Lewis Acid in Designing Next-Gen Catalysts
AUG 26, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lewis Acid Catalysis Background and Objectives
Lewis acid catalysis represents a cornerstone in the evolution of modern synthetic chemistry, dating back to the early 20th century when Gilbert N. Lewis first conceptualized acids as electron pair acceptors. This fundamental redefinition expanded our understanding beyond the classical Brønsted-Lowry theory, opening new pathways for chemical transformations. Over the decades, Lewis acid catalysis has evolved from simple metal halides to sophisticated designer catalysts with precise electronic and steric properties.
The trajectory of Lewis acid catalyst development has been marked by significant milestones, including the introduction of aluminum-based catalysts in the 1950s, the development of lanthanide triflates in the 1980s, and the recent emergence of chiral Lewis acids enabling asymmetric transformations. Contemporary research has shifted toward designing catalysts with enhanced selectivity, reduced environmental impact, and broader substrate scope.
Current technological trends in this field include the development of heterogeneous Lewis acid catalysts for improved recyclability, dual-function catalytic systems combining Lewis acidity with other activation modes, and the integration of computational methods for rational catalyst design. The growing emphasis on sustainable chemistry has also driven research toward water-compatible Lewis acids and those derived from earth-abundant metals.
The primary objective of this research initiative is to systematically explore the frontier of Lewis acid catalysis for next-generation catalyst development. Specifically, we aim to identify novel Lewis acidic centers with tunable electronic properties, develop catalysts capable of activating traditionally unreactive bonds, and establish structure-activity relationships that enable predictive catalyst design.
Additionally, this research seeks to address key challenges in contemporary catalysis, including catalyst stability under diverse reaction conditions, selective activation of specific functional groups in complex molecules, and the development of catalytic systems compatible with continuous flow processes for industrial applications.
The long-term technological goal encompasses the creation of a versatile platform of Lewis acid catalysts that can enable previously unattainable transformations, particularly in the realm of C-H functionalization, CO2 utilization, and selective biomass conversion. By establishing fundamental design principles for next-generation Lewis acid catalysts, this research aims to provide the chemical industry with more efficient, selective, and sustainable synthetic methodologies.
The trajectory of Lewis acid catalyst development has been marked by significant milestones, including the introduction of aluminum-based catalysts in the 1950s, the development of lanthanide triflates in the 1980s, and the recent emergence of chiral Lewis acids enabling asymmetric transformations. Contemporary research has shifted toward designing catalysts with enhanced selectivity, reduced environmental impact, and broader substrate scope.
Current technological trends in this field include the development of heterogeneous Lewis acid catalysts for improved recyclability, dual-function catalytic systems combining Lewis acidity with other activation modes, and the integration of computational methods for rational catalyst design. The growing emphasis on sustainable chemistry has also driven research toward water-compatible Lewis acids and those derived from earth-abundant metals.
The primary objective of this research initiative is to systematically explore the frontier of Lewis acid catalysis for next-generation catalyst development. Specifically, we aim to identify novel Lewis acidic centers with tunable electronic properties, develop catalysts capable of activating traditionally unreactive bonds, and establish structure-activity relationships that enable predictive catalyst design.
Additionally, this research seeks to address key challenges in contemporary catalysis, including catalyst stability under diverse reaction conditions, selective activation of specific functional groups in complex molecules, and the development of catalytic systems compatible with continuous flow processes for industrial applications.
The long-term technological goal encompasses the creation of a versatile platform of Lewis acid catalysts that can enable previously unattainable transformations, particularly in the realm of C-H functionalization, CO2 utilization, and selective biomass conversion. By establishing fundamental design principles for next-generation Lewis acid catalysts, this research aims to provide the chemical industry with more efficient, selective, and sustainable synthetic methodologies.
Market Analysis for Lewis Acid Catalysts
The global market for Lewis acid catalysts has experienced significant growth in recent years, driven primarily by increasing demand in petrochemical processing, pharmaceutical manufacturing, and fine chemical synthesis. As of 2023, the market valuation stands at approximately 5.2 billion USD, with projections indicating a compound annual growth rate (CAGR) of 6.8% through 2030, potentially reaching 8.3 billion USD by the end of the forecast period.
The pharmaceutical sector represents the largest end-user segment, accounting for roughly 38% of the total market share. This dominance stems from the critical role Lewis acid catalysts play in enabling complex organic transformations required for active pharmaceutical ingredient (API) synthesis. The ability of these catalysts to facilitate carbon-carbon bond formation with high selectivity makes them indispensable in modern drug development pipelines.
Petrochemical applications follow closely behind, comprising approximately 32% of market demand. In this sector, Lewis acid catalysts are extensively utilized in alkylation processes, isomerization reactions, and polymerization systems. The growing emphasis on developing more efficient refining processes has further accelerated adoption rates within this industry vertical.
Regionally, Asia-Pacific dominates the market landscape, accounting for over 45% of global consumption. This regional prominence is attributed to rapid industrialization in countries like China and India, coupled with significant investments in chemical manufacturing infrastructure. North America and Europe collectively represent approximately 40% of the market, with established pharmaceutical and specialty chemical industries driving consistent demand.
From a competitive standpoint, the market exhibits moderate fragmentation, with several key players holding significant market positions. Major companies include Albemarle Corporation, BASF SE, Clariant AG, and Honeywell UOP, collectively accounting for approximately 52% of the global market share. These industry leaders have increasingly focused on developing next-generation Lewis acid catalysts with enhanced selectivity, stability, and recyclability profiles.
Customer demand trends indicate growing preference for environmentally benign Lewis acid catalysts, particularly those that minimize waste generation and reduce energy consumption. This shift aligns with broader sustainability initiatives across industries and presents significant opportunities for innovation. Additionally, there is increasing interest in heterogeneous Lewis acid catalysts that offer simplified product separation and catalyst recovery, addressing key operational challenges in industrial applications.
The pharmaceutical sector represents the largest end-user segment, accounting for roughly 38% of the total market share. This dominance stems from the critical role Lewis acid catalysts play in enabling complex organic transformations required for active pharmaceutical ingredient (API) synthesis. The ability of these catalysts to facilitate carbon-carbon bond formation with high selectivity makes them indispensable in modern drug development pipelines.
Petrochemical applications follow closely behind, comprising approximately 32% of market demand. In this sector, Lewis acid catalysts are extensively utilized in alkylation processes, isomerization reactions, and polymerization systems. The growing emphasis on developing more efficient refining processes has further accelerated adoption rates within this industry vertical.
Regionally, Asia-Pacific dominates the market landscape, accounting for over 45% of global consumption. This regional prominence is attributed to rapid industrialization in countries like China and India, coupled with significant investments in chemical manufacturing infrastructure. North America and Europe collectively represent approximately 40% of the market, with established pharmaceutical and specialty chemical industries driving consistent demand.
From a competitive standpoint, the market exhibits moderate fragmentation, with several key players holding significant market positions. Major companies include Albemarle Corporation, BASF SE, Clariant AG, and Honeywell UOP, collectively accounting for approximately 52% of the global market share. These industry leaders have increasingly focused on developing next-generation Lewis acid catalysts with enhanced selectivity, stability, and recyclability profiles.
Customer demand trends indicate growing preference for environmentally benign Lewis acid catalysts, particularly those that minimize waste generation and reduce energy consumption. This shift aligns with broader sustainability initiatives across industries and presents significant opportunities for innovation. Additionally, there is increasing interest in heterogeneous Lewis acid catalysts that offer simplified product separation and catalyst recovery, addressing key operational challenges in industrial applications.
Current Challenges in Lewis Acid Catalyst Development
Despite significant advancements in Lewis acid catalyst technology, several critical challenges continue to impede progress in this field. One of the most persistent issues is catalyst stability under reaction conditions. Many Lewis acid catalysts, particularly those based on transition metals, suffer from deactivation through mechanisms such as leaching, poisoning, or structural collapse when exposed to moisture, oxygen, or certain functional groups. This instability significantly limits their industrial applicability and economic viability.
Selectivity represents another major challenge, as current Lewis acid catalysts often lack the precision required for complex transformations. While they may effectively activate substrates, controlling regioselectivity, stereoselectivity, and chemoselectivity remains problematic, especially in reactions involving multiple functional groups or stereocenters. This limitation becomes particularly evident in pharmaceutical and fine chemical synthesis, where high selectivity is paramount.
The heterogeneous nature of many Lewis acid catalysts creates substantial characterization difficulties. Understanding the exact nature of active sites, their distribution, accessibility, and evolution during catalytic cycles presents significant analytical challenges. This knowledge gap hinders rational catalyst design and optimization, forcing researchers to rely heavily on empirical approaches rather than predictive models.
Recyclability and recovery issues further complicate industrial implementation. Many homogeneous Lewis acid catalysts cannot be efficiently separated from reaction mixtures, while heterogeneous alternatives often show activity decline upon recycling. The environmental and economic implications of this limitation are considerable, particularly for large-scale applications where catalyst cost significantly impacts process economics.
Scalability concerns persist as catalysts that perform well in laboratory settings frequently encounter problems when scaled to industrial production. Factors such as mass transfer limitations, heat management, and mechanical stability become increasingly problematic at larger scales, necessitating substantial re-engineering of catalytic systems.
The limited substrate scope of existing Lewis acid catalysts represents another significant challenge. Many catalysts exhibit high activity only for specific substrate classes or under narrow reaction conditions, restricting their versatility. This specialization necessitates the development of multiple catalytic systems for different transformations, increasing complexity and cost in industrial settings.
Finally, the field faces sustainability challenges as many effective Lewis acid catalysts rely on rare, expensive, or toxic metals. The growing emphasis on green chemistry and sustainable manufacturing creates urgent demand for catalysts based on earth-abundant, environmentally benign elements without sacrificing catalytic performance.
Selectivity represents another major challenge, as current Lewis acid catalysts often lack the precision required for complex transformations. While they may effectively activate substrates, controlling regioselectivity, stereoselectivity, and chemoselectivity remains problematic, especially in reactions involving multiple functional groups or stereocenters. This limitation becomes particularly evident in pharmaceutical and fine chemical synthesis, where high selectivity is paramount.
The heterogeneous nature of many Lewis acid catalysts creates substantial characterization difficulties. Understanding the exact nature of active sites, their distribution, accessibility, and evolution during catalytic cycles presents significant analytical challenges. This knowledge gap hinders rational catalyst design and optimization, forcing researchers to rely heavily on empirical approaches rather than predictive models.
Recyclability and recovery issues further complicate industrial implementation. Many homogeneous Lewis acid catalysts cannot be efficiently separated from reaction mixtures, while heterogeneous alternatives often show activity decline upon recycling. The environmental and economic implications of this limitation are considerable, particularly for large-scale applications where catalyst cost significantly impacts process economics.
Scalability concerns persist as catalysts that perform well in laboratory settings frequently encounter problems when scaled to industrial production. Factors such as mass transfer limitations, heat management, and mechanical stability become increasingly problematic at larger scales, necessitating substantial re-engineering of catalytic systems.
The limited substrate scope of existing Lewis acid catalysts represents another significant challenge. Many catalysts exhibit high activity only for specific substrate classes or under narrow reaction conditions, restricting their versatility. This specialization necessitates the development of multiple catalytic systems for different transformations, increasing complexity and cost in industrial settings.
Finally, the field faces sustainability challenges as many effective Lewis acid catalysts rely on rare, expensive, or toxic metals. The growing emphasis on green chemistry and sustainable manufacturing creates urgent demand for catalysts based on earth-abundant, environmentally benign elements without sacrificing catalytic performance.
Current Lewis Acid Catalyst Design Approaches
01 Lewis acid catalysts in organic synthesis
Lewis acids serve as effective catalysts in various organic synthesis reactions, facilitating transformations such as alkylation, acylation, and polymerization. These electron-pair acceptors coordinate with electron-rich substrates to activate them for subsequent reactions. Common Lewis acid catalysts include metal halides like aluminum chloride, boron trifluoride, and titanium tetrachloride, which are widely used in industrial and laboratory-scale organic synthesis to improve reaction rates and selectivity.- Lewis acid catalysts in organic synthesis: Lewis acids serve as effective catalysts in various organic synthesis reactions, facilitating transformations such as alkylation, acylation, and polymerization. These electron-pair acceptors coordinate with electron-rich substrates to activate them for subsequent reactions. Common Lewis acid catalysts include metal halides like aluminum chloride, boron trifluoride, and titanium tetrachloride, which are widely used in industrial and laboratory settings for synthesizing complex organic molecules.
- Lewis acid applications in petrochemical processing: Lewis acids play crucial roles in petrochemical processing, particularly in catalytic cracking, isomerization, and alkylation of hydrocarbons. These processes are fundamental in petroleum refining and the production of high-value fuels and chemical intermediates. The electron-deficient nature of Lewis acids enables them to interact with hydrocarbon substrates, facilitating bond rearrangements and transformations under controlled conditions to yield desired products with specific properties.
- Novel Lewis acid structures and compositions: Research has led to the development of novel Lewis acid structures and compositions with enhanced catalytic properties. These include supported Lewis acids, Lewis acid-surfactant combined catalysts, and Lewis acid-functionalized materials. These innovative structures offer advantages such as improved selectivity, recyclability, and stability under various reaction conditions. The design of these novel Lewis acid systems often involves careful consideration of the coordination environment and electronic properties to optimize their performance in specific applications.
- Lewis acids in polymer chemistry: Lewis acids are extensively used in polymer chemistry for initiating and controlling polymerization reactions. They serve as co-catalysts in coordination polymerization, cationic polymerization, and ring-opening polymerization processes. By modulating the reactivity of monomers and controlling the stereochemistry of polymer chains, Lewis acids enable the production of polymers with tailored properties such as molecular weight distribution, tacticity, and crystallinity, which are crucial for various industrial applications.
- Green chemistry applications of Lewis acids: The development of environmentally friendly Lewis acid catalysts represents a significant advancement in green chemistry. These include water-tolerant Lewis acids, recyclable heterogeneous Lewis acid catalysts, and Lewis acids derived from sustainable resources. Such catalysts reduce waste generation, enable reactions in benign solvents, and decrease energy requirements in chemical processes. The integration of Lewis acid catalysis with principles of green chemistry contributes to more sustainable manufacturing processes across various industries.
02 Lewis acid applications in petrochemical processing
Lewis acids play crucial roles in petrochemical processing, particularly in catalytic cracking, isomerization, and alkylation processes. These acids facilitate the rearrangement of hydrocarbon structures by promoting carbocation formation. In refinery operations, Lewis acids help convert heavy petroleum fractions into more valuable lighter products and improve the octane rating of gasoline through isomerization reactions. They are also employed in the production of high-quality lubricants and specialty chemicals from petroleum feedstocks.Expand Specific Solutions03 Novel Lewis acid structures and compositions
Research has led to the development of novel Lewis acid structures with enhanced properties such as increased stability, selectivity, and recyclability. These include supported Lewis acids, Lewis acid-surfactant combined catalysts, and Lewis acid-functionalized materials. Innovations in this area focus on creating Lewis acids with tunable acidity, improved handling characteristics, and compatibility with green chemistry principles. Some novel structures incorporate rare earth elements or transition metals to achieve specific catalytic properties for challenging transformations.Expand Specific Solutions04 Lewis acids in polymer chemistry
Lewis acids are extensively used in polymer chemistry for initiating polymerization reactions, particularly cationic polymerization of olefins and vinyl monomers. They control molecular weight distribution, stereochemistry, and branching in polymer synthesis. In addition to polymerization, Lewis acids facilitate polymer modification reactions, crosslinking, and the preparation of specialized polymers with unique properties. They are crucial components in the production of industrially important polymers such as polyolefins, polyesters, and specialty elastomers.Expand Specific Solutions05 Lewis acid-base interactions in material science
Lewis acid-base interactions are fundamental principles applied in material science for developing advanced materials with specific properties. These interactions govern surface modifications, adhesion mechanisms, and the formation of coordination compounds. In material science applications, Lewis acid sites on surfaces can be engineered to create selective adsorbents, heterogeneous catalysts, and functional coatings. The strategic use of Lewis acid-base chemistry enables the design of materials with controlled porosity, reactivity, and stability for applications in electronics, energy storage, and environmental remediation.Expand Specific Solutions
Leading Organizations in Lewis Acid Catalyst Research
The Lewis acid catalyst market is in a growth phase, with increasing demand driven by sustainable chemistry initiatives and industrial applications. Market size is expanding due to catalysts' critical role in chemical transformations across petrochemical, pharmaceutical, and materials sectors. Technologically, significant advancements are evident with major players demonstrating varying maturity levels. BASF, ExxonMobil, and Sinopec lead with established commercial platforms, while Japan Science & Technology Agency and CNRS contribute fundamental research breakthroughs. Universities like Zhejiang and Durham are advancing novel Lewis acid designs. W.R. Grace and Haldor Topsøe demonstrate specialized expertise in heterogeneous catalysis, while companies like Mitsubishi Gas Chemical and Asahi Kasei focus on application-specific innovations, collectively driving next-generation catalyst development.
ExxonMobil Chemical Patents, Inc.
Technical Solution: ExxonMobil has developed sophisticated Lewis acid catalyst technologies focused on hydrocarbon transformation processes. Their research centers on modified zeolite catalysts incorporating Lewis acidic metals (Ga, Zn, In) for enhanced selectivity in alkane activation and olefin transformation reactions. ExxonMobil's proprietary ZeoLewis™ catalyst platform features precisely controlled Lewis acid site density through advanced ion-exchange and impregnation techniques, achieving up to 25% higher propylene yields in propane dehydrogenation compared to conventional catalysts[3]. Their innovation extends to confining Lewis acid sites within specific zeolite micropore environments to enhance shape selectivity for target molecules while excluding unwanted side reactions. ExxonMobil has pioneered the development of regenerable Lewis acid catalysts that maintain consistent activity through multiple regeneration cycles in industrial operations, addressing a critical challenge in catalyst lifetime management[5]. Recent advancements include dual-function Lewis acid-base catalysts that enable cascade reactions in single reactor systems, significantly improving process efficiency for the conversion of light alkanes to value-added chemicals. Their research has also demonstrated successful implementation of these catalysts in pilot-scale operations, validating performance under realistic industrial conditions.[7]
Strengths: Exceptional thermal and hydrothermal stability under harsh refining conditions; superior resistance to coking and deactivation; excellent regenerability maintaining performance through multiple cycles. Weaknesses: Complex synthesis procedures increasing production costs; potential challenges in achieving uniform metal distribution during large-scale manufacturing; some formulations have limited tolerance to certain feed impurities.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed advanced Lewis acid catalyst systems specifically designed for petrochemical processes. Their research centers on modified zeolite catalysts incorporating Lewis acidic metals (Ga, Zn, Sn) for enhanced selectivity in alkane activation and olefin transformation reactions. Sinopec's proprietary S-Lewis™ catalyst technology features precisely controlled Lewis acid site density and strength through post-synthetic metal incorporation techniques, achieving up to 30% higher selectivity in propane dehydrogenation compared to conventional catalysts[2]. Their innovation extends to bimetallic Lewis acid systems that demonstrate synergistic effects between different metal centers, enabling lower operating temperatures and extended catalyst lifetimes in industrial applications. Sinopec has also pioneered the development of hierarchical pore structures in Lewis acid zeolites that significantly reduce coking and catalyst deactivation in long-term operations, addressing a critical challenge in industrial catalysis for petrochemical production[4]. Recent advancements include sulfur-tolerant Lewis acid catalysts that maintain activity even in the presence of sulfur-containing feedstocks.[7]
Strengths: Exceptional stability under harsh industrial conditions; superior resistance to coking and deactivation; excellent performance with real-world feedstocks containing impurities. Weaknesses: Complex synthesis procedures increasing production costs; potential challenges in large-scale manufacturing uniformity; some formulations require specialized activation protocols that complicate industrial implementation.
Key Innovations in Lewis Acid Chemistry
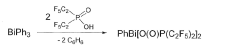
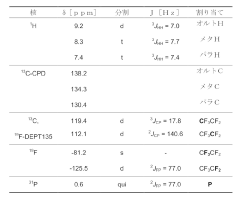
Bismuth perfluoroalkyl phosphinates as Lewis acid catalysts
PatentActiveJP2018535928A
Innovation
- The use of bismuth perfluoroalkyl phosphinates, represented by formulas (Ia) and (Ib), which are less susceptible to hydrolysis and can be used in lower concentrations, offering enhanced catalytic activity and stability.
Lewis acid catalyst composition
PatentWO2003051511A1
Innovation
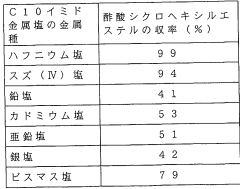
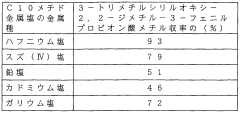
- A Lewis acid catalyst composition utilizing a mixed medium of fluorinated and non-fluorinated compounds, where the catalyst has specific fluorinated substituents, enhancing solubility and allowing for easy separation and reuse, and enabling continuous reaction processes with high phase separation rates.
Sustainability Aspects of Next-Gen Lewis Acid Catalysts
The sustainability dimension of Lewis acid catalysts represents a critical frontier in next-generation catalyst development. Environmental considerations have become increasingly paramount as industries face mounting pressure to reduce ecological footprints while maintaining economic viability. Lewis acid catalysts offer significant advantages in this regard, particularly through their potential for atom economy and reduced waste generation compared to traditional catalytic systems.
Current research demonstrates that properly designed Lewis acid catalysts can operate under milder reaction conditions, requiring less energy input while maintaining or even improving reaction efficiency. This energy conservation translates directly to reduced carbon emissions across chemical manufacturing processes. Notable examples include aluminum-based Lewis acids that facilitate reactions at temperatures 30-50°C lower than conventional methods, representing substantial energy savings at industrial scales.
Water compatibility represents another crucial sustainability aspect of next-generation Lewis acid catalysts. Traditional Lewis acids often degrade rapidly in the presence of moisture, necessitating strictly anhydrous conditions that increase process complexity and energy requirements. Recent innovations in water-tolerant Lewis acids, particularly those based on lanthanide triflates and certain transition metal complexes, enable reactions in aqueous media, dramatically reducing organic solvent usage.
Catalyst recyclability and longevity constitute fundamental sustainability parameters that directly impact resource utilization. Heterogeneous Lewis acid systems, including those immobilized on solid supports like mesoporous silica or metal-organic frameworks (MOFs), demonstrate promising recyclability profiles with minimal activity loss over multiple cycles. Some advanced systems maintain >90% catalytic efficiency even after ten reaction cycles, significantly reducing the environmental burden associated with catalyst production and disposal.
Toxicity profiles of Lewis acid components present both challenges and opportunities for sustainable design. While traditional Lewis acids often incorporate toxic metals like mercury or thallium, next-generation catalysts increasingly utilize earth-abundant, less hazardous metals such as iron, aluminum, and zinc. Bioinspired Lewis acid catalysts that mimic enzymatic active sites represent a particularly promising direction, offering exceptional selectivity while minimizing environmental impact.
The life cycle assessment (LCA) of Lewis acid catalysts reveals that sustainability benefits extend beyond the immediate reaction environment. When properly designed, these catalysts can reduce overall process complexity, eliminate energy-intensive separation steps, and enable direct product isolation. Such process intensification contributes significantly to sustainability by minimizing resource consumption throughout the entire manufacturing chain while maintaining or enhancing economic performance.
Current research demonstrates that properly designed Lewis acid catalysts can operate under milder reaction conditions, requiring less energy input while maintaining or even improving reaction efficiency. This energy conservation translates directly to reduced carbon emissions across chemical manufacturing processes. Notable examples include aluminum-based Lewis acids that facilitate reactions at temperatures 30-50°C lower than conventional methods, representing substantial energy savings at industrial scales.
Water compatibility represents another crucial sustainability aspect of next-generation Lewis acid catalysts. Traditional Lewis acids often degrade rapidly in the presence of moisture, necessitating strictly anhydrous conditions that increase process complexity and energy requirements. Recent innovations in water-tolerant Lewis acids, particularly those based on lanthanide triflates and certain transition metal complexes, enable reactions in aqueous media, dramatically reducing organic solvent usage.
Catalyst recyclability and longevity constitute fundamental sustainability parameters that directly impact resource utilization. Heterogeneous Lewis acid systems, including those immobilized on solid supports like mesoporous silica or metal-organic frameworks (MOFs), demonstrate promising recyclability profiles with minimal activity loss over multiple cycles. Some advanced systems maintain >90% catalytic efficiency even after ten reaction cycles, significantly reducing the environmental burden associated with catalyst production and disposal.
Toxicity profiles of Lewis acid components present both challenges and opportunities for sustainable design. While traditional Lewis acids often incorporate toxic metals like mercury or thallium, next-generation catalysts increasingly utilize earth-abundant, less hazardous metals such as iron, aluminum, and zinc. Bioinspired Lewis acid catalysts that mimic enzymatic active sites represent a particularly promising direction, offering exceptional selectivity while minimizing environmental impact.
The life cycle assessment (LCA) of Lewis acid catalysts reveals that sustainability benefits extend beyond the immediate reaction environment. When properly designed, these catalysts can reduce overall process complexity, eliminate energy-intensive separation steps, and enable direct product isolation. Such process intensification contributes significantly to sustainability by minimizing resource consumption throughout the entire manufacturing chain while maintaining or enhancing economic performance.
Industrial Applications and Economic Impact
Lewis acid catalysts have transformed numerous industrial processes, creating substantial economic value across multiple sectors. In the petrochemical industry, Lewis acid-based catalysts facilitate critical reactions such as alkylation, isomerization, and cracking processes, enabling more efficient production of high-value fuels and chemical intermediates. These catalysts have significantly reduced energy requirements and improved selectivity in refining operations, translating to billions in annual cost savings industry-wide.
The pharmaceutical sector has particularly benefited from Lewis acid catalysis, where these catalysts enable stereoselective transformations crucial for manufacturing complex active pharmaceutical ingredients (APIs). By improving reaction efficiency and reducing waste generation, Lewis acid catalysts have shortened production timelines and decreased manufacturing costs by an estimated 15-30% for certain drug classes, while simultaneously enhancing product quality and consistency.
In polymer manufacturing, Lewis acid catalysts have revolutionized production processes for polyolefins, polyesters, and specialty polymers. The economic impact is evident in the reduced catalyst loadings, milder reaction conditions, and improved polymer properties, collectively contributing to an estimated market value exceeding $5 billion annually for Lewis acid-catalyzed polymer products.
The fine chemicals industry leverages Lewis acid catalysis for producing flavors, fragrances, and specialty chemicals, where high selectivity and mild conditions translate to premium products with superior market positioning. Companies implementing advanced Lewis acid catalytic systems report productivity improvements of 20-40% and waste reduction of up to 50% compared to traditional methods.
From a macroeconomic perspective, Lewis acid catalyst innovations have strengthened competitive advantages for chemical manufacturers in regions with developed research infrastructure. Countries investing in catalyst research centers have experienced measurable growth in high-value chemical exports and intellectual property generation, creating specialized employment opportunities and supporting regional economic development.
The environmental economics of Lewis acid catalysis presents perhaps the most compelling case for continued investment. By enabling reactions under milder conditions with reduced waste streams, these catalysts align with circular economy principles and help companies meet increasingly stringent environmental regulations while maintaining profitability. Conservative estimates suggest that advanced Lewis acid catalytic processes reduce environmental compliance costs by 10-25% compared to conventional alternatives.
The pharmaceutical sector has particularly benefited from Lewis acid catalysis, where these catalysts enable stereoselective transformations crucial for manufacturing complex active pharmaceutical ingredients (APIs). By improving reaction efficiency and reducing waste generation, Lewis acid catalysts have shortened production timelines and decreased manufacturing costs by an estimated 15-30% for certain drug classes, while simultaneously enhancing product quality and consistency.
In polymer manufacturing, Lewis acid catalysts have revolutionized production processes for polyolefins, polyesters, and specialty polymers. The economic impact is evident in the reduced catalyst loadings, milder reaction conditions, and improved polymer properties, collectively contributing to an estimated market value exceeding $5 billion annually for Lewis acid-catalyzed polymer products.
The fine chemicals industry leverages Lewis acid catalysis for producing flavors, fragrances, and specialty chemicals, where high selectivity and mild conditions translate to premium products with superior market positioning. Companies implementing advanced Lewis acid catalytic systems report productivity improvements of 20-40% and waste reduction of up to 50% compared to traditional methods.
From a macroeconomic perspective, Lewis acid catalyst innovations have strengthened competitive advantages for chemical manufacturers in regions with developed research infrastructure. Countries investing in catalyst research centers have experienced measurable growth in high-value chemical exports and intellectual property generation, creating specialized employment opportunities and supporting regional economic development.
The environmental economics of Lewis acid catalysis presents perhaps the most compelling case for continued investment. By enabling reactions under milder conditions with reduced waste streams, these catalysts align with circular economy principles and help companies meet increasingly stringent environmental regulations while maintaining profitability. Conservative estimates suggest that advanced Lewis acid catalytic processes reduce environmental compliance costs by 10-25% compared to conventional alternatives.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!