Protocol optimization for rapid maturation of iPSC-derived cardiomyocytes within microfluidic chips
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
iPSC-CM Maturation Background and Objectives
Induced pluripotent stem cell-derived cardiomyocytes (iPSC-CMs) have revolutionized cardiac research since their introduction in 2006, offering unprecedented opportunities for disease modeling, drug screening, and regenerative medicine. However, a persistent challenge in this field has been the immature phenotype of these cells compared to adult cardiomyocytes, limiting their translational value. Conventional maturation methods typically require 90-120 days of culture, representing a significant bottleneck in research and development pipelines.
The evolution of iPSC-CM technology has progressed through several key phases. Initially, the focus was on differentiation efficiency, moving from embryoid body-based methods to monolayer protocols with defined factors. As differentiation protocols became more reliable, attention shifted to the critical issue of maturation. Early approaches relied on extended time in culture, while more recent strategies have incorporated electrical stimulation, mechanical stretching, and three-dimensional culture systems to accelerate maturation.
Current research trends indicate a convergence of multiple maturation-enhancing factors, with microfluidic technology emerging as a particularly promising platform. Microfluidic chips offer precise control over the cellular microenvironment, including fluid flow, nutrient delivery, and mechanical forces - all critical factors in cardiomyocyte development. The integration of these systems with advanced biomaterials and tissue engineering approaches represents the cutting edge of the field.
The primary technical objective of this research is to develop an optimized protocol that significantly reduces the time required for iPSC-CM maturation within microfluidic chip systems, targeting a maturation period of 14-21 days while achieving physiological characteristics comparable to those observed in 90+ day conventional cultures. This includes appropriate sarcomeric organization, mitochondrial density, calcium handling properties, and electrophysiological responses.
Secondary objectives include establishing quantifiable metrics for assessing maturation status, identifying the minimum combination of factors necessary for accelerated maturation, and ensuring protocol reproducibility across different iPSC lines. Additionally, the protocol should be scalable and compatible with existing high-throughput screening platforms to facilitate industrial applications.
The successful development of rapid maturation protocols would address a critical need in cardiac research, drug development, and personalized medicine. By reducing the time and resources required to generate mature cardiac tissues, such protocols would accelerate drug screening processes, enable more efficient disease modeling, and potentially advance cardiac tissue engineering for therapeutic applications. The ultimate goal is to establish a standardized, efficient system that bridges the gap between in vitro models and native cardiac physiology.
The evolution of iPSC-CM technology has progressed through several key phases. Initially, the focus was on differentiation efficiency, moving from embryoid body-based methods to monolayer protocols with defined factors. As differentiation protocols became more reliable, attention shifted to the critical issue of maturation. Early approaches relied on extended time in culture, while more recent strategies have incorporated electrical stimulation, mechanical stretching, and three-dimensional culture systems to accelerate maturation.
Current research trends indicate a convergence of multiple maturation-enhancing factors, with microfluidic technology emerging as a particularly promising platform. Microfluidic chips offer precise control over the cellular microenvironment, including fluid flow, nutrient delivery, and mechanical forces - all critical factors in cardiomyocyte development. The integration of these systems with advanced biomaterials and tissue engineering approaches represents the cutting edge of the field.
The primary technical objective of this research is to develop an optimized protocol that significantly reduces the time required for iPSC-CM maturation within microfluidic chip systems, targeting a maturation period of 14-21 days while achieving physiological characteristics comparable to those observed in 90+ day conventional cultures. This includes appropriate sarcomeric organization, mitochondrial density, calcium handling properties, and electrophysiological responses.
Secondary objectives include establishing quantifiable metrics for assessing maturation status, identifying the minimum combination of factors necessary for accelerated maturation, and ensuring protocol reproducibility across different iPSC lines. Additionally, the protocol should be scalable and compatible with existing high-throughput screening platforms to facilitate industrial applications.
The successful development of rapid maturation protocols would address a critical need in cardiac research, drug development, and personalized medicine. By reducing the time and resources required to generate mature cardiac tissues, such protocols would accelerate drug screening processes, enable more efficient disease modeling, and potentially advance cardiac tissue engineering for therapeutic applications. The ultimate goal is to establish a standardized, efficient system that bridges the gap between in vitro models and native cardiac physiology.
Market Analysis for iPSC-CM Technologies
The global market for iPSC-derived cardiomyocytes (iPSC-CMs) has experienced significant growth in recent years, driven by increasing demand for more physiologically relevant cardiac models in drug discovery, toxicity testing, and regenerative medicine. The market size for iPSC-CM technologies was valued at approximately $1.2 billion in 2022 and is projected to grow at a compound annual growth rate (CAGR) of 12.5% through 2028.
Pharmaceutical and biotechnology companies represent the largest market segment, accounting for nearly 60% of the total market share. These companies utilize iPSC-CMs primarily for cardiotoxicity screening and drug efficacy testing, which has become increasingly important following several high-profile drug withdrawals due to unforeseen cardiac side effects.
Academic research institutions constitute the second-largest market segment at 25%, focusing on basic cardiac biology research and disease modeling. The remaining market share is divided among contract research organizations (CROs) and regenerative medicine companies developing cell-based therapies for cardiac diseases.
Geographically, North America dominates the market with approximately 45% share, followed by Europe (30%) and Asia-Pacific (20%). The Asia-Pacific region, particularly China, Japan, and South Korea, is expected to witness the fastest growth due to increasing investments in stem cell research and favorable regulatory environments.
The microfluidic chip segment within the iPSC-CM market is experiencing particularly rapid growth, with a CAGR of 15.8%. This acceleration is attributed to the advantages microfluidic systems offer in creating more physiologically relevant microenvironments and enabling high-throughput screening capabilities.
Key market drivers include the rising prevalence of cardiovascular diseases globally, increasing R&D investments in drug discovery, growing adoption of personalized medicine approaches, and technological advancements in iPSC generation and differentiation protocols. The push for alternatives to animal testing in preclinical studies has also significantly boosted market demand.
Major challenges limiting market expansion include high production costs, technical difficulties in achieving fully mature cardiomyocyte phenotypes, standardization issues, and regulatory uncertainties surrounding iPSC-derived products. The average cost of producing clinical-grade iPSC-CMs remains high at $8,000-$15,000 per million cells, creating a significant barrier to widespread adoption.
Recent market trends indicate growing interest in organ-on-chip technologies that incorporate iPSC-CMs, increasing focus on automation and scalability of production processes, and rising demand for patient-specific iPSC-CMs for precision medicine applications. The development of protocols for rapid maturation of iPSC-CMs within microfluidic chips directly addresses current market needs and could potentially unlock significant commercial opportunities in drug discovery and personalized medicine sectors.
Pharmaceutical and biotechnology companies represent the largest market segment, accounting for nearly 60% of the total market share. These companies utilize iPSC-CMs primarily for cardiotoxicity screening and drug efficacy testing, which has become increasingly important following several high-profile drug withdrawals due to unforeseen cardiac side effects.
Academic research institutions constitute the second-largest market segment at 25%, focusing on basic cardiac biology research and disease modeling. The remaining market share is divided among contract research organizations (CROs) and regenerative medicine companies developing cell-based therapies for cardiac diseases.
Geographically, North America dominates the market with approximately 45% share, followed by Europe (30%) and Asia-Pacific (20%). The Asia-Pacific region, particularly China, Japan, and South Korea, is expected to witness the fastest growth due to increasing investments in stem cell research and favorable regulatory environments.
The microfluidic chip segment within the iPSC-CM market is experiencing particularly rapid growth, with a CAGR of 15.8%. This acceleration is attributed to the advantages microfluidic systems offer in creating more physiologically relevant microenvironments and enabling high-throughput screening capabilities.
Key market drivers include the rising prevalence of cardiovascular diseases globally, increasing R&D investments in drug discovery, growing adoption of personalized medicine approaches, and technological advancements in iPSC generation and differentiation protocols. The push for alternatives to animal testing in preclinical studies has also significantly boosted market demand.
Major challenges limiting market expansion include high production costs, technical difficulties in achieving fully mature cardiomyocyte phenotypes, standardization issues, and regulatory uncertainties surrounding iPSC-derived products. The average cost of producing clinical-grade iPSC-CMs remains high at $8,000-$15,000 per million cells, creating a significant barrier to widespread adoption.
Recent market trends indicate growing interest in organ-on-chip technologies that incorporate iPSC-CMs, increasing focus on automation and scalability of production processes, and rising demand for patient-specific iPSC-CMs for precision medicine applications. The development of protocols for rapid maturation of iPSC-CMs within microfluidic chips directly addresses current market needs and could potentially unlock significant commercial opportunities in drug discovery and personalized medicine sectors.
Current Challenges in iPSC-CM Maturation
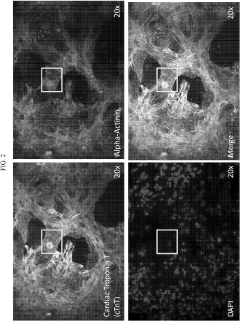
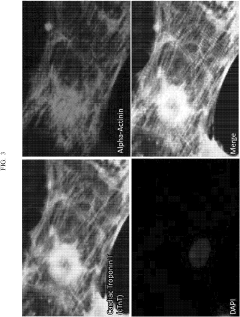
Despite significant advancements in induced pluripotent stem cell-derived cardiomyocyte (iPSC-CM) technology, the maturation of these cells remains a critical bottleneck in cardiac tissue engineering. Current iPSC-CMs typically exhibit immature phenotypes characterized by disorganized sarcomeres, underdeveloped T-tubule networks, and fetal-like electrophysiological properties. These immature characteristics significantly limit their application in disease modeling, drug screening, and regenerative medicine.
The traditional static culture methods fail to recapitulate the dynamic mechanical and electrical stimuli present in the native cardiac microenvironment. Consequently, iPSC-CMs cultured under standard conditions lack proper calcium handling properties and demonstrate reduced contractile force compared to adult cardiomyocytes. Additionally, their metabolic profile remains predominantly glycolytic rather than shifting to fatty acid oxidation, which is characteristic of mature cardiac cells.
Microfluidic platforms offer promising solutions but face several technical challenges. The optimization of flow rates within these systems is particularly problematic, as excessive shear stress can damage cells while insufficient perfusion fails to provide adequate maturation signals. Furthermore, the establishment of appropriate oxygen gradients within microfluidic chips remains difficult to control and monitor in real-time.
Current maturation protocols are also hampered by their extended timeframes, often requiring 4-6 weeks or longer. This prolonged duration increases experimental costs, raises contamination risks, and delays research outcomes. The variability between cell lines and differentiation batches further complicates protocol standardization, resulting in inconsistent maturation outcomes across experiments.
The integration of electrical stimulation within microfluidic systems presents additional technical hurdles. Electrode materials must be biocompatible while maintaining conductivity over extended culture periods. Moreover, determining optimal stimulation parameters (frequency, amplitude, and waveform) for different developmental stages remains largely empirical rather than evidence-based.
Another significant challenge is the lack of standardized assessment metrics for cardiomyocyte maturation. Researchers employ various parameters—structural, functional, and molecular—making cross-study comparisons difficult. This absence of consensus impedes the systematic optimization of maturation protocols and slows progress in the field.
The scalability of microfluidic approaches also presents a substantial obstacle. While these systems excel at providing controlled microenvironments, their limited throughput restricts applications requiring large cell numbers. Additionally, the complex fabrication processes and specialized expertise required for microfluidic chip design and operation create barriers to widespread adoption in standard laboratory settings.
The traditional static culture methods fail to recapitulate the dynamic mechanical and electrical stimuli present in the native cardiac microenvironment. Consequently, iPSC-CMs cultured under standard conditions lack proper calcium handling properties and demonstrate reduced contractile force compared to adult cardiomyocytes. Additionally, their metabolic profile remains predominantly glycolytic rather than shifting to fatty acid oxidation, which is characteristic of mature cardiac cells.
Microfluidic platforms offer promising solutions but face several technical challenges. The optimization of flow rates within these systems is particularly problematic, as excessive shear stress can damage cells while insufficient perfusion fails to provide adequate maturation signals. Furthermore, the establishment of appropriate oxygen gradients within microfluidic chips remains difficult to control and monitor in real-time.
Current maturation protocols are also hampered by their extended timeframes, often requiring 4-6 weeks or longer. This prolonged duration increases experimental costs, raises contamination risks, and delays research outcomes. The variability between cell lines and differentiation batches further complicates protocol standardization, resulting in inconsistent maturation outcomes across experiments.
The integration of electrical stimulation within microfluidic systems presents additional technical hurdles. Electrode materials must be biocompatible while maintaining conductivity over extended culture periods. Moreover, determining optimal stimulation parameters (frequency, amplitude, and waveform) for different developmental stages remains largely empirical rather than evidence-based.
Another significant challenge is the lack of standardized assessment metrics for cardiomyocyte maturation. Researchers employ various parameters—structural, functional, and molecular—making cross-study comparisons difficult. This absence of consensus impedes the systematic optimization of maturation protocols and slows progress in the field.
The scalability of microfluidic approaches also presents a substantial obstacle. While these systems excel at providing controlled microenvironments, their limited throughput restricts applications requiring large cell numbers. Additionally, the complex fabrication processes and specialized expertise required for microfluidic chip design and operation create barriers to widespread adoption in standard laboratory settings.
Current Microfluidic Approaches for CM Maturation
01 Biochemical factors for iPSC-derived cardiomyocyte maturation
Various biochemical factors can be used to enhance the maturation of iPSC-derived cardiomyocytes. These include growth factors, hormones, and small molecules that regulate key signaling pathways involved in cardiac development. The addition of specific factors such as triiodothyronine (T3), insulin-like growth factor-1 (IGF-1), and dexamethasone to culture media can promote structural and functional maturation of cardiomyocytes, leading to improved contractile properties and metabolic profiles that more closely resemble adult cardiomyocytes.- Maturation factors and signaling pathways: Various factors and signaling pathways can be manipulated to enhance the maturation of iPSC-derived cardiomyocytes. These include growth factors, hormones, and small molecules that activate specific cellular pathways involved in cardiac development and maturation. By modulating these pathways, researchers can promote the structural and functional maturation of cardiomyocytes to more closely resemble adult cardiac cells.
- Electrical and mechanical stimulation techniques: Applying electrical and mechanical stimulation to iPSC-derived cardiomyocytes can significantly enhance their maturation. Electrical pacing mimics the natural electrical activity of the heart, while mechanical stress simulates the physical forces experienced by cardiac cells in vivo. These stimulation techniques promote the development of organized sarcomeres, improved calcium handling, and enhanced contractile properties in the cardiomyocytes.
- 3D culture systems and tissue engineering approaches: Three-dimensional culture systems and tissue engineering approaches provide a more physiologically relevant environment for iPSC-derived cardiomyocyte maturation. These include the use of biomaterials, scaffolds, and organoid technologies that better mimic the native cardiac microenvironment. Such systems allow for cell-cell and cell-matrix interactions that are crucial for proper cardiomyocyte development and maturation.
- Metabolic conditioning and substrate utilization: Manipulating the metabolic environment and substrate availability can drive iPSC-derived cardiomyocytes toward a more mature phenotype. Adult cardiomyocytes primarily rely on fatty acid oxidation rather than glycolysis for energy production. By shifting the metabolic profile of iPSC-derived cardiomyocytes through specific culture conditions and supplements, researchers can promote a more adult-like metabolic state that contributes to overall cellular maturation.
- Co-culture with supporting cell types: Co-culturing iPSC-derived cardiomyocytes with other cardiac or supporting cell types can enhance their maturation. Cardiac fibroblasts, endothelial cells, and other non-myocytes provide paracrine factors and establish intercellular connections that promote cardiomyocyte development. These heterotypic cellular interactions better recapitulate the complex cellular composition of the native heart tissue and facilitate more complete cardiomyocyte maturation.
02 Mechanical and electrical stimulation techniques
Mechanical and electrical stimulation methods can significantly enhance the maturation of iPSC-derived cardiomyocytes. These techniques mimic the physiological environment of the heart by providing cyclic stretch, electrical pacing, or both. Such stimulation promotes the alignment of sarcomeres, increases the expression of ion channels, and improves calcium handling properties. These approaches result in cardiomyocytes with enhanced contractile function, more mature electrophysiological properties, and improved response to pharmacological agents.Expand Specific Solutions03 3D culture systems and tissue engineering approaches
Three-dimensional culture systems and tissue engineering approaches provide a more physiologically relevant environment for iPSC-derived cardiomyocyte maturation. These include the use of hydrogels, scaffolds, and organoid systems that allow for cell-cell and cell-matrix interactions similar to those in native cardiac tissue. Such 3D environments promote the development of more mature structural organization, improved contractile function, and enhanced electrophysiological properties compared to traditional 2D culture systems.Expand Specific Solutions04 Co-culture with supporting cell types
Co-culturing iPSC-derived cardiomyocytes with supporting cell types such as cardiac fibroblasts, endothelial cells, or stromal cells can enhance their maturation. These supporting cells provide paracrine factors, extracellular matrix components, and direct cell-cell interactions that promote cardiomyocyte development. This approach results in improved structural organization, enhanced contractile function, and more mature electrophysiological and metabolic profiles of the cardiomyocytes.Expand Specific Solutions05 Metabolic conditioning for enhanced maturation
Metabolic conditioning strategies can be employed to enhance the maturation of iPSC-derived cardiomyocytes. These approaches include modulating substrate availability, oxygen tension, and metabolic pathway activity to shift cardiomyocyte metabolism from glycolysis toward fatty acid oxidation, which is characteristic of adult cardiomyocytes. Techniques such as glucose restriction, fatty acid supplementation, and hypoxia-normoxia cycling can promote more mature metabolic profiles and improve functional properties of iPSC-derived cardiomyocytes.Expand Specific Solutions
Leading Research Groups and Companies
The iPSC-derived cardiomyocyte maturation in microfluidic chips market is in an early growth phase, characterized by intensive research and emerging commercial applications. The global market for organ-on-chip technologies is projected to reach $220 million by 2025, with cardiac models representing a significant segment. Academic institutions dominate this landscape, with Harvard University, Zhejiang University, and Columbia University leading fundamental research. Commercial entities like Emulate, FUJIFILM Cellular Dynamics, and Agilent Technologies are advancing the technology toward standardization. Healthcare organizations including Cedars-Sinai and University Health Network are bridging research-to-clinical application gaps. The technology is approaching early commercial maturity, with companies developing proprietary protocols that enhance cardiomyocyte functionality and physiological relevance for drug discovery and personalized medicine applications.
President & Fellows of Harvard College
Technical Solution: Harvard researchers have developed sophisticated protocols for accelerating iPSC-derived cardiomyocyte maturation in microfluidic environments. Their approach integrates advanced biomaterials engineering with precisely controlled mechanical and electrical stimulation. The Harvard protocol utilizes a staged differentiation process beginning with monolayer culture and small molecule-mediated cardiac induction, followed by transfer to microfluidic chips with engineered substrate stiffness gradients that mimic developmental mechanical cues. Their system incorporates programmable electrical stimulation patterns that evolve over the maturation period, beginning with low frequency stimulation (0.5 Hz) and progressively increasing to physiological rates (1-2 Hz). A key innovation in their approach is the incorporation of anisotropic topographical cues within the microfluidic channels that guide cellular alignment and promote the formation of organized sarcomeric structures. The protocol also features precisely timed introduction of T3 thyroid hormone, fatty acids, and other metabolic modulators that shift cardiomyocyte metabolism from glycolysis toward fatty acid oxidation, a hallmark of mature cardiac tissue. Harvard's system allows for continuous non-invasive monitoring of cardiomyocyte function through integrated sensors that measure contractile force, calcium transients, and action potential characteristics.
Strengths: Comprehensive integration of multiple maturation factors (mechanical, electrical, biochemical); sophisticated engineering approaches for creating physiologically relevant microenvironments; extensive validation using functional and molecular maturation markers. Weaknesses: Complex technical implementation may limit accessibility to specialized laboratories; higher technical expertise required for successful implementation; may require specialized equipment not widely available.
The Children's Medical Center Corp.
Technical Solution: The Children's Medical Center Corporation (Boston Children's Hospital) has developed innovative protocols for enhancing iPSC-derived cardiomyocyte maturation in microfluidic systems. Their approach focuses on recreating developmental cues within controlled microenvironments to accelerate functional maturation. The protocol begins with optimized cardiac differentiation using modulation of Wnt signaling pathways, followed by transfer of early-stage cardiomyocytes to custom microfluidic devices. Within these devices, cells experience precisely controlled hemodynamic forces that mimic developmental fluid dynamics. Their system incorporates pulsatile flow patterns that gradually increase in intensity to simulate the developing cardiovascular system. A distinguishing feature of their approach is the incorporation of endothelial cells in co-culture configurations that enable paracrine signaling between cell types, enhancing cardiomyocyte maturation through physiologically relevant cell-cell interactions. The protocol includes precise temporal control of oxygen tension gradients that transition from hypoxic conditions mimicking early development to normoxic conditions representing mature tissue environments. Their microfluidic platform also enables the controlled delivery of maturation factors including triiodothyronine (T3), glucocorticoids, and fatty acids in physiologically relevant sequences and concentrations.
Strengths: Strong focus on developmental biology principles to guide protocol design; sophisticated co-culture capabilities that better represent in vivo cellular interactions; extensive validation using patient-derived iPSCs for disease modeling applications. Weaknesses: Protocols optimized primarily for pediatric applications may require adaptation for adult cardiomyocyte phenotypes; complex co-culture systems increase variability; higher technical complexity in implementation.
Key Innovations in Rapid Maturation Protocols
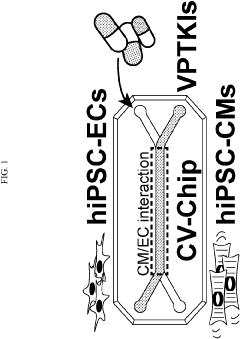
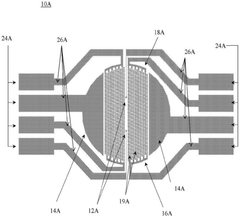
Multi-lineage cardiovascular microfluidic organ-chip
PatentPendingUS20230159896A1
Innovation
- A multi-lineage, fully-integrated cardiovascular organ-chip using human induced pluripotent stem cell-derived cardiomyocytes and endothelial cells, which mimics the complex spatial distribution of the adult human heart, allowing for early and cost-effective drug testing and cardiotoxicity assessment.
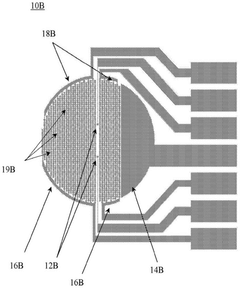
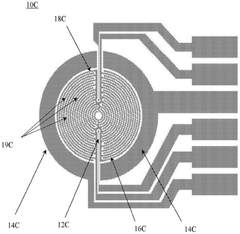
Methods and systems for functional maturation of iPSC- and ESC-derived cardiomyocytes
PatentActiveCN110582569B
Innovation
- By expanding the electricity pace and monitoring characteristics of the XCelligence Cardiocr system, the electrical method is used to induce the maturity of myocardial cells, and monitor the mature process by monitoring cell-base impedance and extracellular records.
Scalability and Manufacturing Considerations
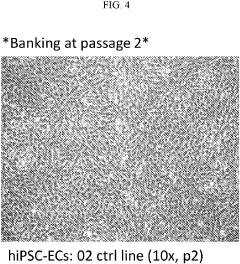
The scalability of iPSC-derived cardiomyocyte maturation protocols within microfluidic chips represents a critical consideration for translating laboratory innovations into commercially viable applications. Current laboratory-scale protocols typically produce limited quantities of mature cardiomyocytes, which is insufficient for industrial applications requiring millions to billions of cells. Scaling production requires careful optimization of microfluidic chip design to maintain consistent fluid dynamics, nutrient distribution, and electrical stimulation across larger surface areas.
Manufacturing considerations must address the standardization of chip fabrication processes. Current microfluidic chips often utilize polydimethylsiloxane (PDMS) due to its optical clarity and gas permeability. However, PDMS presents challenges for mass production, including batch-to-batch variability and limited automation compatibility. Alternative materials such as thermoplastics (polystyrene, polycarbonate) offer superior manufacturing scalability through injection molding techniques, though they may require modification to achieve comparable biocompatibility and gas exchange properties.
Cost analysis reveals significant economic barriers to widespread adoption. The current expense of producing mature cardiomyocytes in microfluidic systems exceeds $10,000 per million cells, primarily driven by growth factors, specialized media, and quality control processes. Economies of scale could potentially reduce costs by 60-70%, but would require substantial initial capital investment in automated manufacturing infrastructure.
Quality control represents another critical manufacturing consideration. Establishing robust, non-destructive methods for assessing cardiomyocyte maturation in real-time across large-scale systems remains challenging. Emerging technologies incorporating integrated sensors for continuous monitoring of electrophysiological activity, contractile force, and metabolic parameters show promise but require further development for manufacturing environments.
Regulatory compliance adds complexity to manufacturing scale-up. Current Good Manufacturing Practice (cGMP) guidelines for cell therapy products demand rigorous documentation, validation, and consistency in production processes. Microfluidic platforms must demonstrate reproducibility across multiple manufacturing lots while maintaining sterility throughout extended culture periods.
Supply chain management for microfluidic cardiomyocyte maturation systems presents unique challenges. The integration of biological components (cells, growth factors) with precision-engineered microfluidic components requires coordinated sourcing strategies and quality assurance protocols. Developing robust cryopreservation methods for mature cardiomyocytes would significantly enhance supply chain flexibility and product shelf-life, though current protocols show limited efficacy for fully mature cells.
Manufacturing considerations must address the standardization of chip fabrication processes. Current microfluidic chips often utilize polydimethylsiloxane (PDMS) due to its optical clarity and gas permeability. However, PDMS presents challenges for mass production, including batch-to-batch variability and limited automation compatibility. Alternative materials such as thermoplastics (polystyrene, polycarbonate) offer superior manufacturing scalability through injection molding techniques, though they may require modification to achieve comparable biocompatibility and gas exchange properties.
Cost analysis reveals significant economic barriers to widespread adoption. The current expense of producing mature cardiomyocytes in microfluidic systems exceeds $10,000 per million cells, primarily driven by growth factors, specialized media, and quality control processes. Economies of scale could potentially reduce costs by 60-70%, but would require substantial initial capital investment in automated manufacturing infrastructure.
Quality control represents another critical manufacturing consideration. Establishing robust, non-destructive methods for assessing cardiomyocyte maturation in real-time across large-scale systems remains challenging. Emerging technologies incorporating integrated sensors for continuous monitoring of electrophysiological activity, contractile force, and metabolic parameters show promise but require further development for manufacturing environments.
Regulatory compliance adds complexity to manufacturing scale-up. Current Good Manufacturing Practice (cGMP) guidelines for cell therapy products demand rigorous documentation, validation, and consistency in production processes. Microfluidic platforms must demonstrate reproducibility across multiple manufacturing lots while maintaining sterility throughout extended culture periods.
Supply chain management for microfluidic cardiomyocyte maturation systems presents unique challenges. The integration of biological components (cells, growth factors) with precision-engineered microfluidic components requires coordinated sourcing strategies and quality assurance protocols. Developing robust cryopreservation methods for mature cardiomyocytes would significantly enhance supply chain flexibility and product shelf-life, though current protocols show limited efficacy for fully mature cells.
Regulatory Pathway for Clinical Translation
The regulatory pathway for translating iPSC-derived cardiomyocytes (iPSC-CMs) within microfluidic chips from laboratory research to clinical applications involves navigating complex regulatory frameworks across different jurisdictions. Understanding these pathways is crucial for successful clinical translation of optimized maturation protocols.
In the United States, the Food and Drug Administration (FDA) classifies these technologies under combination products, requiring comprehensive review through multiple regulatory centers. The Center for Biologics Evaluation and Research (CBER) oversees the cellular components, while the Center for Devices and Radiological Health (CDRH) regulates the microfluidic platform. Developers must engage in early consultation with the FDA through the pre-submission program to establish appropriate regulatory classification and testing requirements.
European regulatory pathways operate under the Advanced Therapy Medicinal Products (ATMP) framework governed by the European Medicines Agency (EMA). The combination of cells and microfluidic devices necessitates compliance with both the Medical Device Regulation (MDR) and ATMP regulations. The EMA offers the Innovation Task Force consultation process to guide developers through these complex regulatory intersections.
Japan has established an expedited pathway through the Pharmaceuticals and Medical Devices Agency (PMDA) under the Sakigake designation system, potentially accelerating approval for innovative technologies like optimized iPSC-CM protocols. This pathway can significantly reduce time-to-market for breakthrough therapies.
Clinical translation requires robust preclinical validation data demonstrating safety and efficacy. This includes comprehensive characterization of iPSC-CM maturation, functional assessment, and thorough evaluation of potential tumorigenicity and immunogenicity risks. Good Manufacturing Practice (GMP) compliance is mandatory, with particular attention to quality control measures for both the cellular components and microfluidic systems.
Regulatory submissions must address specific considerations for iPSC-derived products, including source cell characterization, reprogramming methods, differentiation protocols, and maturation techniques. The microfluidic component requires validation of materials biocompatibility, sterilization methods, and shelf-life stability.
Adaptive licensing approaches are increasingly available for regenerative medicine products, allowing conditional approval based on preliminary efficacy data while requiring post-market surveillance. This pathway may be particularly suitable for rapidly evolving technologies like optimized iPSC-CM maturation protocols.
Successful regulatory navigation requires multidisciplinary expertise spanning regulatory affairs, quality assurance, clinical research, and manufacturing. Early engagement with regulatory bodies through scientific advice meetings can significantly streamline the approval process and align development strategies with regulatory expectations.
In the United States, the Food and Drug Administration (FDA) classifies these technologies under combination products, requiring comprehensive review through multiple regulatory centers. The Center for Biologics Evaluation and Research (CBER) oversees the cellular components, while the Center for Devices and Radiological Health (CDRH) regulates the microfluidic platform. Developers must engage in early consultation with the FDA through the pre-submission program to establish appropriate regulatory classification and testing requirements.
European regulatory pathways operate under the Advanced Therapy Medicinal Products (ATMP) framework governed by the European Medicines Agency (EMA). The combination of cells and microfluidic devices necessitates compliance with both the Medical Device Regulation (MDR) and ATMP regulations. The EMA offers the Innovation Task Force consultation process to guide developers through these complex regulatory intersections.
Japan has established an expedited pathway through the Pharmaceuticals and Medical Devices Agency (PMDA) under the Sakigake designation system, potentially accelerating approval for innovative technologies like optimized iPSC-CM protocols. This pathway can significantly reduce time-to-market for breakthrough therapies.
Clinical translation requires robust preclinical validation data demonstrating safety and efficacy. This includes comprehensive characterization of iPSC-CM maturation, functional assessment, and thorough evaluation of potential tumorigenicity and immunogenicity risks. Good Manufacturing Practice (GMP) compliance is mandatory, with particular attention to quality control measures for both the cellular components and microfluidic systems.
Regulatory submissions must address specific considerations for iPSC-derived products, including source cell characterization, reprogramming methods, differentiation protocols, and maturation techniques. The microfluidic component requires validation of materials biocompatibility, sterilization methods, and shelf-life stability.
Adaptive licensing approaches are increasingly available for regenerative medicine products, allowing conditional approval based on preliminary efficacy data while requiring post-market surveillance. This pathway may be particularly suitable for rapidly evolving technologies like optimized iPSC-CM maturation protocols.
Successful regulatory navigation requires multidisciplinary expertise spanning regulatory affairs, quality assurance, clinical research, and manufacturing. Early engagement with regulatory bodies through scientific advice meetings can significantly streamline the approval process and align development strategies with regulatory expectations.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!