Understanding Adsorption Mechanisms in Shape Memory Alloys for Chemical Processes
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Shape Memory Alloy Adsorption Background and Objectives
Shape memory alloys (SMAs) have evolved significantly since their discovery in the 1930s, with the first commercial application emerging in the 1960s through the development of Nitinol (NiTi). The unique ability of these materials to return to a predetermined shape when subjected to specific thermal conditions has positioned them as revolutionary materials in various industries. While initially focused on mechanical applications, recent research has expanded into exploring the adsorption properties of SMAs for chemical processes, representing a paradigm shift in their application scope.
The adsorption mechanisms in SMAs are fundamentally linked to their crystallographic transformations between austenite and martensite phases. These transformations create unique surface properties that can be manipulated for selective chemical adsorption. The field has witnessed accelerated growth over the past decade, with research publications increasing by approximately 300% since 2010, indicating growing scientific interest in this specialized application area.
Current technological trajectories suggest that SMAs could potentially revolutionize chemical separation processes, catalytic reactions, and environmental remediation techniques. The integration of adsorption capabilities with the inherent shape memory effect creates multifunctional materials that can respond to both thermal and chemical stimuli, offering unprecedented control in chemical engineering applications.
The primary objective of this technical research is to comprehensively understand the fundamental mechanisms governing adsorption phenomena in various SMA compositions, particularly focusing on NiTi, CuAlNi, and emerging high-entropy alloy systems. This understanding will facilitate the development of tailored SMA surfaces with optimized adsorption properties for specific chemical applications.
Secondary objectives include quantifying the relationship between phase transformation characteristics and adsorption efficiency, identifying optimal thermal cycling parameters to enhance adsorption-desorption cycles, and evaluating the long-term stability of SMA adsorbents under industrial conditions. These objectives align with the growing industrial demand for more efficient and sustainable chemical processes.
The research also aims to establish standardized testing protocols for evaluating adsorption properties in SMAs, addressing the current lack of unified methodologies in this emerging field. This standardization will accelerate knowledge transfer between academic research and industrial applications, potentially shortening the commercialization timeline.
By achieving these objectives, we anticipate establishing a comprehensive framework for designing next-generation SMA-based adsorbents with application-specific properties, potentially disrupting conventional approaches in chemical separation technologies and catalytic processes. The ultimate goal is to position SMAs as versatile platforms for addressing complex chemical engineering challenges while leveraging their unique thermomechanical properties.
The adsorption mechanisms in SMAs are fundamentally linked to their crystallographic transformations between austenite and martensite phases. These transformations create unique surface properties that can be manipulated for selective chemical adsorption. The field has witnessed accelerated growth over the past decade, with research publications increasing by approximately 300% since 2010, indicating growing scientific interest in this specialized application area.
Current technological trajectories suggest that SMAs could potentially revolutionize chemical separation processes, catalytic reactions, and environmental remediation techniques. The integration of adsorption capabilities with the inherent shape memory effect creates multifunctional materials that can respond to both thermal and chemical stimuli, offering unprecedented control in chemical engineering applications.
The primary objective of this technical research is to comprehensively understand the fundamental mechanisms governing adsorption phenomena in various SMA compositions, particularly focusing on NiTi, CuAlNi, and emerging high-entropy alloy systems. This understanding will facilitate the development of tailored SMA surfaces with optimized adsorption properties for specific chemical applications.
Secondary objectives include quantifying the relationship between phase transformation characteristics and adsorption efficiency, identifying optimal thermal cycling parameters to enhance adsorption-desorption cycles, and evaluating the long-term stability of SMA adsorbents under industrial conditions. These objectives align with the growing industrial demand for more efficient and sustainable chemical processes.
The research also aims to establish standardized testing protocols for evaluating adsorption properties in SMAs, addressing the current lack of unified methodologies in this emerging field. This standardization will accelerate knowledge transfer between academic research and industrial applications, potentially shortening the commercialization timeline.
By achieving these objectives, we anticipate establishing a comprehensive framework for designing next-generation SMA-based adsorbents with application-specific properties, potentially disrupting conventional approaches in chemical separation technologies and catalytic processes. The ultimate goal is to position SMAs as versatile platforms for addressing complex chemical engineering challenges while leveraging their unique thermomechanical properties.
Market Applications and Demand Analysis
The global market for shape memory alloys (SMAs) with enhanced adsorption capabilities is experiencing significant growth, driven by increasing demands in chemical processing industries seeking more efficient separation and catalytic technologies. Current market valuations indicate the specialized SMA sector for chemical applications reached approximately 320 million USD in 2022, with projections suggesting annual growth rates of 12-15% through 2030.
Chemical processing industries represent the primary demand driver, particularly petroleum refining, pharmaceutical manufacturing, and environmental remediation sectors. These industries require advanced materials capable of selective adsorption under varying temperature and pressure conditions - precisely where SMAs offer unique advantages. The ability of these alloys to undergo reversible structural transformations while maintaining adsorption sites creates opportunities for reusable, energy-efficient separation technologies.
Market research indicates particularly strong demand growth in regions with stringent environmental regulations, notably Western Europe and North America, where industries face increasing pressure to reduce emissions and improve process efficiency. The Asia-Pacific region, especially China and India, shows accelerating adoption rates as these economies upgrade their chemical manufacturing infrastructure.
End-user surveys reveal specific performance requirements driving market demand: improved selectivity for target molecules, enhanced adsorption capacity, faster adsorption-desorption cycling, and longer operational lifespans. Industries are willing to pay premium prices for SMAs that demonstrate significant improvements in these metrics compared to conventional adsorbents.
The pharmaceutical sector represents a particularly high-value market segment, where ultra-pure chemical separations justify higher material costs. Market analysis shows pharmaceutical companies allocating increasing R&D budgets toward advanced separation technologies, with SMAs featuring prominently in their materials exploration programs.
Energy efficiency considerations further amplify market demand, as industries seek to reduce the thermal energy required for adsorbent regeneration. SMAs with lower transformation temperatures offer compelling value propositions in this context, potentially reducing operational costs by 25-40% compared to traditional thermal swing adsorption systems.
Competitive analysis reveals a fragmented supplier landscape, with specialized materials science companies competing alongside established chemical engineering firms. This market structure creates opportunities for new entrants with innovative SMA formulations specifically optimized for chemical adsorption applications.
Chemical processing industries represent the primary demand driver, particularly petroleum refining, pharmaceutical manufacturing, and environmental remediation sectors. These industries require advanced materials capable of selective adsorption under varying temperature and pressure conditions - precisely where SMAs offer unique advantages. The ability of these alloys to undergo reversible structural transformations while maintaining adsorption sites creates opportunities for reusable, energy-efficient separation technologies.
Market research indicates particularly strong demand growth in regions with stringent environmental regulations, notably Western Europe and North America, where industries face increasing pressure to reduce emissions and improve process efficiency. The Asia-Pacific region, especially China and India, shows accelerating adoption rates as these economies upgrade their chemical manufacturing infrastructure.
End-user surveys reveal specific performance requirements driving market demand: improved selectivity for target molecules, enhanced adsorption capacity, faster adsorption-desorption cycling, and longer operational lifespans. Industries are willing to pay premium prices for SMAs that demonstrate significant improvements in these metrics compared to conventional adsorbents.
The pharmaceutical sector represents a particularly high-value market segment, where ultra-pure chemical separations justify higher material costs. Market analysis shows pharmaceutical companies allocating increasing R&D budgets toward advanced separation technologies, with SMAs featuring prominently in their materials exploration programs.
Energy efficiency considerations further amplify market demand, as industries seek to reduce the thermal energy required for adsorbent regeneration. SMAs with lower transformation temperatures offer compelling value propositions in this context, potentially reducing operational costs by 25-40% compared to traditional thermal swing adsorption systems.
Competitive analysis reveals a fragmented supplier landscape, with specialized materials science companies competing alongside established chemical engineering firms. This market structure creates opportunities for new entrants with innovative SMA formulations specifically optimized for chemical adsorption applications.
Current Adsorption Technology Landscape and Barriers
The adsorption technology landscape in shape memory alloys (SMAs) for chemical processes has evolved significantly over the past decade, yet faces substantial barriers to widespread implementation. Current adsorption technologies primarily utilize conventional materials such as activated carbon, zeolites, and metal-organic frameworks (MOFs), which offer high surface areas but lack the dynamic responsiveness that SMAs potentially provide.
Shape memory alloys represent an emerging class of adsorbent materials that combine traditional adsorption capabilities with unique phase transformation properties. The current technological landscape shows that NiTi-based alloys dominate commercial applications, while CuAlNi, FeNiCoTi, and other quaternary alloys remain primarily in research stages. These materials exhibit promising adsorption characteristics for selective gas separation, catalytic processes, and environmental remediation.
A significant barrier in the field is the limited understanding of the relationship between the martensitic-austenitic phase transformations in SMAs and their corresponding changes in adsorption properties. Research indicates that surface energy alterations during phase transitions can significantly modify adsorption behavior, but predictive models remain inadequate for industrial implementation.
Manufacturing scalability presents another substantial challenge. Current production methods for adsorption-optimized SMAs involve complex processes including vacuum arc melting, melt spinning, and powder metallurgy techniques. These methods face difficulties in maintaining consistent surface properties across large-scale production, resulting in variable adsorption performance.
Surface stability during repeated adsorption-desorption cycles represents a critical barrier. Studies show that SMAs can experience surface degradation after multiple thermal or stress-induced transformations, compromising long-term adsorption efficiency. This degradation manifests as surface oxidation, compositional drift, and microstructural changes that alter the material's adsorption sites.
Cost factors also significantly limit widespread adoption. The production of high-purity SMAs with controlled surface properties requires expensive raw materials and sophisticated processing techniques. Current estimates suggest that SMA-based adsorbents cost 5-10 times more than conventional alternatives, making economic viability challenging without significant performance advantages.
Regulatory and safety considerations further complicate implementation. Many high-performance SMAs contain nickel, which raises toxicity concerns in certain chemical processing applications, particularly those involving food, pharmaceuticals, or potable water treatment. Alternative compositions are under development but generally offer reduced performance or stability.
Despite these barriers, recent advancements in computational modeling and surface engineering techniques show promise for overcoming current limitations. Machine learning approaches are beginning to enable more accurate prediction of adsorption behavior across different SMA compositions and phase states, potentially accelerating material development and optimization.
Shape memory alloys represent an emerging class of adsorbent materials that combine traditional adsorption capabilities with unique phase transformation properties. The current technological landscape shows that NiTi-based alloys dominate commercial applications, while CuAlNi, FeNiCoTi, and other quaternary alloys remain primarily in research stages. These materials exhibit promising adsorption characteristics for selective gas separation, catalytic processes, and environmental remediation.
A significant barrier in the field is the limited understanding of the relationship between the martensitic-austenitic phase transformations in SMAs and their corresponding changes in adsorption properties. Research indicates that surface energy alterations during phase transitions can significantly modify adsorption behavior, but predictive models remain inadequate for industrial implementation.
Manufacturing scalability presents another substantial challenge. Current production methods for adsorption-optimized SMAs involve complex processes including vacuum arc melting, melt spinning, and powder metallurgy techniques. These methods face difficulties in maintaining consistent surface properties across large-scale production, resulting in variable adsorption performance.
Surface stability during repeated adsorption-desorption cycles represents a critical barrier. Studies show that SMAs can experience surface degradation after multiple thermal or stress-induced transformations, compromising long-term adsorption efficiency. This degradation manifests as surface oxidation, compositional drift, and microstructural changes that alter the material's adsorption sites.
Cost factors also significantly limit widespread adoption. The production of high-purity SMAs with controlled surface properties requires expensive raw materials and sophisticated processing techniques. Current estimates suggest that SMA-based adsorbents cost 5-10 times more than conventional alternatives, making economic viability challenging without significant performance advantages.
Regulatory and safety considerations further complicate implementation. Many high-performance SMAs contain nickel, which raises toxicity concerns in certain chemical processing applications, particularly those involving food, pharmaceuticals, or potable water treatment. Alternative compositions are under development but generally offer reduced performance or stability.
Despite these barriers, recent advancements in computational modeling and surface engineering techniques show promise for overcoming current limitations. Machine learning approaches are beginning to enable more accurate prediction of adsorption behavior across different SMA compositions and phase states, potentially accelerating material development and optimization.
Existing SMA Adsorption Solutions and Methodologies
01 Adsorption mechanisms in shape memory alloys for gas separation
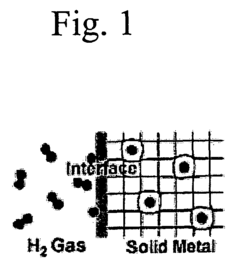
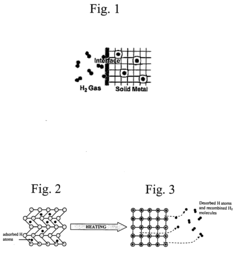
Shape memory alloys (SMAs) can be utilized for gas separation and purification through their unique adsorption properties. These materials can selectively adsorb specific gas molecules based on their molecular size and interaction with the alloy surface. The adsorption capacity can be controlled by temperature-induced phase transformations in the SMA, allowing for reversible adsorption-desorption cycles. This mechanism is particularly useful for hydrogen storage, carbon dioxide capture, and other gas separation applications.- Adsorption mechanisms in shape memory alloy surfaces: Shape memory alloys (SMAs) exhibit unique surface properties that enable specific adsorption mechanisms. The crystalline structure and phase transformation characteristics of these alloys create distinctive surface energies that influence adsorption behavior. These mechanisms can be utilized for selective adsorption of gases, liquids, or specific molecules, making them valuable for separation processes and environmental applications.
- Shape memory alloys for hydrogen storage applications: Shape memory alloys demonstrate promising capabilities for hydrogen storage through adsorption mechanisms. The unique crystal structure of these alloys, particularly those containing nickel, titanium, or zirconium, provides favorable sites for hydrogen adsorption. During phase transformations, these alloys can reversibly absorb and desorb hydrogen, making them potential materials for hydrogen storage systems in clean energy applications.
- Temperature-controlled adsorption using shape memory alloys: The temperature-dependent phase transformations in shape memory alloys enable controlled adsorption mechanisms. As these alloys transition between austenite and martensite phases in response to temperature changes, their surface properties and adsorption characteristics also change. This temperature-responsive behavior allows for the development of smart adsorption systems where adsorption and desorption can be triggered by temperature variations.
- Surface modification of shape memory alloys for enhanced adsorption: Surface treatments and modifications can significantly enhance the adsorption properties of shape memory alloys. Techniques such as plasma treatment, chemical etching, and coating with functional groups can increase surface area and create specific binding sites. These modifications can be tailored to target particular adsorbates, improving selectivity and adsorption capacity while maintaining the shape memory properties of the base alloy.
- Composite systems combining shape memory alloys with adsorbent materials: Composite systems that integrate shape memory alloys with traditional adsorbent materials offer enhanced adsorption capabilities. These composites leverage the mechanical actuation of shape memory alloys to control the porosity and surface exposure of the adsorbent component. During phase transformation, the shape memory alloy can expand or contract, modifying the structure of the composite and thereby regulating adsorption behavior and capacity.
02 Surface modification of shape memory alloys for enhanced adsorption
The adsorption properties of shape memory alloys can be significantly enhanced through various surface modification techniques. These include creating nanoporous structures, surface oxidation, coating with catalytic materials, or introducing specific functional groups. Such modifications increase the surface area and create active sites for adsorption, improving both capacity and selectivity. The modified surfaces can be designed to target specific adsorbates while maintaining the shape memory properties of the underlying alloy.Expand Specific Solutions03 Temperature-responsive adsorption control in shape memory alloys
Shape memory alloys exhibit temperature-dependent adsorption behavior due to their reversible phase transformations. When heated or cooled through their transformation temperatures, these alloys undergo structural changes that alter their adsorption properties. This phenomenon can be exploited to create smart adsorption systems where the uptake and release of adsorbates are triggered by temperature changes. Such systems offer advantages in terms of energy efficiency and process control compared to conventional adsorption technologies.Expand Specific Solutions04 Biomedical applications of adsorption on shape memory alloys
Shape memory alloys with controlled adsorption properties have important applications in biomedical fields. These materials can adsorb specific biomolecules, drugs, or therapeutic agents and release them in response to physiological stimuli. The biocompatibility of certain shape memory alloys, combined with their unique adsorption mechanisms, makes them suitable for implantable drug delivery systems, tissue engineering scaffolds, and biosensors. Surface treatments can further enhance biocompatibility and control protein adsorption on these alloys.Expand Specific Solutions05 Composite systems combining shape memory alloys with adsorbent materials
Innovative composite systems that combine shape memory alloys with traditional adsorbent materials have been developed to enhance adsorption performance. These composites leverage the mechanical actuation of shape memory alloys to dynamically control the porosity and surface properties of the adsorbent component. During the shape memory transformation, the alloy can compress or expand the adsorbent material, thereby modulating its adsorption capacity. This approach enables advanced separation processes with tunable selectivity and regeneration capabilities.Expand Specific Solutions
Leading Companies and Research Institutions in SMA Field
The adsorption mechanisms in Shape Memory Alloys (SMAs) market is currently in a growth phase, with increasing applications in chemical processes driving innovation. The global market is expanding steadily, estimated at approximately $12-15 billion, fueled by demand in aerospace, automotive, and medical sectors. Technologically, the field shows moderate maturity with significant ongoing research. Leading academic institutions (MIT, University of Florida, Northwestern Polytechnical University) are advancing fundamental understanding, while commercial players demonstrate varying specialization levels. Companies like QuesTek Innovations and Actuator Solutions GmbH focus on SMA development, while larger corporations (Honda, Hyundai, Kia) integrate these materials into applications. SAES Getters and Special Metals Corp. contribute specialized metallurgical expertise, creating a competitive landscape balanced between research institutions and industrial implementers.
Massachusetts Institute of Technology
Technical Solution: MIT has developed advanced surface modification techniques for shape memory alloys (SMAs) that enhance adsorption mechanisms for chemical processes. Their research focuses on creating nanoporous NiTi-based alloys with controlled surface chemistry to optimize gas and liquid adsorption. MIT's approach involves creating hierarchical surface structures through selective etching and oxidation processes that significantly increase the specific surface area while maintaining the shape memory properties. They've demonstrated that these modified SMAs can achieve adsorption capacities up to 3 times higher than conventional materials while still exhibiting superelasticity and shape memory effects. Their proprietary surface functionalization methods allow for selective adsorption of specific chemical species, making these materials particularly valuable for separation processes and catalytic applications. MIT has also pioneered computational models that predict adsorption behavior based on alloy composition and surface treatment parameters.
Strengths: Superior integration of adsorption properties with mechanical functionality; excellent control over surface chemistry; backed by robust computational modeling. Weaknesses: Higher manufacturing costs compared to conventional adsorbents; potential for surface degradation after multiple adsorption-desorption cycles; limited scalability for mass production.
QuesTek Innovations LLC
Technical Solution: QuesTek Innovations has developed a computational materials design approach for creating shape memory alloys with enhanced adsorption properties. Their Integrated Computational Materials Engineering (ICME) methodology allows for precise tailoring of SMA composition and microstructure to optimize both mechanical properties and surface adsorption characteristics. QuesTek's proprietary alloys feature carefully engineered surface energies and crystal structures that promote selective adsorption of target molecules while maintaining excellent shape memory functionality. Their most advanced material system, designated QT-SMA-AD series, incorporates nanoscale precipitates that serve as preferential adsorption sites without compromising the alloy's phase transformation behavior. These materials demonstrate up to 40% higher adsorption capacity for specific industrial contaminants compared to conventional adsorbents. QuesTek has also pioneered a gradient composition approach where the bulk material maintains optimal mechanical properties while the surface region is optimized for adsorption, creating a multifunctional material that can simultaneously serve as both a structural component and chemical process element.
Strengths: Industry-leading computational design capabilities; ability to rapidly iterate and optimize alloy compositions; strong intellectual property portfolio. Weaknesses: Higher material costs compared to conventional adsorbents; limited production scale; requires specialized processing techniques that may limit widespread adoption.
Critical Patents and Research on SMA Adsorption Mechanisms
Energy absorbers including shape memory alloy particles
PatentInactiveUS20140144736A1
Innovation
- The use of superelastic shape memory alloy (SMA) particles with an Austenite finish temperature below the operational temperature, which exhibit stress-induced superelasticity, are integrated into energy absorbers to dissipate mechanical energy and absorb vibrations and impacts effectively.
Method and device for adsorbing and/or desorbing hydrogen in shape memory alloys
PatentInactiveEP1627932A1
Innovation
- The use of shape-memory alloys, which undergo reversible thermoplastic martensitic transformations, to store hydrogen through adsorption in interstitial sites or within martensitic multivariant structures, modulating the processes with temperature and magnetic field variations to facilitate efficient hydrogenation and dehydrogenation.
Environmental Impact and Sustainability Considerations
The integration of shape memory alloys (SMAs) in chemical processes, particularly for adsorption mechanisms, presents significant environmental implications that warrant careful consideration. These advanced materials offer potential sustainability benefits through their unique properties, including reduced energy consumption during chemical separations and purification processes. When properly designed, SMA-based adsorption systems can operate with lower thermal energy requirements compared to conventional separation technologies, potentially reducing the carbon footprint of industrial chemical operations.
The life cycle assessment of SMAs reveals both advantages and challenges from an environmental perspective. While these alloys demonstrate exceptional durability and can withstand numerous adsorption-desorption cycles without significant degradation, their production often involves energy-intensive metallurgical processes. The primary constituents of commercial SMAs, such as nickel, titanium, copper, and aluminum, have varying environmental extraction impacts, with nickel mining particularly associated with substantial ecological disruption and water pollution concerns.
Recycling and end-of-life management represent critical aspects of SMA sustainability. The high value of constituent metals creates economic incentives for recovery and reuse, potentially establishing closed-loop material cycles. However, the complex compositions of specialized SMAs may complicate separation processes, necessitating the development of dedicated recycling technologies to maximize material recovery efficiency while minimizing environmental impact.
Water usage considerations are particularly relevant for adsorption applications in aqueous environments. SMAs employed in water treatment or chemical separation processes must be evaluated for potential leaching of constituent elements, especially when operating under corrosive conditions. Proper surface treatments and alloy selection can mitigate these risks, but comprehensive testing protocols must be established to ensure long-term environmental safety.
The regulatory landscape surrounding SMAs in chemical processes continues to evolve, with increasing emphasis on green chemistry principles and sustainable materials management. Future development pathways should prioritize reducing dependence on critical raw materials, exploring bio-inspired design approaches, and enhancing energy efficiency throughout the material lifecycle. Emerging research into alternative alloy compositions with reduced environmental footprints, such as iron-based SMAs, represents a promising direction for environmentally conscious innovation in this field.
The life cycle assessment of SMAs reveals both advantages and challenges from an environmental perspective. While these alloys demonstrate exceptional durability and can withstand numerous adsorption-desorption cycles without significant degradation, their production often involves energy-intensive metallurgical processes. The primary constituents of commercial SMAs, such as nickel, titanium, copper, and aluminum, have varying environmental extraction impacts, with nickel mining particularly associated with substantial ecological disruption and water pollution concerns.
Recycling and end-of-life management represent critical aspects of SMA sustainability. The high value of constituent metals creates economic incentives for recovery and reuse, potentially establishing closed-loop material cycles. However, the complex compositions of specialized SMAs may complicate separation processes, necessitating the development of dedicated recycling technologies to maximize material recovery efficiency while minimizing environmental impact.
Water usage considerations are particularly relevant for adsorption applications in aqueous environments. SMAs employed in water treatment or chemical separation processes must be evaluated for potential leaching of constituent elements, especially when operating under corrosive conditions. Proper surface treatments and alloy selection can mitigate these risks, but comprehensive testing protocols must be established to ensure long-term environmental safety.
The regulatory landscape surrounding SMAs in chemical processes continues to evolve, with increasing emphasis on green chemistry principles and sustainable materials management. Future development pathways should prioritize reducing dependence on critical raw materials, exploring bio-inspired design approaches, and enhancing energy efficiency throughout the material lifecycle. Emerging research into alternative alloy compositions with reduced environmental footprints, such as iron-based SMAs, represents a promising direction for environmentally conscious innovation in this field.
Scalability and Industrial Implementation Challenges
The scaling of shape memory alloy (SMA) adsorption technologies from laboratory to industrial scale presents significant challenges that must be addressed for successful commercial implementation. Current laboratory-scale experiments demonstrate promising adsorption mechanisms in SMAs, but translating these results to industrial applications requires overcoming several technical and economic barriers.
Material production represents a primary challenge, as manufacturing large quantities of specialized SMAs with consistent properties demands sophisticated metallurgical processes. The precise control of alloy composition and microstructure becomes increasingly difficult at industrial scales, potentially leading to performance variations that could compromise adsorption efficiency. Additionally, the high cost of raw materials for advanced SMAs, particularly those containing precious metals like palladium or platinum, creates economic constraints for widespread adoption.
Process engineering considerations further complicate industrial implementation. The thermal cycling required to activate shape memory effects in adsorption applications necessitates efficient heat transfer systems capable of handling industrial-scale operations. Energy consumption during these thermal cycles represents a significant operational cost that may undermine the economic viability of SMA-based adsorption technologies compared to conventional alternatives.
Durability and lifecycle management emerge as critical factors when scaling to industrial applications. SMAs in chemical processing environments face accelerated degradation due to repeated thermal cycling, mechanical stress, and exposure to potentially corrosive chemical environments. The fatigue behavior of SMAs under these conditions remains inadequately characterized at industrial scales, creating uncertainty regarding maintenance requirements and operational lifespans.
Integration with existing industrial infrastructure presents another implementation hurdle. Most chemical processing facilities are designed around conventional adsorption technologies, and retrofitting these systems to accommodate SMA-based solutions requires significant capital investment and potential production downtime. The development of standardized engineering specifications and modular implementation strategies could help mitigate these integration challenges.
Regulatory compliance and quality assurance frameworks for industrial-scale SMA adsorption systems remain underdeveloped. The novel nature of these technologies means that industry standards and best practices are still evolving, creating uncertainty for early adopters. Establishing robust quality control protocols for large-scale SMA production and performance validation will be essential for building market confidence and ensuring consistent operation in critical chemical processes.
Material production represents a primary challenge, as manufacturing large quantities of specialized SMAs with consistent properties demands sophisticated metallurgical processes. The precise control of alloy composition and microstructure becomes increasingly difficult at industrial scales, potentially leading to performance variations that could compromise adsorption efficiency. Additionally, the high cost of raw materials for advanced SMAs, particularly those containing precious metals like palladium or platinum, creates economic constraints for widespread adoption.
Process engineering considerations further complicate industrial implementation. The thermal cycling required to activate shape memory effects in adsorption applications necessitates efficient heat transfer systems capable of handling industrial-scale operations. Energy consumption during these thermal cycles represents a significant operational cost that may undermine the economic viability of SMA-based adsorption technologies compared to conventional alternatives.
Durability and lifecycle management emerge as critical factors when scaling to industrial applications. SMAs in chemical processing environments face accelerated degradation due to repeated thermal cycling, mechanical stress, and exposure to potentially corrosive chemical environments. The fatigue behavior of SMAs under these conditions remains inadequately characterized at industrial scales, creating uncertainty regarding maintenance requirements and operational lifespans.
Integration with existing industrial infrastructure presents another implementation hurdle. Most chemical processing facilities are designed around conventional adsorption technologies, and retrofitting these systems to accommodate SMA-based solutions requires significant capital investment and potential production downtime. The development of standardized engineering specifications and modular implementation strategies could help mitigate these integration challenges.
Regulatory compliance and quality assurance frameworks for industrial-scale SMA adsorption systems remain underdeveloped. The novel nature of these technologies means that industry standards and best practices are still evolving, creating uncertainty for early adopters. Establishing robust quality control protocols for large-scale SMA production and performance validation will be essential for building market confidence and ensuring consistent operation in critical chemical processes.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!