Risks and Rewards in Hydrochloric Acid Industrial Use
JUL 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HCl Industrial Use Background and Objectives
Hydrochloric acid (HCl) has been a cornerstone in industrial processes for over a century, playing a crucial role in various sectors including chemical manufacturing, metal processing, and water treatment. The evolution of HCl usage in industry has been marked by significant technological advancements and a growing understanding of its properties and potential applications.
The primary objective of this technical research report is to comprehensively examine the risks and rewards associated with the industrial use of hydrochloric acid. This analysis aims to provide a balanced perspective on the benefits that HCl brings to various industrial processes, while also addressing the inherent challenges and potential hazards of its utilization.
Historically, the industrial production and use of HCl can be traced back to the early 19th century, with the development of the Leblanc process for soda ash production. Since then, its applications have expanded dramatically, driven by the growth of the chemical industry and the increasing demand for efficient and cost-effective industrial processes.
In recent years, the industrial use of HCl has been influenced by several key trends. These include the push for more sustainable and environmentally friendly production methods, the development of advanced materials resistant to HCl corrosion, and the implementation of stricter safety regulations governing the handling and storage of hazardous chemicals.
The technological evolution in HCl production and utilization has led to significant improvements in efficiency and safety. Modern production methods, such as the chlor-alkali process, have made HCl more readily available and economically viable for a wide range of industrial applications. Additionally, advancements in materials science have resulted in the development of corrosion-resistant equipment and containment systems, enabling safer handling and storage of HCl in industrial settings.
As we look towards the future, the industrial use of HCl is expected to continue evolving. Emerging technologies, such as membrane-based processes for HCl recovery and recycling, promise to further enhance the sustainability and economic viability of HCl usage. Moreover, ongoing research into alternative acid catalysts and process intensification techniques may lead to new applications and improved methodologies for HCl utilization in various industries.
This technical research report aims to provide a comprehensive overview of the current state of HCl industrial use, exploring both the opportunities it presents and the challenges that must be addressed. By examining the technological progress, market dynamics, and regulatory landscape surrounding HCl, we seek to offer valuable insights for industry stakeholders and decision-makers navigating the complex landscape of industrial chemical usage.
The primary objective of this technical research report is to comprehensively examine the risks and rewards associated with the industrial use of hydrochloric acid. This analysis aims to provide a balanced perspective on the benefits that HCl brings to various industrial processes, while also addressing the inherent challenges and potential hazards of its utilization.
Historically, the industrial production and use of HCl can be traced back to the early 19th century, with the development of the Leblanc process for soda ash production. Since then, its applications have expanded dramatically, driven by the growth of the chemical industry and the increasing demand for efficient and cost-effective industrial processes.
In recent years, the industrial use of HCl has been influenced by several key trends. These include the push for more sustainable and environmentally friendly production methods, the development of advanced materials resistant to HCl corrosion, and the implementation of stricter safety regulations governing the handling and storage of hazardous chemicals.
The technological evolution in HCl production and utilization has led to significant improvements in efficiency and safety. Modern production methods, such as the chlor-alkali process, have made HCl more readily available and economically viable for a wide range of industrial applications. Additionally, advancements in materials science have resulted in the development of corrosion-resistant equipment and containment systems, enabling safer handling and storage of HCl in industrial settings.
As we look towards the future, the industrial use of HCl is expected to continue evolving. Emerging technologies, such as membrane-based processes for HCl recovery and recycling, promise to further enhance the sustainability and economic viability of HCl usage. Moreover, ongoing research into alternative acid catalysts and process intensification techniques may lead to new applications and improved methodologies for HCl utilization in various industries.
This technical research report aims to provide a comprehensive overview of the current state of HCl industrial use, exploring both the opportunities it presents and the challenges that must be addressed. By examining the technological progress, market dynamics, and regulatory landscape surrounding HCl, we seek to offer valuable insights for industry stakeholders and decision-makers navigating the complex landscape of industrial chemical usage.
Market Demand Analysis for HCl Applications
The global market for hydrochloric acid (HCl) applications continues to expand, driven by diverse industrial needs and technological advancements. The demand for HCl is primarily fueled by its widespread use in various sectors, including chemical manufacturing, steel pickling, oil well acidizing, and water treatment.
In the chemical industry, HCl serves as a crucial raw material for producing numerous chemicals, such as vinyl chloride monomer (VCM), a key component in PVC manufacturing. The growing construction and automotive industries have significantly boosted PVC demand, consequently increasing the need for HCl. Additionally, the pharmaceutical sector relies heavily on HCl for drug synthesis and purification processes, contributing to market growth.
The steel industry remains a major consumer of HCl, utilizing it in pickling processes to remove rust and scale from steel surfaces. As global steel production continues to rise, particularly in emerging economies, the demand for HCl in this sector is expected to maintain a steady growth trajectory.
Oil and gas exploration activities have also contributed to the increasing demand for HCl. The acid is extensively used in well acidizing operations to enhance oil and gas recovery from reservoirs. With the ongoing exploration of unconventional oil and gas resources, such as shale gas, the demand for HCl in this sector is projected to grow further.
Water treatment applications represent another significant market for HCl. The acid is used in pH adjustment, water purification, and desalination processes. As global water scarcity concerns intensify and regulations on water quality become more stringent, the demand for HCl in water treatment is expected to rise.
The Asia-Pacific region dominates the global HCl market, with China being the largest producer and consumer. Rapid industrialization, urbanization, and infrastructure development in emerging economies like India and Southeast Asian countries are driving regional demand. North America and Europe also maintain substantial market shares, primarily due to their well-established chemical and pharmaceutical industries.
Despite the positive market outlook, environmental concerns and regulatory pressures pose challenges to HCl market growth. Stringent regulations on emissions and waste management have led to increased focus on developing eco-friendly alternatives and improving production processes to minimize environmental impact.
In conclusion, the market demand for HCl applications remains robust, supported by diverse industrial needs and technological advancements. However, industry players must navigate environmental challenges and regulatory landscapes to ensure sustainable growth in the coming years.
In the chemical industry, HCl serves as a crucial raw material for producing numerous chemicals, such as vinyl chloride monomer (VCM), a key component in PVC manufacturing. The growing construction and automotive industries have significantly boosted PVC demand, consequently increasing the need for HCl. Additionally, the pharmaceutical sector relies heavily on HCl for drug synthesis and purification processes, contributing to market growth.
The steel industry remains a major consumer of HCl, utilizing it in pickling processes to remove rust and scale from steel surfaces. As global steel production continues to rise, particularly in emerging economies, the demand for HCl in this sector is expected to maintain a steady growth trajectory.
Oil and gas exploration activities have also contributed to the increasing demand for HCl. The acid is extensively used in well acidizing operations to enhance oil and gas recovery from reservoirs. With the ongoing exploration of unconventional oil and gas resources, such as shale gas, the demand for HCl in this sector is projected to grow further.
Water treatment applications represent another significant market for HCl. The acid is used in pH adjustment, water purification, and desalination processes. As global water scarcity concerns intensify and regulations on water quality become more stringent, the demand for HCl in water treatment is expected to rise.
The Asia-Pacific region dominates the global HCl market, with China being the largest producer and consumer. Rapid industrialization, urbanization, and infrastructure development in emerging economies like India and Southeast Asian countries are driving regional demand. North America and Europe also maintain substantial market shares, primarily due to their well-established chemical and pharmaceutical industries.
Despite the positive market outlook, environmental concerns and regulatory pressures pose challenges to HCl market growth. Stringent regulations on emissions and waste management have led to increased focus on developing eco-friendly alternatives and improving production processes to minimize environmental impact.
In conclusion, the market demand for HCl applications remains robust, supported by diverse industrial needs and technological advancements. However, industry players must navigate environmental challenges and regulatory landscapes to ensure sustainable growth in the coming years.
Current Challenges in HCl Handling and Usage
The industrial use of hydrochloric acid (HCl) presents significant challenges in handling and usage due to its corrosive nature and potential health hazards. One of the primary concerns is the risk of chemical burns and respiratory issues for workers exposed to HCl vapors or liquid. Proper personal protective equipment (PPE) and rigorous safety protocols are essential, but maintaining consistent compliance across diverse industrial settings remains a persistent challenge.
Storage and transportation of HCl pose additional risks. The acid's corrosive properties necessitate specialized containment materials and equipment, which can be costly to implement and maintain. Leaks or spills during transport or storage can lead to environmental contamination and pose severe health risks to nearby communities. The development of more robust, cost-effective containment solutions is an ongoing challenge for the industry.
The disposal of HCl waste and byproducts presents another significant hurdle. Environmental regulations are becoming increasingly stringent, requiring industries to implement more sophisticated waste management and neutralization processes. This not only increases operational costs but also demands continuous innovation in treatment technologies to meet evolving environmental standards.
Corrosion of equipment and infrastructure is a pervasive issue in HCl-intensive industries. The acid's aggressive nature can lead to premature degradation of pipes, tanks, and processing equipment, necessitating frequent maintenance and replacement. This not only impacts operational efficiency but also poses safety risks if corrosion-induced failures occur. The development of more resistant materials and protective coatings remains an active area of research and development.
Process control and monitoring present additional challenges, particularly in maintaining the precise concentrations required for various industrial applications. Fluctuations in HCl concentration can affect product quality and process efficiency. Advanced sensing and control technologies are needed to ensure consistent acid strength and purity, especially in high-precision manufacturing processes.
The energy-intensive nature of HCl production and handling processes contributes to high operational costs and environmental concerns. Improving energy efficiency in acid production, concentration adjustment, and waste treatment processes is a key challenge that intersects with broader sustainability goals in the industrial sector.
Lastly, the regulatory landscape surrounding HCl use is complex and evolving. Industries must navigate a patchwork of local, national, and international regulations governing the production, transport, use, and disposal of HCl. Staying compliant with these regulations while maintaining operational efficiency and cost-effectiveness is an ongoing challenge that requires continuous adaptation and investment in compliance management systems.
Storage and transportation of HCl pose additional risks. The acid's corrosive properties necessitate specialized containment materials and equipment, which can be costly to implement and maintain. Leaks or spills during transport or storage can lead to environmental contamination and pose severe health risks to nearby communities. The development of more robust, cost-effective containment solutions is an ongoing challenge for the industry.
The disposal of HCl waste and byproducts presents another significant hurdle. Environmental regulations are becoming increasingly stringent, requiring industries to implement more sophisticated waste management and neutralization processes. This not only increases operational costs but also demands continuous innovation in treatment technologies to meet evolving environmental standards.
Corrosion of equipment and infrastructure is a pervasive issue in HCl-intensive industries. The acid's aggressive nature can lead to premature degradation of pipes, tanks, and processing equipment, necessitating frequent maintenance and replacement. This not only impacts operational efficiency but also poses safety risks if corrosion-induced failures occur. The development of more resistant materials and protective coatings remains an active area of research and development.
Process control and monitoring present additional challenges, particularly in maintaining the precise concentrations required for various industrial applications. Fluctuations in HCl concentration can affect product quality and process efficiency. Advanced sensing and control technologies are needed to ensure consistent acid strength and purity, especially in high-precision manufacturing processes.
The energy-intensive nature of HCl production and handling processes contributes to high operational costs and environmental concerns. Improving energy efficiency in acid production, concentration adjustment, and waste treatment processes is a key challenge that intersects with broader sustainability goals in the industrial sector.
Lastly, the regulatory landscape surrounding HCl use is complex and evolving. Industries must navigate a patchwork of local, national, and international regulations governing the production, transport, use, and disposal of HCl. Staying compliant with these regulations while maintaining operational efficiency and cost-effectiveness is an ongoing challenge that requires continuous adaptation and investment in compliance management systems.
Existing Safety Measures for HCl Industrial Use
01 Production and purification of hydrochloric acid
Various methods and processes for producing and purifying hydrochloric acid, including industrial-scale production techniques and purification steps to obtain high-quality acid for different applications.- Production methods of hydrochloric acid: Various methods are employed for the production of hydrochloric acid, including direct synthesis from hydrogen and chlorine, as a byproduct in chlorination processes, and through the reaction of sulfuric acid with sodium chloride. These methods are optimized for efficiency and purity in industrial settings.
- Purification and concentration techniques: Techniques for purifying and concentrating hydrochloric acid involve distillation, membrane separation, and adsorption processes. These methods aim to remove impurities and achieve desired concentration levels for various industrial applications.
- Applications in chemical processing: Hydrochloric acid is widely used in chemical processing, including metal treatment, pH regulation, and as a reagent in various chemical reactions. Its versatility makes it a crucial component in many industrial processes and manufacturing operations.
- Safety and handling considerations: Proper safety measures and handling procedures are essential when working with hydrochloric acid due to its corrosive nature. This includes the use of specialized equipment, protective gear, and storage solutions to minimize risks associated with its use and transportation.
- Environmental impact and waste management: Managing the environmental impact of hydrochloric acid production and use involves developing recycling methods, neutralization techniques, and proper disposal practices. These approaches aim to minimize the acid's effect on ecosystems and comply with environmental regulations.
02 Applications in chemical processing
Hydrochloric acid is widely used in chemical processing, including as a catalyst, reagent, or pH adjuster in various industrial processes such as metal treatment, water treatment, and pharmaceutical manufacturing.Expand Specific Solutions03 Safety and handling of hydrochloric acid
Equipment, methods, and systems for safely handling, storing, and transporting hydrochloric acid, including specialized containers, protective gear, and safety protocols to prevent accidents and exposure.Expand Specific Solutions04 Environmental impact and waste management
Techniques for managing hydrochloric acid waste, reducing environmental impact, and implementing recycling or neutralization processes to minimize the ecological footprint of industrial hydrochloric acid use.Expand Specific Solutions05 Analytical methods and quality control
Procedures and instruments for analyzing the concentration, purity, and composition of hydrochloric acid, as well as quality control measures to ensure consistent product specifications for various industrial and laboratory applications.Expand Specific Solutions
Key Players in HCl Production and Utilization
The industrial use of hydrochloric acid presents both risks and rewards in a mature market with steady growth. The global hydrochloric acid market is expected to reach $1.5 billion by 2025, driven by demand in various industries. Technologically, the production and handling of hydrochloric acid are well-established, with companies like Dorf Ketal Chemicals FZE and Fluid Energy Group Ltd. offering specialized solutions. However, environmental and safety concerns persist, prompting innovation in safer handling and waste management techniques. Firms such as Industrie De Nora SpA and Zhejiang Runtu New Materials Co. Ltd. are developing advanced technologies for acid recovery and recycling, addressing both economic and environmental challenges in the industry.
Dorf Ketal Chemicals FZE
Technical Solution: Dorf Ketal has developed innovative solutions for hydrochloric acid (HCl) management in industrial processes. Their approach focuses on minimizing corrosion risks while maximizing process efficiency. They have introduced a range of HCl inhibitors and neutralizers designed to protect equipment and pipelines in various industrial applications. Their technology includes specialized polymer-based formulations that form protective films on metal surfaces, significantly reducing corrosion rates even in high-temperature and high-pressure environments[1]. Additionally, they have developed eco-friendly HCl scavengers that effectively neutralize excess acid without introducing harmful by-products into the system[2].
Strengths: Highly effective corrosion protection, environmentally friendly solutions, and versatility across different industrial applications. Weaknesses: May require frequent reapplication in extreme conditions, and initial implementation costs could be higher than traditional methods.
Fluid Energy Group Ltd.
Technical Solution: Fluid Energy Group has pioneered a revolutionary approach to HCl alternatives with their Enviro-Syn Modified Acid™ technology. This innovation addresses both the risks and rewards of traditional HCl use in industries such as oil and gas, mining, and industrial cleaning. Their modified acid systems maintain the effectiveness of HCl while significantly reducing its hazardous properties. The technology involves altering the molecular structure of HCl to create a slower-reacting, less corrosive substance that still retains its acidic properties[3]. This results in reduced health and safety risks for workers, decreased environmental impact, and extended equipment lifespan. Furthermore, their products have shown improved performance in certain applications, such as enhanced penetration in carbonate formations for well stimulation[4].
Strengths: Significantly reduced health and safety risks, improved environmental profile, and potential for enhanced performance in specific applications. Weaknesses: May not be suitable for all HCl applications, and could require process modifications for implementation.
Innovations in HCl Containment and Transport
Using Synthetic Acid Compositions as Alternatives to Conventional Acids in The Oil And Gas Industry
PatentActiveUS20230086463A1
Innovation
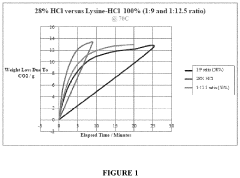
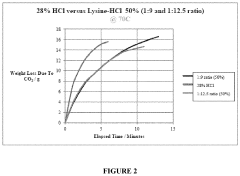
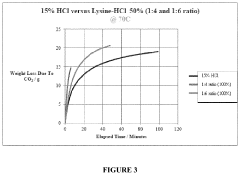
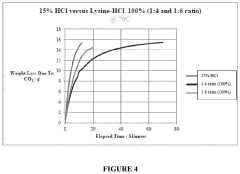
- An aqueous synthetic acid composition comprising lysine and hydrogen chloride in specific molar ratios, which provides low corrosion rates, biodegradability, controlled reaction rates, and thermal stability up to 220°C, reducing toxicity and environmental impact while maintaining the effectiveness of hydrochloric acid.
Synthetic acid compositions and uses thereof
PatentActiveCA2925142A1
Innovation
- A synthetic acid composition comprising urea and hydrogen chloride in a specific molar ratio, combined with metal iodides, alcohols, and phosphonic acids, which reduces corrosion rates, is non-fuming, non-toxic, and biodegradable, offering improved safety and environmental compatibility.
Environmental Impact of HCl Industrial Use
The industrial use of hydrochloric acid (HCl) has significant environmental implications that require careful consideration. HCl emissions from industrial processes can contribute to air pollution, particularly in the form of acid rain. When released into the atmosphere, HCl reacts with water vapor to form hydrochloric acid droplets, which can fall as precipitation and negatively impact ecosystems, water bodies, and infrastructure. This acidification can lead to the degradation of soil quality, harm aquatic life, and accelerate the corrosion of buildings and monuments.
Water pollution is another critical concern associated with HCl industrial use. Improper disposal or accidental spills of HCl can contaminate surface and groundwater sources. The high acidity of HCl can drastically alter the pH of water bodies, making them inhospitable for aquatic organisms and potentially rendering water sources unsuitable for human consumption or agricultural use. Furthermore, HCl can mobilize heavy metals in soil and sediments, leading to secondary contamination of water resources.
The production and transportation of HCl also pose environmental risks. Manufacturing processes often involve energy-intensive operations, contributing to greenhouse gas emissions and climate change. Transportation accidents involving HCl can result in localized environmental disasters, affecting soil, water, and air quality in the affected areas. Such incidents may require extensive remediation efforts and can have long-lasting ecological consequences.
However, it is important to note that the industrial use of HCl also offers environmental benefits when properly managed. HCl plays a crucial role in water treatment processes, helping to neutralize alkaline effluents and remove impurities from water supplies. It is also used in the production of various chemicals that contribute to environmental protection, such as water treatment chemicals and air pollution control agents.
To mitigate the environmental impact of HCl industrial use, stringent regulations and best practices have been implemented in many countries. These include emission control technologies, proper storage and handling protocols, and waste management strategies. Advanced scrubbing systems can effectively capture HCl emissions, while closed-loop processes and recycling initiatives help minimize waste and reduce the overall environmental footprint of HCl use in industry.
Ongoing research and development efforts are focused on developing more environmentally friendly alternatives to HCl in certain applications and improving the efficiency of HCl-based processes to reduce consumption and emissions. These advancements, coupled with responsible industrial practices, are essential for balancing the economic benefits of HCl use with environmental protection goals.
Water pollution is another critical concern associated with HCl industrial use. Improper disposal or accidental spills of HCl can contaminate surface and groundwater sources. The high acidity of HCl can drastically alter the pH of water bodies, making them inhospitable for aquatic organisms and potentially rendering water sources unsuitable for human consumption or agricultural use. Furthermore, HCl can mobilize heavy metals in soil and sediments, leading to secondary contamination of water resources.
The production and transportation of HCl also pose environmental risks. Manufacturing processes often involve energy-intensive operations, contributing to greenhouse gas emissions and climate change. Transportation accidents involving HCl can result in localized environmental disasters, affecting soil, water, and air quality in the affected areas. Such incidents may require extensive remediation efforts and can have long-lasting ecological consequences.
However, it is important to note that the industrial use of HCl also offers environmental benefits when properly managed. HCl plays a crucial role in water treatment processes, helping to neutralize alkaline effluents and remove impurities from water supplies. It is also used in the production of various chemicals that contribute to environmental protection, such as water treatment chemicals and air pollution control agents.
To mitigate the environmental impact of HCl industrial use, stringent regulations and best practices have been implemented in many countries. These include emission control technologies, proper storage and handling protocols, and waste management strategies. Advanced scrubbing systems can effectively capture HCl emissions, while closed-loop processes and recycling initiatives help minimize waste and reduce the overall environmental footprint of HCl use in industry.
Ongoing research and development efforts are focused on developing more environmentally friendly alternatives to HCl in certain applications and improving the efficiency of HCl-based processes to reduce consumption and emissions. These advancements, coupled with responsible industrial practices, are essential for balancing the economic benefits of HCl use with environmental protection goals.
Regulatory Framework for HCl in Industry
The regulatory framework for hydrochloric acid (HCl) in industry is complex and multifaceted, reflecting the substance's hazardous nature and widespread industrial applications. At the international level, the United Nations' Globally Harmonized System of Classification and Labelling of Chemicals (GHS) provides a foundation for standardizing HCl safety information and handling procedures across borders.
In the United States, the Occupational Safety and Health Administration (OSHA) sets stringent guidelines for HCl use in workplaces. These include permissible exposure limits (PELs), requirements for personal protective equipment (PPE), and protocols for emergency response. The Environmental Protection Agency (EPA) regulates HCl under various statutes, including the Clean Air Act and the Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA).
European regulations are governed by the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) framework, which mandates extensive documentation and risk assessment for HCl use. The Classification, Labelling and Packaging (CLP) Regulation aligns EU standards with the GHS, ensuring consistent hazard communication.
In Asia, countries like China and Japan have implemented their own regulatory systems. China's Regulations on Safe Management of Hazardous Chemicals cover HCl production, storage, and transportation. Japan's Industrial Safety and Health Law and Poisonous and Deleterious Substances Control Law provide comprehensive oversight of HCl handling and use.
Specific industry sectors face additional regulations. For instance, the semiconductor industry must adhere to ultra-high purity standards for HCl, as outlined in SEMI guidelines. The pharmaceutical industry is subject to Good Manufacturing Practice (GMP) regulations, which include specific provisions for HCl use in drug production.
Transportation of HCl is heavily regulated globally. The International Maritime Dangerous Goods (IMDG) Code governs sea transport, while air shipments are controlled by the International Air Transport Association's Dangerous Goods Regulations. Road and rail transport regulations vary by country but generally align with UN recommendations.
As environmental concerns grow, many jurisdictions are implementing stricter emissions controls on HCl. This includes mandating the use of scrubbers in industrial processes and setting increasingly stringent air quality standards. The trend towards circular economy principles is also influencing regulations, with a growing emphasis on HCl recycling and recovery in industrial processes.
In the United States, the Occupational Safety and Health Administration (OSHA) sets stringent guidelines for HCl use in workplaces. These include permissible exposure limits (PELs), requirements for personal protective equipment (PPE), and protocols for emergency response. The Environmental Protection Agency (EPA) regulates HCl under various statutes, including the Clean Air Act and the Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA).
European regulations are governed by the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) framework, which mandates extensive documentation and risk assessment for HCl use. The Classification, Labelling and Packaging (CLP) Regulation aligns EU standards with the GHS, ensuring consistent hazard communication.
In Asia, countries like China and Japan have implemented their own regulatory systems. China's Regulations on Safe Management of Hazardous Chemicals cover HCl production, storage, and transportation. Japan's Industrial Safety and Health Law and Poisonous and Deleterious Substances Control Law provide comprehensive oversight of HCl handling and use.
Specific industry sectors face additional regulations. For instance, the semiconductor industry must adhere to ultra-high purity standards for HCl, as outlined in SEMI guidelines. The pharmaceutical industry is subject to Good Manufacturing Practice (GMP) regulations, which include specific provisions for HCl use in drug production.
Transportation of HCl is heavily regulated globally. The International Maritime Dangerous Goods (IMDG) Code governs sea transport, while air shipments are controlled by the International Air Transport Association's Dangerous Goods Regulations. Road and rail transport regulations vary by country but generally align with UN recommendations.
As environmental concerns grow, many jurisdictions are implementing stricter emissions controls on HCl. This includes mandating the use of scrubbers in industrial processes and setting increasingly stringent air quality standards. The trend towards circular economy principles is also influencing regulations, with a growing emphasis on HCl recycling and recovery in industrial processes.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!