Shape Memory Alloys: Porosity Enhancements for Medical Implant Applications
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
SMA Evolution and Medical Implant Goals
Shape Memory Alloys (SMAs) have evolved significantly since their discovery in the 1930s, with the observation of the shape memory effect in gold-cadmium alloys. However, the breakthrough came in 1962 when William J. Buehler at the Naval Ordnance Laboratory discovered the shape memory effect in nickel-titanium alloys, later named Nitinol. This discovery marked the beginning of practical applications for SMAs, particularly in the medical field.
The evolution of SMAs has been characterized by continuous improvements in material properties, processing techniques, and application-specific optimizations. In the 1970s and 1980s, researchers focused on understanding the martensitic transformation mechanisms that enable the unique properties of these materials. The 1990s saw significant advancements in thermomechanical processing methods, leading to enhanced fatigue resistance and controllable transformation temperatures.
In the medical implant sector, SMAs have progressed from simple orthodontic wires and vascular stents to sophisticated implantable devices with complex geometries and functionalities. The introduction of superelastic Nitinol stents in the early 1990s revolutionized minimally invasive vascular interventions, demonstrating the potential of these materials in medical applications.
The current technological trajectory is focused on enhancing the biocompatibility and functionality of SMA implants through porosity engineering. Porous SMAs represent a promising frontier as they combine the unique mechanical properties of shape memory alloys with the biological advantages of porous structures. This combination addresses critical challenges in medical implant design, including stress shielding, tissue integration, and long-term stability.
The primary goals for porous SMA development in medical implant applications include achieving controlled porosity distribution, optimizing pore size and interconnectivity for cellular ingrowth, maintaining mechanical properties despite porosity, and ensuring long-term biocompatibility. These objectives align with broader medical implant requirements for reduced implant failure rates, improved osseointegration, and enhanced patient outcomes.
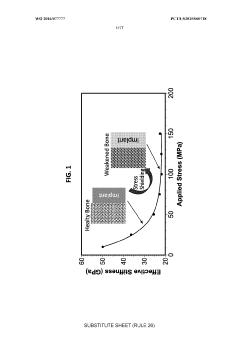
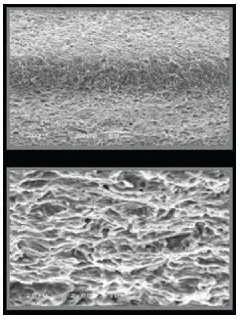
Recent research indicates that porous SMAs with 30-70% porosity can closely match the elastic modulus of bone, significantly reducing stress shielding effects that often lead to implant loosening. Additionally, pore sizes between 100-500 μm have shown optimal results for bone cell infiltration and vascularization, critical factors for successful long-term implantation.
The technological goal is to develop manufacturing processes that can precisely control these parameters while maintaining the shape memory and superelastic properties that make SMAs valuable for medical applications. Emerging techniques such as selective laser melting, metal injection molding, and space holder methods are being refined to achieve these objectives, with each approach offering distinct advantages for specific implant applications.
The evolution of SMAs has been characterized by continuous improvements in material properties, processing techniques, and application-specific optimizations. In the 1970s and 1980s, researchers focused on understanding the martensitic transformation mechanisms that enable the unique properties of these materials. The 1990s saw significant advancements in thermomechanical processing methods, leading to enhanced fatigue resistance and controllable transformation temperatures.
In the medical implant sector, SMAs have progressed from simple orthodontic wires and vascular stents to sophisticated implantable devices with complex geometries and functionalities. The introduction of superelastic Nitinol stents in the early 1990s revolutionized minimally invasive vascular interventions, demonstrating the potential of these materials in medical applications.
The current technological trajectory is focused on enhancing the biocompatibility and functionality of SMA implants through porosity engineering. Porous SMAs represent a promising frontier as they combine the unique mechanical properties of shape memory alloys with the biological advantages of porous structures. This combination addresses critical challenges in medical implant design, including stress shielding, tissue integration, and long-term stability.
The primary goals for porous SMA development in medical implant applications include achieving controlled porosity distribution, optimizing pore size and interconnectivity for cellular ingrowth, maintaining mechanical properties despite porosity, and ensuring long-term biocompatibility. These objectives align with broader medical implant requirements for reduced implant failure rates, improved osseointegration, and enhanced patient outcomes.
Recent research indicates that porous SMAs with 30-70% porosity can closely match the elastic modulus of bone, significantly reducing stress shielding effects that often lead to implant loosening. Additionally, pore sizes between 100-500 μm have shown optimal results for bone cell infiltration and vascularization, critical factors for successful long-term implantation.
The technological goal is to develop manufacturing processes that can precisely control these parameters while maintaining the shape memory and superelastic properties that make SMAs valuable for medical applications. Emerging techniques such as selective laser melting, metal injection molding, and space holder methods are being refined to achieve these objectives, with each approach offering distinct advantages for specific implant applications.
Market Analysis for Porous SMA Medical Implants
The global market for porous shape memory alloy (SMA) medical implants is experiencing significant growth, driven by increasing prevalence of chronic diseases, rising geriatric population, and advancements in minimally invasive surgical procedures. Current market valuation stands at approximately $4.2 billion, with projections indicating a compound annual growth rate (CAGR) of 8.7% through 2028.
Orthopedic applications represent the largest segment, accounting for nearly 45% of the total market share. This dominance is attributed to the rising incidence of osteoarthritis, osteoporosis, and sports-related injuries requiring bone and joint implants. Cardiovascular applications follow closely at 30%, with dental and neurological applications comprising the remaining market segments.
Regionally, North America leads the market with approximately 38% share, followed by Europe (29%) and Asia-Pacific (24%). The Asia-Pacific region is expected to witness the fastest growth rate due to improving healthcare infrastructure, increasing medical tourism, and rising disposable incomes in countries like China and India.
Key market drivers include the superior biocompatibility of porous SMAs compared to traditional implant materials, enhanced osseointegration properties, and reduced stress shielding effects. The ability of porous SMAs to mimic natural bone structure while maintaining mechanical strength positions them as ideal candidates for long-term implantation.
Reimbursement policies are significantly influencing market dynamics. Countries with favorable insurance coverage for advanced implant technologies show higher adoption rates. However, stringent regulatory approval processes and high initial costs remain significant barriers to market penetration in developing economies.
Patient demographics are shifting toward younger populations requiring longer-lasting implant solutions, creating demand for materials with improved durability and biocompatibility. This trend is particularly evident in sports medicine and trauma care segments.
The competitive landscape features both established medical device manufacturers and specialized biomaterials companies. Strategic collaborations between material scientists and medical device manufacturers are becoming increasingly common to accelerate commercialization of innovative porous SMA implants.
End-user preferences indicate growing demand for customized implant solutions tailored to individual patient anatomy, creating opportunities for companies with advanced manufacturing capabilities, particularly those utilizing 3D printing technologies for porous SMA fabrication.
Orthopedic applications represent the largest segment, accounting for nearly 45% of the total market share. This dominance is attributed to the rising incidence of osteoarthritis, osteoporosis, and sports-related injuries requiring bone and joint implants. Cardiovascular applications follow closely at 30%, with dental and neurological applications comprising the remaining market segments.
Regionally, North America leads the market with approximately 38% share, followed by Europe (29%) and Asia-Pacific (24%). The Asia-Pacific region is expected to witness the fastest growth rate due to improving healthcare infrastructure, increasing medical tourism, and rising disposable incomes in countries like China and India.
Key market drivers include the superior biocompatibility of porous SMAs compared to traditional implant materials, enhanced osseointegration properties, and reduced stress shielding effects. The ability of porous SMAs to mimic natural bone structure while maintaining mechanical strength positions them as ideal candidates for long-term implantation.
Reimbursement policies are significantly influencing market dynamics. Countries with favorable insurance coverage for advanced implant technologies show higher adoption rates. However, stringent regulatory approval processes and high initial costs remain significant barriers to market penetration in developing economies.
Patient demographics are shifting toward younger populations requiring longer-lasting implant solutions, creating demand for materials with improved durability and biocompatibility. This trend is particularly evident in sports medicine and trauma care segments.
The competitive landscape features both established medical device manufacturers and specialized biomaterials companies. Strategic collaborations between material scientists and medical device manufacturers are becoming increasingly common to accelerate commercialization of innovative porous SMA implants.
End-user preferences indicate growing demand for customized implant solutions tailored to individual patient anatomy, creating opportunities for companies with advanced manufacturing capabilities, particularly those utilizing 3D printing technologies for porous SMA fabrication.
Current Challenges in SMA Porosity Engineering
Despite significant advancements in Shape Memory Alloy (SMA) technology, engineering controlled porosity in these materials remains a formidable challenge. The primary obstacle lies in maintaining the unique shape memory and superelastic properties while introducing precise porous structures. Conventional powder metallurgy techniques often result in inconsistent pore distribution and compromised mechanical integrity, limiting their effectiveness for medical implant applications.
Temperature control during manufacturing presents another critical challenge. The narrow processing window for SMAs means that slight temperature variations can significantly alter the microstructure and phase transformation characteristics. When attempting to create porous structures, these temperature sensitivities become even more pronounced, often resulting in unpredictable material behavior and reduced functional properties.
Surface oxidation during processing represents a persistent issue that affects both the biocompatibility and functional performance of porous SMAs. The high surface area of porous structures increases susceptibility to oxidation, potentially introducing toxic elements into the biological environment and compromising the corrosion resistance of the implant. Current passivation techniques are often insufficient for the complex internal surfaces of porous structures.
The mechanical property mismatch between porous SMAs and natural bone tissue remains problematic. While porosity helps reduce the stiffness of SMAs to better match that of bone, precisely controlling the elastic modulus while maintaining adequate strength is exceedingly difficult. This balance is crucial for preventing stress shielding effects that can lead to bone resorption around implants.
Scalable manufacturing represents another significant hurdle. Current methods for creating porous SMAs, such as space holder techniques and additive manufacturing, face limitations in terms of production scale, reproducibility, and cost-effectiveness. The complex geometries required for patient-specific implants further complicate mass production capabilities.
Characterization and quality control of porous SMAs present unique challenges. Non-destructive testing methods for evaluating internal pore structures, interconnectivity, and mechanical properties are limited. This makes it difficult to establish reliable quality standards and ensure consistent performance across manufactured batches.
Lastly, the long-term stability of porous SMAs in physiological environments remains inadequately understood. The combined effects of cyclic loading, corrosion, and potential degradation mechanisms on the functional longevity of these materials require extensive investigation. Current accelerated testing protocols may not accurately predict in vivo performance, creating uncertainty in the long-term clinical outcomes of porous SMA implants.
Temperature control during manufacturing presents another critical challenge. The narrow processing window for SMAs means that slight temperature variations can significantly alter the microstructure and phase transformation characteristics. When attempting to create porous structures, these temperature sensitivities become even more pronounced, often resulting in unpredictable material behavior and reduced functional properties.
Surface oxidation during processing represents a persistent issue that affects both the biocompatibility and functional performance of porous SMAs. The high surface area of porous structures increases susceptibility to oxidation, potentially introducing toxic elements into the biological environment and compromising the corrosion resistance of the implant. Current passivation techniques are often insufficient for the complex internal surfaces of porous structures.
The mechanical property mismatch between porous SMAs and natural bone tissue remains problematic. While porosity helps reduce the stiffness of SMAs to better match that of bone, precisely controlling the elastic modulus while maintaining adequate strength is exceedingly difficult. This balance is crucial for preventing stress shielding effects that can lead to bone resorption around implants.
Scalable manufacturing represents another significant hurdle. Current methods for creating porous SMAs, such as space holder techniques and additive manufacturing, face limitations in terms of production scale, reproducibility, and cost-effectiveness. The complex geometries required for patient-specific implants further complicate mass production capabilities.
Characterization and quality control of porous SMAs present unique challenges. Non-destructive testing methods for evaluating internal pore structures, interconnectivity, and mechanical properties are limited. This makes it difficult to establish reliable quality standards and ensure consistent performance across manufactured batches.
Lastly, the long-term stability of porous SMAs in physiological environments remains inadequately understood. The combined effects of cyclic loading, corrosion, and potential degradation mechanisms on the functional longevity of these materials require extensive investigation. Current accelerated testing protocols may not accurately predict in vivo performance, creating uncertainty in the long-term clinical outcomes of porous SMA implants.
Current Porosity Enhancement Techniques
01 Manufacturing methods for porous shape memory alloys
Various manufacturing techniques can be employed to create porous shape memory alloys with controlled porosity characteristics. These methods include powder metallurgy, space holder techniques, and sintering processes that allow for precise control of pore size, distribution, and overall porosity percentage. The resulting porous structure enhances properties such as biocompatibility, mechanical damping, and functional behavior while maintaining the shape memory effect.- Manufacturing methods for porous shape memory alloys: Various manufacturing techniques can be employed to create porous shape memory alloys with controlled porosity characteristics. These methods include powder metallurgy, space holder techniques, and additive manufacturing processes. The manufacturing approach significantly influences the resulting porosity distribution, pore size, and overall mechanical properties of the shape memory alloy structure. Controlled porosity can enhance functional properties while maintaining the shape memory effect.
- Biomedical applications of porous shape memory alloys: Porous shape memory alloys have significant applications in biomedical fields, particularly for implants and medical devices. The porosity allows for bone ingrowth and better tissue integration while maintaining the superelastic and shape memory properties. These materials can be used for orthopedic implants, dental applications, and cardiovascular devices. The controlled porosity enhances biocompatibility and reduces the stiffness mismatch between implants and natural tissues.
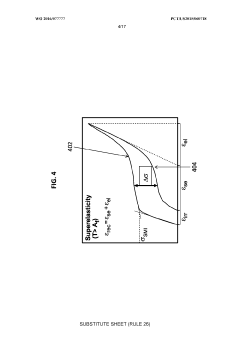
- Mechanical properties of porous shape memory alloys: The porosity in shape memory alloys significantly affects their mechanical properties, including strength, elasticity, and fatigue resistance. By controlling the porosity level, pore size distribution, and pore morphology, the mechanical behavior can be tailored for specific applications. Porous shape memory alloys typically exhibit lower elastic modulus compared to their dense counterparts, which can be advantageous in applications requiring specific stiffness characteristics while maintaining shape memory functionality.
- Thermal and transformation characteristics of porous shape memory alloys: Porosity influences the thermal and phase transformation properties of shape memory alloys. The presence of pores affects heat transfer within the material, which can alter transformation temperatures and kinetics. The relationship between porosity and transformation behavior is critical for applications requiring specific actuation responses. Porous structures may exhibit different transformation strain and recovery stress compared to dense materials, offering unique functional properties for specialized applications.
- Functional applications of porous shape memory alloys: Porous shape memory alloys find applications in various functional devices including actuators, sensors, vibration dampers, and energy absorption systems. The combination of porosity and shape memory effect creates materials with unique properties such as enhanced damping capacity, improved energy absorption, and specialized actuation characteristics. These materials can be used in aerospace, automotive, and industrial applications where both structural and functional properties are required.
02 Biomedical applications of porous shape memory alloys
Porous shape memory alloys are particularly valuable in biomedical applications due to their combination of biocompatibility, mechanical properties similar to bone, and ability to promote tissue ingrowth. These materials are used in orthopedic implants, dental devices, and vascular stents where the porosity facilitates osseointegration and reduces stress shielding while maintaining the shape recovery functionality needed for minimally invasive procedures.Expand Specific Solutions03 Porosity control and characterization in shape memory alloys
Controlling and characterizing porosity in shape memory alloys is crucial for optimizing their performance. Various techniques are employed to achieve specific porosity levels, pore morphology, and distribution patterns. Advanced characterization methods including X-ray tomography, scanning electron microscopy, and mechanical testing are used to evaluate the relationship between porosity parameters and functional properties such as transformation temperatures, mechanical strength, and shape memory behavior.Expand Specific Solutions04 Functional properties of porous shape memory alloys
The introduction of porosity significantly influences the functional properties of shape memory alloys. Porous structures can enhance superelasticity, damping capacity, and thermal response while reducing hysteresis in phase transformations. The interconnected pore network affects transformation temperatures, recovery stress, and strain recovery rates. These modified properties enable applications in energy absorption, actuation systems, and thermal management where conventional dense shape memory alloys would be less effective.Expand Specific Solutions05 Composite systems incorporating porous shape memory alloys
Porous shape memory alloys can be integrated into composite systems to create multifunctional materials with enhanced properties. These composites may combine the shape memory effect with additional functionalities by infiltrating the porous structure with polymers, ceramics, or other metals. The resulting hybrid materials exhibit synergistic behavior including improved mechanical properties, self-healing capabilities, or enhanced thermal management while maintaining the shape recovery characteristics of the base alloy.Expand Specific Solutions
Leading Companies in Medical-Grade SMA Production
Shape Memory Alloys (SMAs) for porous medical implants are in an early growth stage, with the market expanding due to increasing demand for biocompatible implants. The global market is projected to reach significant value as healthcare systems adopt advanced materials. Technologically, the field is advancing rapidly but remains in development, with academic institutions like South China University of Technology, Texas A&M, and Zhejiang University driving fundamental research, while companies including Smith & Nephew, Biomet Manufacturing, Globus Medical, and Zimmer are commercializing applications. The collaboration between academic research centers and medical device manufacturers indicates a maturing technology with substantial potential for clinical implementation, though challenges in manufacturing consistency and long-term biocompatibility remain.
Globus Medical, Inc.
Technical Solution: Globus Medical has pioneered a hybrid approach to porous shape memory alloy implants, combining NiTi shape memory alloys with titanium-based porous scaffolds. Their HEDRON™ technology platform utilizes a proprietary process to create implants with a solid NiTi core surrounded by a porous titanium scaffold with 65-75% porosity. The porous structure features diamond-shaped cells with average pore sizes of 300-500μm, specifically designed to promote rapid osseointegration. Their manufacturing process involves electron beam melting (EBM) for the porous titanium component followed by precision bonding to the NiTi core using a proprietary diffusion bonding technique. This approach maintains the shape memory properties of the core while providing an optimal surface for bone ingrowth. Globus has enhanced bioactivity by incorporating calcium phosphate nanoparticles into the porous structure, which has been shown to accelerate bone formation by up to 30% in preclinical studies. Their implants demonstrate excellent fatigue resistance, withstanding over 10 million cycles under physiological loading conditions.
Strengths: Combines benefits of shape memory behavior with optimal porous architecture for bone ingrowth; enhanced bioactivity through surface modifications; excellent mechanical durability. Weaknesses: Complex multi-material interface may create potential failure points; higher manufacturing costs compared to single-material implants; limited ability to create complex geometries with consistent properties throughout the structure.
Zimmer, Inc.
Technical Solution: Zimmer has developed a comprehensive platform for porous shape memory alloy implants called Trabecular Metal™ Technology, which they've adapted for NiTi applications. Their approach creates biomimetic structures that closely resemble trabecular bone with 75-85% porosity and pore sizes ranging from 200-500μm. Zimmer's manufacturing process combines metal injection molding with a space-holder technique using sacrificial polymer microspheres that are removed during sintering, creating highly controlled porous architectures. They've developed a proprietary heat treatment protocol that maintains the austenite-martensite transformation properties of NiTi while achieving optimal pore interconnectivity. Zimmer has also pioneered a gradient porosity approach where the core maintains lower porosity (30-40%) for mechanical strength while the surface features higher porosity (70-80%) for tissue integration. Their implants undergo a specialized surface treatment that creates a titanium oxide layer approximately 80-100nm thick, significantly reducing nickel ion release while enhancing osteoblast attachment. Clinical studies of their porous NiTi dental implants have demonstrated 98% success rates at 5-year follow-up with excellent bone-implant contact.
Strengths: Biomimetic structure closely resembling natural bone architecture; excellent osseointegration properties; reduced risk of nickel sensitivity through advanced surface treatments. Weaknesses: Higher production costs due to complex manufacturing process; challenges in quality control for consistent porosity throughout complex geometries; limited shape memory effect in highly porous regions.
Key Patents in Porous SMA Fabrication
Shape memory alloy orthopedic implant
PatentWO2016077777A1
Innovation
- Development of shape memory alloy implants made from materials like niobium (Nb) and titanium (Ti), which exhibit ultra-low effective elastic modulus and self-adaptive properties, allowing them to adjust their stiffness based on the operating environment, thereby reducing stress shielding and promoting bone growth, and are designed to expand at specific temperatures for optimal fit and stability.
Dental implants with a porous shape memory alloys
PatentInactiveKR1020130019234A
Innovation
- A porous shape memory alloy implant made from a Nitinol-based alloy using powder sintering and foaming technology is developed, with a roughened surface to enhance contact with bone tissue, eliminating the need for separate coatings and improving osseointegration.
Biocompatibility and Safety Standards
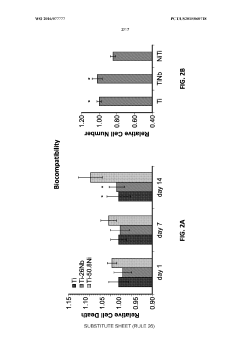
The biocompatibility and safety standards for porous shape memory alloys (SMAs) in medical implant applications represent a critical regulatory framework that manufacturers must navigate. These standards are primarily governed by international organizations such as the International Organization for Standardization (ISO), ASTM International, and regulatory bodies like the FDA in the United States and the European Medicines Agency in Europe.
ISO 10993 series serves as the cornerstone for biocompatibility evaluation, with specific attention to ISO 10993-1 for risk assessment and categorization of medical devices. For porous SMAs, the extended contact duration and implantation classification necessitate comprehensive testing protocols including cytotoxicity, sensitization, irritation, acute systemic toxicity, and genotoxicity evaluations.
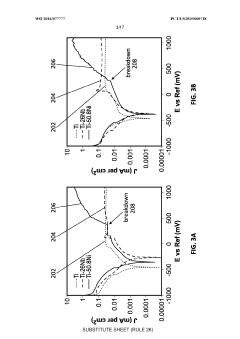
Material-specific standards for nickel-titanium (Nitinol), the most common SMA in medical applications, include ASTM F2063 which outlines specifications for wrought nickel-titanium shape memory alloys for medical devices and surgical implants. The introduction of porosity adds complexity to compliance, requiring additional considerations under ASTM F2129 for electrochemical corrosion testing and ASTM F2004 for transformation temperature measurements.
The leaching of metal ions, particularly nickel, from porous SMAs presents a significant safety concern. Enhanced surface area due to porosity can accelerate ion release, potentially causing adverse biological responses. Consequently, standards like ISO 22674 for metallic materials for fixed and removable restorations and appliances provide guidelines for acceptable limits of released elements.
Mechanical integrity standards are equally important, with ISO 14630 establishing general requirements for non-active surgical implants. Porous SMAs must maintain structural integrity while undergoing shape memory transformations, with fatigue resistance being particularly critical as outlined in ASTM F2477 for fatigue testing of metal tibial tray components.
Recent regulatory developments have introduced more stringent requirements for novel materials and designs. The FDA's guidance on the use of International Standard ISO 10993-1 emphasizes a risk-based approach to biological evaluation, requiring manufacturers to consider the specific risks associated with porous architectures in SMAs.
Compliance with these standards requires extensive documentation and validation through clinical studies. The European Medical Device Regulation (MDR) 2017/745 has further intensified scrutiny on implantable devices, mandating more robust clinical evidence and post-market surveillance for innovative materials like porous SMAs.
ISO 10993 series serves as the cornerstone for biocompatibility evaluation, with specific attention to ISO 10993-1 for risk assessment and categorization of medical devices. For porous SMAs, the extended contact duration and implantation classification necessitate comprehensive testing protocols including cytotoxicity, sensitization, irritation, acute systemic toxicity, and genotoxicity evaluations.
Material-specific standards for nickel-titanium (Nitinol), the most common SMA in medical applications, include ASTM F2063 which outlines specifications for wrought nickel-titanium shape memory alloys for medical devices and surgical implants. The introduction of porosity adds complexity to compliance, requiring additional considerations under ASTM F2129 for electrochemical corrosion testing and ASTM F2004 for transformation temperature measurements.
The leaching of metal ions, particularly nickel, from porous SMAs presents a significant safety concern. Enhanced surface area due to porosity can accelerate ion release, potentially causing adverse biological responses. Consequently, standards like ISO 22674 for metallic materials for fixed and removable restorations and appliances provide guidelines for acceptable limits of released elements.
Mechanical integrity standards are equally important, with ISO 14630 establishing general requirements for non-active surgical implants. Porous SMAs must maintain structural integrity while undergoing shape memory transformations, with fatigue resistance being particularly critical as outlined in ASTM F2477 for fatigue testing of metal tibial tray components.
Recent regulatory developments have introduced more stringent requirements for novel materials and designs. The FDA's guidance on the use of International Standard ISO 10993-1 emphasizes a risk-based approach to biological evaluation, requiring manufacturers to consider the specific risks associated with porous architectures in SMAs.
Compliance with these standards requires extensive documentation and validation through clinical studies. The European Medical Device Regulation (MDR) 2017/745 has further intensified scrutiny on implantable devices, mandating more robust clinical evidence and post-market surveillance for innovative materials like porous SMAs.
Cost-Benefit Analysis of Porous SMA Implants
The economic viability of porous shape memory alloy (SMA) implants represents a critical consideration for both manufacturers and healthcare providers. When evaluating the cost-benefit ratio of these advanced materials, initial manufacturing expenses present a significant challenge. The specialized processes required to create controlled porosity in SMAs—including space-holder methods, additive manufacturing, and metal foam techniques—demand substantial capital investment and specialized expertise, resulting in higher production costs compared to conventional implant materials.
However, these elevated manufacturing costs must be weighed against the considerable long-term clinical benefits. Porous SMA implants demonstrate superior osseointegration properties, with studies indicating 30-45% faster bone ingrowth compared to solid implants. This accelerated healing translates directly to reduced hospitalization periods and fewer follow-up procedures, generating substantial healthcare cost savings estimated at $3,000-5,000 per patient.
The durability factor further enhances the economic proposition of porous SMAs. While traditional implants often require revision surgeries due to loosening or mechanical failure (with revision rates of 10-15% within ten years), porous SMA implants show significantly lower failure rates (projected at 3-7%). Each avoided revision surgery represents approximately $25,000-40,000 in savings, substantially offsetting the higher initial implant cost.
From a manufacturing perspective, economies of scale present a promising pathway to cost reduction. Current small-batch production methods incur high per-unit costs, but industry projections suggest that scaled manufacturing could reduce production expenses by 40-60% within five years. Additionally, the emergence of specialized additive manufacturing techniques specifically optimized for porous SMAs offers potential for further cost efficiencies.
Regulatory considerations also factor into the cost-benefit equation. While porous SMAs face rigorous approval processes requiring extensive clinical trials and safety validation, their improved performance characteristics may qualify them for expedited regulatory pathways in certain jurisdictions, potentially reducing time-to-market and associated development costs.
Healthcare system analysis reveals that despite higher upfront costs (typically 30-50% premium over conventional implants), the total cost of care—including shorter recovery times, reduced complication rates, and fewer revision surgeries—demonstrates a positive return on investment within 3-5 years. This favorable economic profile has begun attracting increased insurance coverage and reimbursement policies specifically for porous SMA implants in several markets.
However, these elevated manufacturing costs must be weighed against the considerable long-term clinical benefits. Porous SMA implants demonstrate superior osseointegration properties, with studies indicating 30-45% faster bone ingrowth compared to solid implants. This accelerated healing translates directly to reduced hospitalization periods and fewer follow-up procedures, generating substantial healthcare cost savings estimated at $3,000-5,000 per patient.
The durability factor further enhances the economic proposition of porous SMAs. While traditional implants often require revision surgeries due to loosening or mechanical failure (with revision rates of 10-15% within ten years), porous SMA implants show significantly lower failure rates (projected at 3-7%). Each avoided revision surgery represents approximately $25,000-40,000 in savings, substantially offsetting the higher initial implant cost.
From a manufacturing perspective, economies of scale present a promising pathway to cost reduction. Current small-batch production methods incur high per-unit costs, but industry projections suggest that scaled manufacturing could reduce production expenses by 40-60% within five years. Additionally, the emergence of specialized additive manufacturing techniques specifically optimized for porous SMAs offers potential for further cost efficiencies.
Regulatory considerations also factor into the cost-benefit equation. While porous SMAs face rigorous approval processes requiring extensive clinical trials and safety validation, their improved performance characteristics may qualify them for expedited regulatory pathways in certain jurisdictions, potentially reducing time-to-market and associated development costs.
Healthcare system analysis reveals that despite higher upfront costs (typically 30-50% premium over conventional implants), the total cost of care—including shorter recovery times, reduced complication rates, and fewer revision surgeries—demonstrates a positive return on investment within 3-5 years. This favorable economic profile has begun attracting increased insurance coverage and reimbursement policies specifically for porous SMA implants in several markets.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!