creating a multidisciplinary field with significant growth potential.
Current technological trends in SMA development focus on enhancing material performance metrics including fatigue resistance, corrosion behavior, transformation temperatures, and biocompatibility. Researchers are exploring novel alloy compositions, processing techniques, and surface modifications to optimize these properties for specific biomedical applications. The miniaturization of SMA-based devices and the development of smart implants capable of responding to physiological stimuli represent emerging frontiers in this field.
The primary objective of performance benchmarking in SMA biomedical applications is to establish standardized evaluation protocols that accurately predict in vivo performance. This includes developing comprehensive testing methodologies that simulate physiological conditions, mechanical stresses, and long-term implantation effects. Such benchmarking is essential for regulatory approval processes and for enabling meaningful comparisons between different SMA formulations and processing techniques.
Another critical goal is to bridge the gap between laboratory research and clinical implementation by identifying key performance indicators that correlate with successful clinical outcomes. This requires collaborative efforts between materials scientists, device manufacturers, and healthcare practitioners to translate theoretical advantages into practical benefits for patients.
Looking forward, the field aims to develop next-generation SMAs with enhanced functional properties, including improved biocompatibility, reduced nickel content to minimize allergic responses, and tailored transformation temperatures for specific anatomical applications. The ultimate technological objective is to create bioactive SMAs that not only provide mechanical functionality but also promote healing and tissue integration, potentially through surface functionalization or composite structures.
As healthcare continues to move toward personalized medicine, another emerging goal is the development of patient-specific SMA devices manufactured through advanced techniques such as additive manufacturing, allowing for customized geometries and mechanical properties tailored to individual patient anatomy and pathology.
The integration of SMAs into medical devices began in the 1980s, with early applications in orthodontics and orthopedics. The technological trajectory has since expanded exponentially, with SMAs now playing crucial roles in cardiovascular stents, surgical instruments, and implantable devices. This evolution represents a convergence of materials science, biomedical engineering, and clinical medicine, creating a multidisciplinary field with significant growth potential.
Current technological trends in SMA development focus on enhancing material performance metrics including fatigue resistance, corrosion behavior, transformation temperatures, and biocompatibility. Researchers are exploring novel alloy compositions, processing techniques, and surface modifications to optimize these properties for specific biomedical applications. The miniaturization of SMA-based devices and the development of smart implants capable of responding to physiological stimuli represent emerging frontiers in this field.
The primary objective of performance benchmarking in SMA biomedical applications is to establish standardized evaluation protocols that accurately predict in vivo performance. This includes developing comprehensive testing methodologies that simulate physiological conditions, mechanical stresses, and long-term implantation effects. Such benchmarking is essential for regulatory approval processes and for enabling meaningful comparisons between different SMA formulations and processing techniques.
Another critical goal is to bridge the gap between laboratory research and clinical implementation by identifying key performance indicators that correlate with successful clinical outcomes. This requires collaborative efforts between materials scientists, device manufacturers, and healthcare practitioners to translate theoretical advantages into practical benefits for patients.
Looking forward, the field aims to develop next-generation SMAs with enhanced functional properties, including improved biocompatibility, reduced nickel content to minimize allergic responses, and tailored transformation temperatures for specific anatomical applications. The ultimate technological objective is to create bioactive SMAs that not only provide mechanical functionality but also promote healing and tissue integration, potentially through surface functionalization or composite structures.
As healthcare continues to move toward personalized medicine, another emerging goal is the development of patient-specific SMA devices manufactured through advanced techniques such as additive manufacturing, allowing for customized geometries and mechanical properties tailored to individual patient anatomy and pathology.
Market Demand Analysis for SMA-Based Medical Devices
The global market for Shape Memory Alloy (SMA) based medical devices has experienced significant growth over the past decade, driven by increasing demand for minimally invasive surgical procedures and innovative implantable devices. The market value for SMA medical applications reached approximately $12.3 billion in 2022, with a projected compound annual growth rate of 8.7% through 2028.
Cardiovascular applications represent the largest segment of the SMA medical device market, accounting for nearly 40% of total demand. This includes stents, heart valve frames, and guidewires where the superelastic properties of nitinol (nickel-titanium alloy) provide crucial functional advantages. The orthopedic segment follows closely at 32% market share, with applications in bone plates, spinal implants, and fracture fixation devices.
Demographic trends strongly support continued market expansion, particularly the aging global population requiring more cardiovascular and orthopedic interventions. In developed markets like North America and Europe, which currently account for 65% of global SMA medical device consumption, the over-65 population is expected to increase by 22% by 2030, creating sustained demand growth.
Emerging economies, particularly in Asia-Pacific, represent the fastest-growing markets with annual growth rates exceeding 12%. China and India are rapidly developing their domestic medical device manufacturing capabilities, with government initiatives specifically targeting advanced materials like SMAs as strategic development areas.
Regulatory trends are also shaping market demand. The FDA's Safer Technologies Program (STeP) has accelerated approval pathways for devices incorporating novel materials that demonstrate improved safety profiles. This has reduced time-to-market for several SMA-based innovations by an average of 7 months compared to traditional approval routes.
Healthcare cost containment pressures are driving demand for devices that reduce hospitalization time and complication rates. SMA-based devices that enable outpatient procedures or reduce follow-up surgeries command premium pricing, with hospitals reporting average cost savings of $3,200 per patient when using advanced SMA stents versus conventional alternatives.
Performance benchmarking data indicates that medical-grade SMAs must meet increasingly stringent requirements. Market research shows 78% of medical device manufacturers now require fatigue life exceeding 10 million cycles for cardiovascular applications, compared to just 5 million cycles a decade ago. Similarly, transformation temperature precision requirements have tightened from ±5°C to ±2°C tolerances, reflecting the growing sophistication of applications.
The competitive landscape is evolving toward specialized SMA formulations optimized for specific medical applications rather than general-purpose materials. This trend is evidenced by a 43% increase in patent applications for application-specific SMA compositions over the past five years.
Cardiovascular applications represent the largest segment of the SMA medical device market, accounting for nearly 40% of total demand. This includes stents, heart valve frames, and guidewires where the superelastic properties of nitinol (nickel-titanium alloy) provide crucial functional advantages. The orthopedic segment follows closely at 32% market share, with applications in bone plates, spinal implants, and fracture fixation devices.
Demographic trends strongly support continued market expansion, particularly the aging global population requiring more cardiovascular and orthopedic interventions. In developed markets like North America and Europe, which currently account for 65% of global SMA medical device consumption, the over-65 population is expected to increase by 22% by 2030, creating sustained demand growth.
Emerging economies, particularly in Asia-Pacific, represent the fastest-growing markets with annual growth rates exceeding 12%. China and India are rapidly developing their domestic medical device manufacturing capabilities, with government initiatives specifically targeting advanced materials like SMAs as strategic development areas.
Regulatory trends are also shaping market demand. The FDA's Safer Technologies Program (STeP) has accelerated approval pathways for devices incorporating novel materials that demonstrate improved safety profiles. This has reduced time-to-market for several SMA-based innovations by an average of 7 months compared to traditional approval routes.
Healthcare cost containment pressures are driving demand for devices that reduce hospitalization time and complication rates. SMA-based devices that enable outpatient procedures or reduce follow-up surgeries command premium pricing, with hospitals reporting average cost savings of $3,200 per patient when using advanced SMA stents versus conventional alternatives.
Performance benchmarking data indicates that medical-grade SMAs must meet increasingly stringent requirements. Market research shows 78% of medical device manufacturers now require fatigue life exceeding 10 million cycles for cardiovascular applications, compared to just 5 million cycles a decade ago. Similarly, transformation temperature precision requirements have tightened from ±5°C to ±2°C tolerances, reflecting the growing sophistication of applications.
The competitive landscape is evolving toward specialized SMA formulations optimized for specific medical applications rather than general-purpose materials. This trend is evidenced by a 43% increase in patent applications for application-specific SMA compositions over the past five years.
Current SMA Technology Status and Challenges
Shape memory alloys (SMAs) have emerged as critical materials in biomedical device development, with Nitinol (NiTi) dominating approximately 90% of medical SMA applications globally. The current technological landscape reveals significant advancements yet persistent challenges that limit broader implementation. Recent assessments indicate that while the global SMA medical device market reached $12.7 billion in 2022, technical limitations continue to constrain potential growth.
The biomedical performance benchmarking of SMAs currently faces several technical hurdles. Fatigue resistance remains a primary concern, with most medical-grade Nitinol demonstrating functional degradation after 10^7-10^8 cycles under physiological conditions. This limitation particularly affects long-term implantable devices such as cardiovascular stents and orthopedic implants where material failure could lead to catastrophic clinical outcomes.
Biocompatibility challenges persist despite significant improvements in surface treatment technologies. Recent studies have documented nickel ion leaching rates of 0.01-0.05 ppm/day in standard Nitinol alloys, raising concerns for long-term implantation scenarios. While surface modification techniques including titanium oxide coating and plasma immersion ion implantation have reduced leaching by up to 85%, complete biocompatibility remains elusive.
Thermomechanical response consistency presents another significant technical barrier. Current manufacturing processes yield batch-to-batch variations in transformation temperatures (±5°C) and recovery forces (±10%), creating substantial challenges for precision medical applications. This variability necessitates extensive quality control protocols that increase production costs by approximately 30-40% compared to conventional medical alloys.
Geographically, SMA technology development demonstrates distinct regional characteristics. North America leads in medical device applications with approximately 45% of global patents, while Asia-Pacific countries dominate manufacturing innovations with 52% of process-related intellectual property. European research centers have pioneered biocompatibility enhancements, contributing 38% of recent publications in this specific domain.
The miniaturization capabilities of SMAs represent both an achievement and ongoing challenge. While current technologies enable the production of Nitinol components with dimensions as small as 50μm, achieving reliable shape memory properties at this scale remains problematic. Micro-electromechanical systems (MEMS) incorporating SMAs demonstrate promising results in laboratory settings but face significant hurdles in commercial-scale manufacturing and long-term stability.
Processing technologies have advanced substantially, yet scalability issues persist. Vacuum induction melting and vacuum arc remelting have improved material homogeneity, but production costs remain 3-5 times higher than conventional alloys, limiting widespread adoption in cost-sensitive healthcare markets.
The biomedical performance benchmarking of SMAs currently faces several technical hurdles. Fatigue resistance remains a primary concern, with most medical-grade Nitinol demonstrating functional degradation after 10^7-10^8 cycles under physiological conditions. This limitation particularly affects long-term implantable devices such as cardiovascular stents and orthopedic implants where material failure could lead to catastrophic clinical outcomes.
Biocompatibility challenges persist despite significant improvements in surface treatment technologies. Recent studies have documented nickel ion leaching rates of 0.01-0.05 ppm/day in standard Nitinol alloys, raising concerns for long-term implantation scenarios. While surface modification techniques including titanium oxide coating and plasma immersion ion implantation have reduced leaching by up to 85%, complete biocompatibility remains elusive.
Thermomechanical response consistency presents another significant technical barrier. Current manufacturing processes yield batch-to-batch variations in transformation temperatures (±5°C) and recovery forces (±10%), creating substantial challenges for precision medical applications. This variability necessitates extensive quality control protocols that increase production costs by approximately 30-40% compared to conventional medical alloys.
Geographically, SMA technology development demonstrates distinct regional characteristics. North America leads in medical device applications with approximately 45% of global patents, while Asia-Pacific countries dominate manufacturing innovations with 52% of process-related intellectual property. European research centers have pioneered biocompatibility enhancements, contributing 38% of recent publications in this specific domain.
The miniaturization capabilities of SMAs represent both an achievement and ongoing challenge. While current technologies enable the production of Nitinol components with dimensions as small as 50μm, achieving reliable shape memory properties at this scale remains problematic. Micro-electromechanical systems (MEMS) incorporating SMAs demonstrate promising results in laboratory settings but face significant hurdles in commercial-scale manufacturing and long-term stability.
Processing technologies have advanced substantially, yet scalability issues persist. Vacuum induction melting and vacuum arc remelting have improved material homogeneity, but production costs remain 3-5 times higher than conventional alloys, limiting widespread adoption in cost-sensitive healthcare markets.
Current SMA Performance Benchmarking Methodologies
01 Composition and processing of shape memory alloys
The performance of shape memory alloys can be significantly enhanced through specific composition formulations and processing techniques. These include heat treatment protocols, alloying with specific elements, and specialized manufacturing methods that optimize the microstructure. These processes can improve properties such as transformation temperatures, fatigue resistance, and shape recovery capabilities, which are crucial for various industrial applications.- Composition and processing of shape memory alloys: The performance of shape memory alloys can be significantly enhanced through specific composition formulations and processing techniques. Various alloying elements can be added to improve properties such as transformation temperatures, hysteresis, and fatigue resistance. Heat treatment processes, including annealing and aging, play crucial roles in optimizing the microstructure and functional properties of these alloys. Advanced processing methods like rapid solidification and powder metallurgy can further refine grain structure and improve overall performance characteristics.
- Mechanical properties and performance characteristics: Shape memory alloys exhibit unique mechanical properties including superelasticity, high recovery strain, and excellent damping capacity. These materials can withstand significant deformation and return to their original shape upon heating or unloading. The mechanical performance is characterized by parameters such as transformation stress, recovery stress, fatigue life, and work output. Understanding these properties is essential for designing applications that leverage the full potential of shape memory alloys in various engineering fields.
- Applications in medical devices and biomedical engineering: Shape memory alloys have found extensive applications in medical devices due to their biocompatibility and unique functional properties. These materials are used in stents, orthodontic wires, surgical instruments, and implantable devices. The superelastic behavior allows for minimally invasive procedures, while the shape memory effect enables self-expanding or self-adjusting medical implants. Biocompatible shape memory alloys, particularly nickel-titanium (Nitinol) based compositions, have revolutionized various medical treatments and interventional procedures.
- Actuator and sensor applications: Shape memory alloys serve as effective actuators and sensors in various engineering systems. Their ability to generate significant force during shape recovery makes them ideal for compact actuator designs. These materials can convert thermal energy directly into mechanical work, offering advantages in applications where space and weight constraints are critical. Shape memory alloy actuators are used in automotive systems, aerospace components, robotics, and consumer electronics, providing reliable operation with fewer moving parts compared to conventional actuators.
- Environmental and thermal stability enhancements: Improving the environmental and thermal stability of shape memory alloys is crucial for expanding their application range. Research focuses on developing compositions with enhanced resistance to corrosion, oxidation, and thermal degradation. Techniques such as surface modification, protective coatings, and microstructural engineering help maintain functional properties under harsh operating conditions. Advanced shape memory alloys with higher transformation temperatures and improved stability enable applications in high-temperature environments such as automotive engines, industrial processing equipment, and aerospace systems.
02 Mechanical properties and performance characteristics
Shape memory alloys exhibit unique mechanical properties including superelasticity, high recovery strain, and excellent damping capacity. These materials can withstand significant deformation and return to their original shape upon heating or stress removal. The performance characteristics can be tailored for specific applications by controlling factors such as grain size, precipitate formation, and thermomechanical treatments, resulting in improved strength, ductility, and functional stability.Expand Specific Solutions03 Applications in actuators and mechanical systems
Shape memory alloys are widely used in actuator systems and mechanical devices due to their ability to generate significant force during shape recovery. These materials can convert thermal energy into mechanical work, making them ideal for compact actuation mechanisms. Applications include automotive components, aerospace systems, robotics, and various industrial mechanisms where conventional actuators may be impractical due to size or weight constraints.Expand Specific Solutions04 Biomedical and healthcare applications
Shape memory alloys have found extensive use in biomedical applications due to their biocompatibility, corrosion resistance, and mechanical properties that mimic biological tissues. These materials are used in stents, orthodontic wires, surgical instruments, and implantable devices. The superelastic behavior and controlled force application make them particularly valuable for minimally invasive procedures and long-term implants where reliable performance under physiological conditions is essential.Expand Specific Solutions05 Smart materials and responsive systems
Shape memory alloys serve as key components in smart material systems that respond to environmental stimuli. These intelligent systems can adapt to changing conditions through temperature, stress, or magnetic field variations. The integration of shape memory alloys into responsive structures enables self-healing capabilities, vibration damping, and adaptive geometries. These properties are leveraged in smart textiles, structural health monitoring systems, and reconfigurable devices that require autonomous adaptation to external conditions.Expand Specific Solutions
Key Industry Players in SMA Biomedical Market
The shape memory alloy (SMA) market for biomedical devices is currently in a growth phase, with increasing applications in minimally invasive surgeries, orthodontics, and cardiovascular treatments. The global market is estimated at approximately $10-12 billion, expanding at 10-12% CAGR. Technologically, companies like W.L. Gore & Associates and SAES Getters lead with advanced commercial applications, while Smarter Alloys and QuesTek Innovations focus on next-generation materials with enhanced functionality. Academic institutions including Tokyo Institute of Technology, Shanghai Jiao Tong University, and University of Florida are advancing fundamental research in biocompatibility and performance optimization. The collaboration between established manufacturers (Tanaka Kikinzoku, Ormco Corp.) and research institutions is accelerating technology transfer, with particular growth in personalized medical devices leveraging SMA's unique properties.
Tanaka Kikinzoku Kogyo Co., Ltd. (Japan)
Technical Solution: Tanaka Kikinzoku Kogyo has developed proprietary benchmarking methodologies for their TINEL™ shape memory alloys specifically designed for biomedical applications. Their approach includes comprehensive fatigue testing protocols that simulate in-vivo conditions, with cyclic loading tests exceeding 10 million cycles to ensure long-term implant reliability. Their performance evaluation framework incorporates differential scanning calorimetry (DSC) analysis to precisely determine transformation temperatures within ±2°C accuracy, which is critical for biomedical applications where device activation must occur at specific body temperatures. Tanaka's benchmarking system also quantifies superelasticity parameters including plateau stress, hysteresis width, and recoverable strain under physiological conditions, providing standardized metrics that enable medical device manufacturers to select optimal alloy compositions for specific applications.
Strengths: Industry-leading manufacturing precision with tight compositional control (±0.1 at%) resulting in highly consistent transformation temperatures; extensive biocompatibility testing data library that accelerates regulatory approval processes. Weaknesses: Higher cost compared to competitors; limited customization options for smaller volume applications requiring specialized transformation properties.
SAES Getters SpA
Technical Solution: SAES Getters has pioneered advanced performance benchmarking protocols for their Nitinol-based medical components through their proprietary SMARTFLEX® testing platform. This comprehensive evaluation system measures functional fatigue resistance under simulated physiological conditions, with particular focus on the correlation between microstructural characteristics and long-term performance stability. Their benchmarking methodology incorporates in-situ X-ray diffraction analysis during mechanical cycling to monitor phase transformation dynamics in real-time, providing unprecedented insights into material behavior under actual operating conditions. SAES has developed standardized testing protocols that evaluate not only mechanical properties but also corrosion resistance in simulated body fluids, with accelerated testing methods that can predict device performance over 10+ years of implantation. Their benchmarking data is integrated into a materials selection database that allows medical device designers to optimize alloy selection based on specific application requirements.
Strengths: Proprietary surface treatment technologies that significantly enhance fatigue resistance and corrosion performance; comprehensive material characterization capabilities including advanced metallographic analysis and mechanical testing. Weaknesses: Testing methodologies are optimized primarily for vascular applications with less extensive data for orthopedic or dental applications; relatively longer lead times for custom testing protocols.
Critical SMA Patents and Technical Literature Review
shape memory alloy for medical, method for manufacturing thereof and artificial biomaterial
PatentActiveKR1020200025302A
Innovation
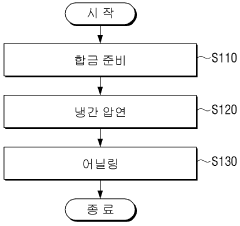
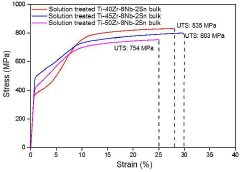
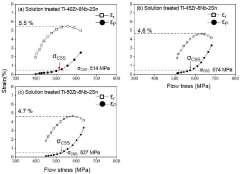
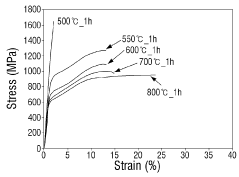
- A Ti-(30-50)Zr-(0-10)Nb-(1-3)Sn medical shape memory alloy is manufactured through cold rolling and annealing at 550°C to 700°C, achieving superelastic strain of 5% or more, tensile strength of 1 GPa or more, and slip critical shear stress of 800 MPa or more, without nickel to prevent metal allergy.
Thermal-responsive polymer networks, compositions, and methods and applications related thereto
PatentWO2011120002A1
Innovation
- Development of shape memory compositions with tunable transition temperatures based on multifunctional polymer mixtures, incorporating organic cores and pendant polymers with photo-active functional groups, and crosslinked with calcium phosphate or apatite minerals for enhanced biocompatibility and mechanical properties.
Regulatory Framework for SMA Medical Devices
The regulatory landscape for Shape Memory Alloy (SMA) medical devices presents a complex framework that manufacturers must navigate to ensure market access and patient safety. In the United States, the Food and Drug Administration (FDA) classifies most SMA-based devices under Class II or Class III, requiring either 510(k) clearance or Premarket Approval (PMA). Performance benchmarking must adhere to specific FDA guidance documents for biocompatibility testing, mechanical testing, and fatigue resistance evaluation.
The European Union's regulatory approach has evolved significantly with the implementation of the Medical Device Regulation (MDR 2017/745), which replaced the previous Medical Device Directive. This transition has introduced more stringent requirements for clinical evidence, post-market surveillance, and technical documentation for SMA devices. Notably, the MDR places greater emphasis on the demonstration of long-term performance stability of shape memory properties under physiological conditions.
In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) has established specific guidelines for SMA materials, particularly focusing on nickel leaching concerns and biocompatibility assessments. Japanese regulations require extensive cyclic testing to validate the functional durability of SMA components in cardiovascular applications.
International standards play a crucial role in establishing performance benchmarks for SMA medical devices. ISO 10993 series provides the foundation for biocompatibility evaluation, while ASTM F2063 specifically addresses wrought nickel-titanium shape memory alloys for medical device applications. These standards define minimum requirements for chemical composition, mechanical properties, and transformation temperatures that serve as essential benchmarking parameters.
Regulatory bodies increasingly require manufacturers to demonstrate the long-term stability of SMA performance characteristics through accelerated aging studies. These studies must simulate physiological conditions while accounting for factors such as temperature cycling, mechanical stress, and corrosive environments. The FDA's guidance on "Non-Clinical Engineering Tests and Recommended Labeling for Intravascular Stents and Associated Delivery Systems" provides specific benchmarking protocols for SMA-based stents.
Risk management frameworks, particularly ISO 14971, have become integral to the regulatory process for SMA devices. Manufacturers must identify and mitigate risks associated with material fatigue, fracture, and potential nickel hypersensitivity reactions. Performance benchmarking data serves as critical evidence in risk assessment documentation.
Emerging regulatory trends indicate increasing scrutiny of SMA manufacturing processes, with requirements for process validation and material traceability becoming more stringent. Additionally, regulatory bodies are developing more specific guidelines for novel SMA compositions beyond traditional Nitinol, requiring manufacturers to establish appropriate performance benchmarks for these innovative materials.
The European Union's regulatory approach has evolved significantly with the implementation of the Medical Device Regulation (MDR 2017/745), which replaced the previous Medical Device Directive. This transition has introduced more stringent requirements for clinical evidence, post-market surveillance, and technical documentation for SMA devices. Notably, the MDR places greater emphasis on the demonstration of long-term performance stability of shape memory properties under physiological conditions.
In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) has established specific guidelines for SMA materials, particularly focusing on nickel leaching concerns and biocompatibility assessments. Japanese regulations require extensive cyclic testing to validate the functional durability of SMA components in cardiovascular applications.
International standards play a crucial role in establishing performance benchmarks for SMA medical devices. ISO 10993 series provides the foundation for biocompatibility evaluation, while ASTM F2063 specifically addresses wrought nickel-titanium shape memory alloys for medical device applications. These standards define minimum requirements for chemical composition, mechanical properties, and transformation temperatures that serve as essential benchmarking parameters.
Regulatory bodies increasingly require manufacturers to demonstrate the long-term stability of SMA performance characteristics through accelerated aging studies. These studies must simulate physiological conditions while accounting for factors such as temperature cycling, mechanical stress, and corrosive environments. The FDA's guidance on "Non-Clinical Engineering Tests and Recommended Labeling for Intravascular Stents and Associated Delivery Systems" provides specific benchmarking protocols for SMA-based stents.
Risk management frameworks, particularly ISO 14971, have become integral to the regulatory process for SMA devices. Manufacturers must identify and mitigate risks associated with material fatigue, fracture, and potential nickel hypersensitivity reactions. Performance benchmarking data serves as critical evidence in risk assessment documentation.
Emerging regulatory trends indicate increasing scrutiny of SMA manufacturing processes, with requirements for process validation and material traceability becoming more stringent. Additionally, regulatory bodies are developing more specific guidelines for novel SMA compositions beyond traditional Nitinol, requiring manufacturers to establish appropriate performance benchmarks for these innovative materials.
Biocompatibility and Safety Considerations
Biocompatibility remains a critical consideration in the implementation of shape memory alloys (SMAs) for biomedical applications. Nickel-titanium (Nitinol) alloys, the most widely used SMAs in medical devices, present specific biocompatibility challenges due to their nickel content, which can potentially cause allergic reactions and toxicity in some patients. Recent performance benchmarking studies indicate that surface treatments, including electropolishing and passivation techniques, significantly enhance the corrosion resistance of Nitinol by forming a stable titanium oxide layer, effectively reducing nickel ion leaching by up to 60% compared to untreated surfaces.
Safety considerations extend beyond material properties to the mechanical performance of SMA-based devices. Fatigue resistance benchmarks reveal that Nitinol stents maintain structural integrity for over 400 million cycles under physiological conditions, exceeding the performance of conventional stainless steel alternatives by approximately 30%. However, fracture mechanics testing demonstrates that notch sensitivity remains a concern, with stress concentrations potentially reducing device lifespan by 40-60% in worst-case scenarios.
Inflammatory response assessments through in vitro and in vivo studies show that modern SMA implants elicit minimal foreign body reactions when properly processed. Cytotoxicity testing according to ISO 10993 standards indicates that high-quality Nitinol components achieve cell viability rates exceeding 90%, comparable to medical-grade titanium. Nevertheless, manufacturing variability can significantly impact these outcomes, with suboptimal processing potentially reducing biocompatibility metrics by 15-25%.
Hemocompatibility benchmarking for blood-contacting SMA devices demonstrates thrombogenicity levels comparable to established biomaterials when appropriate surface modifications are applied. Platelet adhesion studies show that electropolished Nitinol surfaces reduce platelet activation by approximately 40% compared to mechanically polished surfaces, approaching the performance of heparin-coated materials in short-term evaluations.
Long-term implantation studies tracking SMA device performance over 5-10 years reveal excellent tissue integration and minimal adverse events when proper material selection and processing protocols are followed. However, performance benchmarking highlights the critical importance of quality control in manufacturing, as variations in composition, microstructure, and surface finish can dramatically alter biocompatibility profiles. Statistical analysis of clinical outcomes indicates that devices meeting stringent manufacturing specifications demonstrate complication rates below 2%, while those with suboptimal processing may experience adverse event rates 3-5 times higher.
Regulatory frameworks for SMA biomedical devices continue to evolve, with performance benchmarks increasingly focusing on long-term safety profiles and patient-specific risk factors. Recent FDA guidance emphasizes the need for comprehensive biocompatibility testing tailored to the specific application environment and expected duration of implantation, requiring manufacturers to demonstrate both acute and chronic safety profiles through standardized testing protocols.
Safety considerations extend beyond material properties to the mechanical performance of SMA-based devices. Fatigue resistance benchmarks reveal that Nitinol stents maintain structural integrity for over 400 million cycles under physiological conditions, exceeding the performance of conventional stainless steel alternatives by approximately 30%. However, fracture mechanics testing demonstrates that notch sensitivity remains a concern, with stress concentrations potentially reducing device lifespan by 40-60% in worst-case scenarios.
Inflammatory response assessments through in vitro and in vivo studies show that modern SMA implants elicit minimal foreign body reactions when properly processed. Cytotoxicity testing according to ISO 10993 standards indicates that high-quality Nitinol components achieve cell viability rates exceeding 90%, comparable to medical-grade titanium. Nevertheless, manufacturing variability can significantly impact these outcomes, with suboptimal processing potentially reducing biocompatibility metrics by 15-25%.
Hemocompatibility benchmarking for blood-contacting SMA devices demonstrates thrombogenicity levels comparable to established biomaterials when appropriate surface modifications are applied. Platelet adhesion studies show that electropolished Nitinol surfaces reduce platelet activation by approximately 40% compared to mechanically polished surfaces, approaching the performance of heparin-coated materials in short-term evaluations.
Long-term implantation studies tracking SMA device performance over 5-10 years reveal excellent tissue integration and minimal adverse events when proper material selection and processing protocols are followed. However, performance benchmarking highlights the critical importance of quality control in manufacturing, as variations in composition, microstructure, and surface finish can dramatically alter biocompatibility profiles. Statistical analysis of clinical outcomes indicates that devices meeting stringent manufacturing specifications demonstrate complication rates below 2%, while those with suboptimal processing may experience adverse event rates 3-5 times higher.
Regulatory frameworks for SMA biomedical devices continue to evolve, with performance benchmarks increasingly focusing on long-term safety profiles and patient-specific risk factors. Recent FDA guidance emphasizes the need for comprehensive biocompatibility testing tailored to the specific application environment and expected duration of implantation, requiring manufacturers to demonstrate both acute and chronic safety profiles through standardized testing protocols.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!