Electrochemical Compression Materials: Membranes And Electrode Choices
SEP 12, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electrochemical Compression Technology Background and Objectives
Electrochemical compression technology represents a revolutionary approach to gas compression that offers significant advantages over traditional mechanical methods. This technology has evolved from early electrochemical concepts developed in the 1970s to increasingly sophisticated systems capable of achieving high compression ratios with minimal moving parts. The fundamental principle leverages electrochemical reactions to transport and compress gases, particularly hydrogen, through selective membranes under the influence of an electric field.
The evolution of this technology has been closely tied to advancements in fuel cell research, as many of the core materials and principles overlap. Early systems suffered from efficiency limitations and material degradation issues, but recent breakthroughs in membrane and electrode materials have dramatically improved performance metrics. The technology has progressed from laboratory curiosities to commercially viable systems for specific applications, particularly in the hydrogen energy sector.
Current research focuses intensively on optimizing the critical components: membranes and electrodes. These materials determine compression efficiency, durability, and overall system performance. Proton exchange membranes (PEMs) have dominated the field, with Nafion being the industry standard, though newer materials including hydrocarbon-based membranes show promising characteristics for specific compression applications.
The electrode materials present equally important research opportunities, with catalyst composition and structure significantly impacting reaction kinetics and energy efficiency. Traditional platinum-based catalysts are being challenged by novel alloys and non-precious metal alternatives that could substantially reduce system costs while maintaining performance.
The primary technical objectives in this field include developing membrane materials with enhanced proton conductivity while maintaining mechanical stability under pressure differentials, creating electrode structures that minimize polarization losses, and designing integrated systems that achieve higher compression ratios with reduced energy input. Additionally, researchers aim to extend operational lifetimes by addressing degradation mechanisms in both membranes and electrodes.
Market drivers for this technology include the growing hydrogen economy, which requires efficient compression solutions for storage and transportation, as well as industrial applications requiring ultra-pure gas compression without contamination risks. Environmental regulations favoring emission-free technologies further accelerate development in this space.
The convergence of materials science, electrochemistry, and mechanical engineering in this field presents unique interdisciplinary challenges and opportunities. Success in advancing electrochemical compression technology could revolutionize multiple industries while supporting global decarbonization efforts through more efficient hydrogen infrastructure.
The evolution of this technology has been closely tied to advancements in fuel cell research, as many of the core materials and principles overlap. Early systems suffered from efficiency limitations and material degradation issues, but recent breakthroughs in membrane and electrode materials have dramatically improved performance metrics. The technology has progressed from laboratory curiosities to commercially viable systems for specific applications, particularly in the hydrogen energy sector.
Current research focuses intensively on optimizing the critical components: membranes and electrodes. These materials determine compression efficiency, durability, and overall system performance. Proton exchange membranes (PEMs) have dominated the field, with Nafion being the industry standard, though newer materials including hydrocarbon-based membranes show promising characteristics for specific compression applications.
The electrode materials present equally important research opportunities, with catalyst composition and structure significantly impacting reaction kinetics and energy efficiency. Traditional platinum-based catalysts are being challenged by novel alloys and non-precious metal alternatives that could substantially reduce system costs while maintaining performance.
The primary technical objectives in this field include developing membrane materials with enhanced proton conductivity while maintaining mechanical stability under pressure differentials, creating electrode structures that minimize polarization losses, and designing integrated systems that achieve higher compression ratios with reduced energy input. Additionally, researchers aim to extend operational lifetimes by addressing degradation mechanisms in both membranes and electrodes.
Market drivers for this technology include the growing hydrogen economy, which requires efficient compression solutions for storage and transportation, as well as industrial applications requiring ultra-pure gas compression without contamination risks. Environmental regulations favoring emission-free technologies further accelerate development in this space.
The convergence of materials science, electrochemistry, and mechanical engineering in this field presents unique interdisciplinary challenges and opportunities. Success in advancing electrochemical compression technology could revolutionize multiple industries while supporting global decarbonization efforts through more efficient hydrogen infrastructure.
Market Analysis for Electrochemical Compression Applications
The electrochemical compression market is experiencing significant growth driven by increasing demand for clean energy solutions and sustainable technologies. Current market valuations indicate that the global electrochemical compression sector reached approximately 320 million USD in 2022, with projections suggesting a compound annual growth rate of 9.7% through 2030. This growth trajectory is primarily fueled by applications in hydrogen refueling stations, energy storage systems, and industrial gas processing.
Hydrogen energy applications represent the largest market segment, accounting for roughly 45% of the total electrochemical compression market. This dominance stems from the global push toward hydrogen as a clean energy carrier and the expansion of hydrogen refueling infrastructure across North America, Europe, and parts of Asia. Countries like Japan, Germany, and South Korea have implemented substantial subsidies and incentives for hydrogen technology deployment, further stimulating market growth.
The refrigeration and cooling sector constitutes the second-largest application area, representing approximately 30% of the market. Electrochemical compression offers significant advantages over traditional vapor compression systems, including higher energy efficiency, reduced environmental impact, and the absence of harmful refrigerants. This segment is expected to grow at the fastest rate among all applications, driven by stringent environmental regulations and the phase-out of hydrofluorocarbon refrigerants.
Regional analysis reveals that North America currently leads the market with a 38% share, followed by Europe (32%) and Asia-Pacific (25%). However, the Asia-Pacific region is anticipated to exhibit the highest growth rate over the forecast period, primarily due to rapid industrialization, increasing energy demands, and substantial government investments in clean energy technologies in countries like China and India.
Customer demand patterns indicate a growing preference for electrochemical compression systems with higher efficiency, longer operational lifetimes, and reduced maintenance requirements. End-users are increasingly willing to pay premium prices for systems that demonstrate superior membrane and electrode performance, highlighting the critical importance of materials research in this field.
Market barriers include high initial capital costs, limited awareness of electrochemical compression technology among potential end-users, and competition from established conventional compression technologies. The average cost of electrochemical compression systems remains 30-40% higher than traditional mechanical compressors, though this gap is narrowing as production scales increase and material innovations reduce manufacturing costs.
Hydrogen energy applications represent the largest market segment, accounting for roughly 45% of the total electrochemical compression market. This dominance stems from the global push toward hydrogen as a clean energy carrier and the expansion of hydrogen refueling infrastructure across North America, Europe, and parts of Asia. Countries like Japan, Germany, and South Korea have implemented substantial subsidies and incentives for hydrogen technology deployment, further stimulating market growth.
The refrigeration and cooling sector constitutes the second-largest application area, representing approximately 30% of the market. Electrochemical compression offers significant advantages over traditional vapor compression systems, including higher energy efficiency, reduced environmental impact, and the absence of harmful refrigerants. This segment is expected to grow at the fastest rate among all applications, driven by stringent environmental regulations and the phase-out of hydrofluorocarbon refrigerants.
Regional analysis reveals that North America currently leads the market with a 38% share, followed by Europe (32%) and Asia-Pacific (25%). However, the Asia-Pacific region is anticipated to exhibit the highest growth rate over the forecast period, primarily due to rapid industrialization, increasing energy demands, and substantial government investments in clean energy technologies in countries like China and India.
Customer demand patterns indicate a growing preference for electrochemical compression systems with higher efficiency, longer operational lifetimes, and reduced maintenance requirements. End-users are increasingly willing to pay premium prices for systems that demonstrate superior membrane and electrode performance, highlighting the critical importance of materials research in this field.
Market barriers include high initial capital costs, limited awareness of electrochemical compression technology among potential end-users, and competition from established conventional compression technologies. The average cost of electrochemical compression systems remains 30-40% higher than traditional mechanical compressors, though this gap is narrowing as production scales increase and material innovations reduce manufacturing costs.
Current Membrane and Electrode Materials: Status and Challenges
The current landscape of electrochemical compression materials is characterized by significant advancements in both membrane and electrode technologies, yet substantial challenges remain. Proton exchange membranes (PEMs), particularly Nafion, dominate the field due to their excellent proton conductivity and chemical stability. However, these perfluorosulfonic acid-based membranes suffer from high cost, limited temperature range (typically below 100°C), and performance degradation under low humidity conditions. Alternative membranes such as hydrocarbon-based polymers (SPEEK, SPES) offer cost advantages but generally exhibit lower conductivity and durability.
Anion exchange membranes (AEMs) represent an emerging alternative with potential cost benefits and improved electrochemical performance. However, AEMs currently face significant stability challenges in alkaline environments, with degradation mechanisms affecting both the polymer backbone and functional groups. Recent research has focused on developing more stable quaternary ammonium functionalized polymers and exploring novel polymer architectures to enhance mechanical properties.
For electrode materials, platinum and platinum-group metals remain the gold standard catalysts, particularly for hydrogen compression applications. The high cost of these materials presents a major barrier to widespread commercialization. Platinum loading reduction strategies include alloying with transition metals (Pt-Ni, Pt-Co) and developing core-shell nanostructures, which have shown promising activity while reducing precious metal content.
Non-precious metal catalysts based on transition metal oxides, nitrides, and carbon-supported materials have demonstrated potential for certain electrochemical compression applications but generally exhibit lower activity and stability compared to platinum-based counterparts. The electrode-membrane interface also presents significant challenges, with issues related to three-phase boundary formation, catalyst utilization, and interfacial resistance affecting overall system performance.
Recent innovations in electrode fabrication techniques include direct deposition methods, spray coating, and electrospinning to create optimized electrode structures. These approaches aim to improve mass transport properties while maintaining high electronic and ionic conductivity. Additionally, composite membranes incorporating inorganic fillers (such as metal oxides, MOFs, and graphene derivatives) have shown enhanced mechanical stability and conductivity under variable operating conditions.
The durability of both membrane and electrode materials remains a critical challenge, with chemical degradation, mechanical stress, and contamination effects limiting long-term operation. Current research efforts focus on developing accelerated stress tests and in-situ characterization techniques to better understand degradation mechanisms and develop mitigation strategies for next-generation materials.
Anion exchange membranes (AEMs) represent an emerging alternative with potential cost benefits and improved electrochemical performance. However, AEMs currently face significant stability challenges in alkaline environments, with degradation mechanisms affecting both the polymer backbone and functional groups. Recent research has focused on developing more stable quaternary ammonium functionalized polymers and exploring novel polymer architectures to enhance mechanical properties.
For electrode materials, platinum and platinum-group metals remain the gold standard catalysts, particularly for hydrogen compression applications. The high cost of these materials presents a major barrier to widespread commercialization. Platinum loading reduction strategies include alloying with transition metals (Pt-Ni, Pt-Co) and developing core-shell nanostructures, which have shown promising activity while reducing precious metal content.
Non-precious metal catalysts based on transition metal oxides, nitrides, and carbon-supported materials have demonstrated potential for certain electrochemical compression applications but generally exhibit lower activity and stability compared to platinum-based counterparts. The electrode-membrane interface also presents significant challenges, with issues related to three-phase boundary formation, catalyst utilization, and interfacial resistance affecting overall system performance.
Recent innovations in electrode fabrication techniques include direct deposition methods, spray coating, and electrospinning to create optimized electrode structures. These approaches aim to improve mass transport properties while maintaining high electronic and ionic conductivity. Additionally, composite membranes incorporating inorganic fillers (such as metal oxides, MOFs, and graphene derivatives) have shown enhanced mechanical stability and conductivity under variable operating conditions.
The durability of both membrane and electrode materials remains a critical challenge, with chemical degradation, mechanical stress, and contamination effects limiting long-term operation. Current research efforts focus on developing accelerated stress tests and in-situ characterization techniques to better understand degradation mechanisms and develop mitigation strategies for next-generation materials.
State-of-the-Art Membrane and Electrode Material Solutions
01 Proton exchange membranes for electrochemical compression
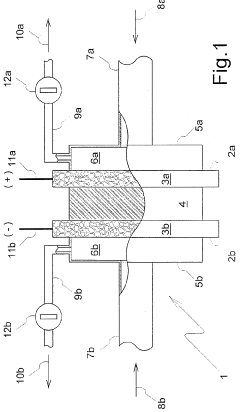
Proton exchange membranes (PEMs) are critical components in electrochemical compression systems, facilitating the selective transport of protons while blocking the passage of gases. These membranes typically consist of perfluorosulfonic acid polymers that provide high proton conductivity and chemical stability. Advanced PEM materials enhance compression efficiency by reducing electrical resistance and improving gas separation capabilities, which is essential for applications in hydrogen compression and storage systems.- Membrane materials for electrochemical compression: Specialized membrane materials are crucial for efficient electrochemical compression systems. These membranes facilitate ion transport while maintaining separation between electrodes. Advanced polymer electrolyte membranes with high proton conductivity and mechanical stability are particularly important for hydrogen compression applications. Perfluorosulfonic acid (PFSA) membranes and composite membranes incorporating inorganic fillers have shown improved performance in terms of gas permeability, mechanical strength, and durability under compression conditions.
- Electrode materials and catalysts for electrochemical compression: Electrode materials and catalysts significantly impact the efficiency of electrochemical compression systems. Noble metal catalysts like platinum and palladium are commonly used for their excellent catalytic activity. Recent developments include nanostructured electrodes with high surface area and novel catalyst compositions that reduce noble metal loading while maintaining performance. Carbon-supported catalysts and metal alloys have shown promising results in enhancing reaction kinetics and reducing overpotential during compression operations, leading to improved energy efficiency.
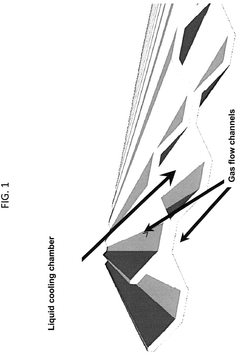
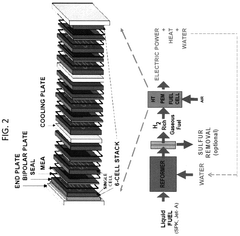
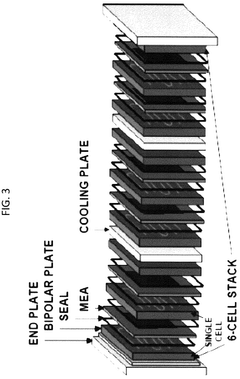
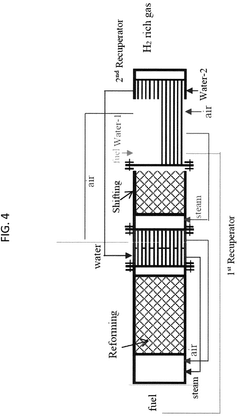
- Electrochemical cell configurations for compression applications: Various electrochemical cell configurations have been developed specifically for compression applications. These designs focus on optimizing gas flow channels, electrode-membrane interfaces, and overall cell geometry to enhance compression efficiency. Stack designs with multiple cells in series or parallel arrangements allow for achieving higher compression ratios. Innovations include integrated cooling systems to manage heat generated during compression and specialized sealing technologies to prevent gas leakage at high pressures.
- Composite and hybrid materials for enhanced performance: Composite and hybrid materials combine the advantages of different material classes to overcome limitations of single-component systems in electrochemical compression. These include polymer-ceramic composites for membranes with improved mechanical stability and reduced gas crossover, and hybrid electrode structures incorporating both carbon-based materials and metal oxides. Such materials demonstrate superior performance in terms of durability under pressure cycling, resistance to chemical degradation, and maintenance of electrochemical activity over extended operation periods.
- System integration and control strategies: Effective system integration and control strategies are essential for optimizing electrochemical compression performance. This includes advanced sensing and monitoring technologies to track key parameters such as pressure, temperature, and humidity across the system. Adaptive control algorithms adjust operating conditions in real-time to maintain efficiency under varying loads. Integration approaches also address balance-of-plant components like heat exchangers, water management systems, and power conditioning equipment that support the core electrochemical compression process.
02 Electrode materials and catalysts for electrochemical compression
Electrode materials play a crucial role in electrochemical compression systems, with catalysts being particularly important for facilitating electrochemical reactions. Noble metals like platinum and their alloys are commonly used as catalysts due to their high activity and stability. Recent developments include the use of nanostructured catalysts and carbon-supported materials to increase the active surface area while reducing the amount of precious metals required, thereby improving performance and reducing costs in electrochemical compression applications.Expand Specific Solutions03 Membrane electrode assemblies (MEAs) for electrochemical compression
Membrane electrode assemblies (MEAs) integrate the membrane and electrode components into a single functional unit for electrochemical compression systems. The design of MEAs focuses on optimizing the interface between the membrane and electrodes to enhance proton transport, gas diffusion, and overall system efficiency. Advanced fabrication techniques include hot-pressing, spray coating, and direct deposition methods to create robust MEAs with improved durability and performance under the high-pressure conditions typical of electrochemical compression applications.Expand Specific Solutions04 Novel materials for enhanced electrochemical compression efficiency
Research on novel materials aims to overcome limitations of conventional electrochemical compression systems. These include composite membranes incorporating inorganic fillers to enhance mechanical strength and conductivity, ionic liquids as electrolytes for operation at extreme temperatures, and metal-organic frameworks for improved gas selectivity. These advanced materials contribute to higher compression ratios, lower energy consumption, and extended operational lifetimes in applications ranging from refrigeration systems to hydrogen purification and storage.Expand Specific Solutions05 System integration and design considerations for electrochemical compression
Effective integration of membranes and electrodes into complete electrochemical compression systems requires careful consideration of operating parameters, flow field designs, and sealing technologies. Key design aspects include thermal management to prevent membrane dehydration, pressure distribution systems to maintain uniform compression, and cell stacking configurations to achieve desired pressure ratios. Advanced system designs incorporate sensors and control systems to optimize performance under varying conditions, enabling applications in clean energy technologies, gas separation processes, and next-generation cooling systems.Expand Specific Solutions
Leading Companies and Research Institutions in the Field
Electrochemical compression materials research, particularly focusing on membranes and electrode choices, is currently in a growth phase with increasing market adoption. The global market is expanding rapidly due to rising demand for clean energy solutions and hydrogen technologies, with projections indicating significant growth over the next decade. Technologically, the field shows varying maturity levels across applications. Leading companies like W.L. Gore & Associates and 3M have established strong positions in membrane technology, while Toray Industries and Robert Bosch GmbH are advancing electrode materials. ITM Power and 24M Technologies are innovating in system integration. Academic institutions like the Dalian Institute of Chemical Physics and Rensselaer Polytechnic Institute are contributing fundamental research, creating a competitive landscape balanced between established industrial players and emerging specialized firms.
W. L. Gore & Associates, Inc.
Technical Solution: Gore has pioneered reinforced membrane electrode assemblies (MEAs) specifically engineered for electrochemical compression applications. Their technology centers on expanded polytetrafluoroethylene (ePTFE) reinforced composite membranes that offer exceptional mechanical strength while maintaining high proton conductivity. Gore's proprietary manufacturing process creates a unique microporous structure that enhances gas diffusion while preventing membrane deformation under pressure differentials (up to 30+ bar). Their MEAs incorporate advanced catalyst layer designs with optimized platinum loadings (typically 0.1-0.4 mg/cm²) and ionomer-to-carbon ratios tailored for compression efficiency. The company has developed specialized membrane treatments that improve water management capabilities, allowing for stable operation across varying humidity levels (20-100% RH). Gore's integrated electrode-membrane systems feature controlled interfacial properties that minimize contact resistance and enhance long-term durability in compression applications.
Strengths: Exceptional mechanical stability under pressure cycling; superior durability (demonstrated >20,000 hours operational lifetime); excellent dimensional stability with minimal swelling. Weaknesses: Premium pricing compared to standard membranes; specialized manufacturing requirements limit production scaling; performance optimization required for specific operating conditions.
3M Innovative Properties Co.
Technical Solution: 3M has developed advanced ion exchange membranes specifically designed for electrochemical compression applications. Their technology focuses on perfluorinated sulfonic acid (PFSA) membranes with enhanced proton conductivity and mechanical stability. The company has engineered composite membranes that incorporate reinforcement layers to improve durability while maintaining high ionic conductivity. Their proprietary manufacturing process allows for precise control of membrane thickness (typically 25-175 μm) and equivalent weight (800-1100 g/mol), optimizing performance for specific compression applications. 3M's membranes feature specialized surface treatments that enhance electrode-membrane interfaces, reducing contact resistance and improving overall system efficiency. Recent developments include membranes with modified side chains that demonstrate superior performance under variable humidity conditions, addressing a key challenge in electrochemical compression systems.
Strengths: Superior durability and chemical stability in harsh operating environments; excellent proton conductivity (>0.10 S/cm under optimal conditions); established manufacturing capabilities for consistent quality. Weaknesses: Higher cost compared to non-fluorinated alternatives; environmental concerns regarding fluoropolymer production and disposal; performance degradation at extreme temperature conditions.
Critical Patents and Research on Electrochemical Compression Materials
Electrochemical hydrogen compressor for operation at pressures exceeding 12,500 psi
PatentActiveUS12119525B2
Innovation
- The development of inorganic-organic composite polymer membranes and liquid-cooled metallic bipolar plates, which enhance proton conduction, mechanical strength, and temperature tolerance, along with an electrochemical hydrogen compressor that uses a composite membrane to efficiently compress and purify hydrogen.
Electrochemical cell with a polymer electrolyte and process for producing these polymer electrolytes
PatentWO1995007553A2
Innovation
- The electrochemical cell employs a membrane film made from terminally sulfonated, radiation-grafted base polymers consisting of substituted and unsubstituted polyolefins, with an intermediate layer of proton-conducting hydrophilic copolymers, which are crosslinked using agents like divinylbenzene, providing enhanced stability and cost-effectiveness.
Environmental Impact and Sustainability Considerations
The environmental impact of electrochemical compression systems is significantly influenced by the materials selected for membranes and electrodes. Traditional compression technologies often rely on refrigerants with high global warming potential (GWP), whereas electrochemical compression can utilize hydrogen or other environmentally benign working fluids, substantially reducing direct emissions.
Membrane materials present varying environmental footprints throughout their lifecycle. Nafion and other perfluorosulfonic acid (PFSA) membranes, while offering excellent performance, contain fluorinated compounds that persist in the environment and pose end-of-life disposal challenges. Recent research into hydrocarbon-based and composite membranes demonstrates promising alternatives with reduced environmental impact while maintaining acceptable performance metrics.
Electrode materials similarly warrant careful sustainability consideration. Platinum group metals (PGMs), commonly used as catalysts, face resource scarcity concerns and involve energy-intensive mining operations with significant ecological disruption. Research into reduced-PGM and PGM-free catalysts, including transition metal compounds and carbon-based materials, represents a critical pathway toward more sustainable electrochemical compression systems.
Manufacturing processes for these materials contribute substantially to their overall environmental footprint. Energy-intensive production methods for specialized polymers and catalyst materials can offset the operational environmental benefits of electrochemical compression technology. Emerging green synthesis approaches and additive manufacturing techniques offer potential for reducing embodied energy and associated emissions in material production.
Durability and lifetime considerations directly impact sustainability through material replacement frequency and system efficiency over time. Membrane degradation mechanisms, including chemical attack and mechanical stress, necessitate replacement and generate waste. Research into self-healing membranes and more robust electrode structures could significantly extend component lifetimes, reducing material consumption and improving lifecycle sustainability metrics.
Water management within these systems presents both challenges and opportunities for environmental improvement. While water is essential for proton transport in many membrane systems, water purification requirements can increase system complexity and energy consumption. Developments in low-humidity operation membranes could reduce water requirements, particularly valuable in water-scarce regions.
End-of-life considerations and circular economy principles are increasingly important in material selection. Recyclability of membrane and electrode materials remains limited by complex composite structures and contamination issues. Research into design-for-disassembly approaches and recovery methods for valuable components like platinum catalysts could significantly improve the overall sustainability profile of electrochemical compression technologies.
Membrane materials present varying environmental footprints throughout their lifecycle. Nafion and other perfluorosulfonic acid (PFSA) membranes, while offering excellent performance, contain fluorinated compounds that persist in the environment and pose end-of-life disposal challenges. Recent research into hydrocarbon-based and composite membranes demonstrates promising alternatives with reduced environmental impact while maintaining acceptable performance metrics.
Electrode materials similarly warrant careful sustainability consideration. Platinum group metals (PGMs), commonly used as catalysts, face resource scarcity concerns and involve energy-intensive mining operations with significant ecological disruption. Research into reduced-PGM and PGM-free catalysts, including transition metal compounds and carbon-based materials, represents a critical pathway toward more sustainable electrochemical compression systems.
Manufacturing processes for these materials contribute substantially to their overall environmental footprint. Energy-intensive production methods for specialized polymers and catalyst materials can offset the operational environmental benefits of electrochemical compression technology. Emerging green synthesis approaches and additive manufacturing techniques offer potential for reducing embodied energy and associated emissions in material production.
Durability and lifetime considerations directly impact sustainability through material replacement frequency and system efficiency over time. Membrane degradation mechanisms, including chemical attack and mechanical stress, necessitate replacement and generate waste. Research into self-healing membranes and more robust electrode structures could significantly extend component lifetimes, reducing material consumption and improving lifecycle sustainability metrics.
Water management within these systems presents both challenges and opportunities for environmental improvement. While water is essential for proton transport in many membrane systems, water purification requirements can increase system complexity and energy consumption. Developments in low-humidity operation membranes could reduce water requirements, particularly valuable in water-scarce regions.
End-of-life considerations and circular economy principles are increasingly important in material selection. Recyclability of membrane and electrode materials remains limited by complex composite structures and contamination issues. Research into design-for-disassembly approaches and recovery methods for valuable components like platinum catalysts could significantly improve the overall sustainability profile of electrochemical compression technologies.
Manufacturing Scalability and Cost Analysis
The scalability of manufacturing processes for electrochemical compression materials presents significant challenges that directly impact commercial viability. Current membrane production methods, particularly for proton exchange membranes like Nafion, involve complex chemical processes requiring precise control of temperature, pressure, and chemical composition. These processes often utilize expensive equipment and specialized facilities, creating barriers to mass production and cost reduction.
Electrode manufacturing faces similar scalability issues, with precious metal catalysts like platinum representing a major cost driver. Current production methods typically involve batch processing with limited throughput, contributing to higher unit costs. The transition from laboratory-scale production to industrial-scale manufacturing requires substantial process engineering and capital investment, often exceeding $50-100 million for a commercial-scale facility.
Material costs constitute approximately 40-60% of the total system cost in electrochemical compression systems. Nafion membranes, for instance, currently cost between $500-1000/m², while platinum-based electrodes can cost $2000-4000 per kilogram. These high material costs significantly impact the economic feasibility of widespread adoption, particularly in price-sensitive applications like hydrogen compression for fuel cell vehicles.
Supply chain considerations further complicate manufacturing economics. Key raw materials for advanced membranes and electrodes often face supply constraints or geopolitical risks. For example, platinum group metals are concentrated in a few countries, creating potential supply vulnerabilities. Alternative materials like palladium alloys or non-precious metal catalysts offer potential cost advantages but frequently underperform in durability or efficiency metrics.
Economies of scale represent a critical pathway to cost reduction. Industry analyses suggest that a tenfold increase in production volume could potentially reduce manufacturing costs by 30-50% through improved process efficiency, reduced waste, and amortization of fixed costs. However, achieving such scale requires significant market growth and sustained investment in manufacturing infrastructure.
Recent innovations in manufacturing techniques show promise for improving scalability. Roll-to-roll processing for membrane electrode assemblies, automated catalyst deposition systems, and continuous flow reactors for membrane synthesis all demonstrate potential for increasing throughput while maintaining quality control. These advances could reduce production costs by 15-25% in the near term while enabling further scaling in the future.
Electrode manufacturing faces similar scalability issues, with precious metal catalysts like platinum representing a major cost driver. Current production methods typically involve batch processing with limited throughput, contributing to higher unit costs. The transition from laboratory-scale production to industrial-scale manufacturing requires substantial process engineering and capital investment, often exceeding $50-100 million for a commercial-scale facility.
Material costs constitute approximately 40-60% of the total system cost in electrochemical compression systems. Nafion membranes, for instance, currently cost between $500-1000/m², while platinum-based electrodes can cost $2000-4000 per kilogram. These high material costs significantly impact the economic feasibility of widespread adoption, particularly in price-sensitive applications like hydrogen compression for fuel cell vehicles.
Supply chain considerations further complicate manufacturing economics. Key raw materials for advanced membranes and electrodes often face supply constraints or geopolitical risks. For example, platinum group metals are concentrated in a few countries, creating potential supply vulnerabilities. Alternative materials like palladium alloys or non-precious metal catalysts offer potential cost advantages but frequently underperform in durability or efficiency metrics.
Economies of scale represent a critical pathway to cost reduction. Industry analyses suggest that a tenfold increase in production volume could potentially reduce manufacturing costs by 30-50% through improved process efficiency, reduced waste, and amortization of fixed costs. However, achieving such scale requires significant market growth and sustained investment in manufacturing infrastructure.
Recent innovations in manufacturing techniques show promise for improving scalability. Roll-to-roll processing for membrane electrode assemblies, automated catalyst deposition systems, and continuous flow reactors for membrane synthesis all demonstrate potential for increasing throughput while maintaining quality control. These advances could reduce production costs by 15-25% in the near term while enabling further scaling in the future.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!