Role of Multi-Electron Transfer Pathways in Electrolytic Cells
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electrolytic Cell Evolution and Objectives
Electrolytic cells have undergone significant evolution since their inception in the late 18th century. The fundamental principle of using electrical energy to drive non-spontaneous chemical reactions has remained constant, but the efficiency, scale, and applications of these cells have dramatically expanded. Initially, electrolytic cells were primarily used for metal extraction and purification. However, as our understanding of electrochemistry advanced, their applications diversified into fields such as water treatment, chemical synthesis, and energy storage.
The evolution of electrolytic cells has been driven by the need for more efficient and sustainable industrial processes. Early cells suffered from high energy consumption and limited control over reaction conditions. Subsequent developments focused on improving electrode materials, optimizing cell design, and enhancing electrolyte compositions. These advancements led to increased reaction rates, improved selectivity, and reduced energy requirements.
A significant milestone in the evolution of electrolytic cells was the introduction of membrane technology in the mid-20th century. This innovation allowed for the separation of anodic and cathodic compartments, preventing unwanted side reactions and improving overall efficiency. The development of dimensionally stable anodes (DSA) in the 1960s marked another crucial advancement, particularly in the chlor-alkali industry, by dramatically extending electrode lifetimes and reducing energy consumption.
In recent years, the focus has shifted towards understanding and harnessing multi-electron transfer pathways in electrolytic cells. This approach aims to overcome the limitations of traditional single-electron transfer processes, potentially enabling more complex and efficient electrochemical transformations. The objective is to develop electrolytic systems capable of facilitating the simultaneous transfer of multiple electrons, which could revolutionize various industrial processes and open up new avenues for chemical synthesis and energy conversion.
The current objectives in electrolytic cell research and development are multifaceted. Firstly, there is a strong emphasis on improving energy efficiency to reduce the environmental impact and operational costs of electrolytic processes. This includes the development of novel electrode materials and catalysts that can lower overpotentials and increase reaction rates. Secondly, researchers are working on expanding the range of reactions that can be carried out in electrolytic cells, particularly focusing on the synthesis of complex organic molecules and the production of high-value chemicals.
Another key objective is the integration of renewable energy sources with electrolytic processes. This aims to create more sustainable industrial practices by utilizing excess renewable electricity for chemical production and energy storage. Additionally, there is growing interest in developing electrolytic cells for carbon dioxide reduction, which could play a crucial role in mitigating climate change by converting CO2 into useful chemicals or fuels.
The evolution of electrolytic cells has been driven by the need for more efficient and sustainable industrial processes. Early cells suffered from high energy consumption and limited control over reaction conditions. Subsequent developments focused on improving electrode materials, optimizing cell design, and enhancing electrolyte compositions. These advancements led to increased reaction rates, improved selectivity, and reduced energy requirements.
A significant milestone in the evolution of electrolytic cells was the introduction of membrane technology in the mid-20th century. This innovation allowed for the separation of anodic and cathodic compartments, preventing unwanted side reactions and improving overall efficiency. The development of dimensionally stable anodes (DSA) in the 1960s marked another crucial advancement, particularly in the chlor-alkali industry, by dramatically extending electrode lifetimes and reducing energy consumption.
In recent years, the focus has shifted towards understanding and harnessing multi-electron transfer pathways in electrolytic cells. This approach aims to overcome the limitations of traditional single-electron transfer processes, potentially enabling more complex and efficient electrochemical transformations. The objective is to develop electrolytic systems capable of facilitating the simultaneous transfer of multiple electrons, which could revolutionize various industrial processes and open up new avenues for chemical synthesis and energy conversion.
The current objectives in electrolytic cell research and development are multifaceted. Firstly, there is a strong emphasis on improving energy efficiency to reduce the environmental impact and operational costs of electrolytic processes. This includes the development of novel electrode materials and catalysts that can lower overpotentials and increase reaction rates. Secondly, researchers are working on expanding the range of reactions that can be carried out in electrolytic cells, particularly focusing on the synthesis of complex organic molecules and the production of high-value chemicals.
Another key objective is the integration of renewable energy sources with electrolytic processes. This aims to create more sustainable industrial practices by utilizing excess renewable electricity for chemical production and energy storage. Additionally, there is growing interest in developing electrolytic cells for carbon dioxide reduction, which could play a crucial role in mitigating climate change by converting CO2 into useful chemicals or fuels.
Industrial Demand Analysis
The industrial demand for multi-electron transfer pathways in electrolytic cells has been steadily growing across various sectors. This technology plays a crucial role in enhancing the efficiency and sustainability of electrochemical processes, which are fundamental to numerous industrial applications.
In the energy sector, there is a significant demand for improved electrolytic cells in hydrogen production. As the world shifts towards cleaner energy sources, hydrogen fuel cells are gaining traction. Multi-electron transfer pathways can potentially increase the efficiency of water electrolysis, making hydrogen production more economically viable and environmentally friendly.
The chemical industry also shows a strong interest in this technology. Multi-electron transfer pathways can optimize the production of various chemicals, including chlorine, sodium hydroxide, and aluminum. By improving the efficiency of these electrochemical processes, industries can reduce energy consumption and production costs while increasing output.
In the field of environmental remediation, there is a growing demand for advanced electrolytic cells. Multi-electron transfer pathways can enhance the effectiveness of wastewater treatment processes, particularly in the removal of persistent organic pollutants and heavy metals. This technology offers a promising solution for industries struggling with stringent environmental regulations.
The electronics industry is another sector driving demand for this technology. As electronic devices become more sophisticated, there is a need for more efficient and compact power sources. Multi-electron transfer pathways could lead to the development of improved batteries and supercapacitors, meeting the industry's demand for higher energy density and faster charging capabilities.
Emerging applications in biotechnology and pharmaceuticals are also contributing to the industrial demand. Electrolytic cells with enhanced multi-electron transfer capabilities could revolutionize biosensors and drug delivery systems, opening new avenues for medical diagnostics and treatments.
The automotive industry, particularly the electric vehicle (EV) sector, is showing increased interest in this technology. Improved multi-electron transfer pathways could lead to more efficient EV batteries, addressing range anxiety and charging time concerns that currently hinder widespread EV adoption.
As industries worldwide focus on sustainability and energy efficiency, the demand for technologies that can optimize electrochemical processes is expected to grow. Multi-electron transfer pathways in electrolytic cells offer a promising solution to meet these industrial needs, driving research and development efforts across multiple sectors.
In the energy sector, there is a significant demand for improved electrolytic cells in hydrogen production. As the world shifts towards cleaner energy sources, hydrogen fuel cells are gaining traction. Multi-electron transfer pathways can potentially increase the efficiency of water electrolysis, making hydrogen production more economically viable and environmentally friendly.
The chemical industry also shows a strong interest in this technology. Multi-electron transfer pathways can optimize the production of various chemicals, including chlorine, sodium hydroxide, and aluminum. By improving the efficiency of these electrochemical processes, industries can reduce energy consumption and production costs while increasing output.
In the field of environmental remediation, there is a growing demand for advanced electrolytic cells. Multi-electron transfer pathways can enhance the effectiveness of wastewater treatment processes, particularly in the removal of persistent organic pollutants and heavy metals. This technology offers a promising solution for industries struggling with stringent environmental regulations.
The electronics industry is another sector driving demand for this technology. As electronic devices become more sophisticated, there is a need for more efficient and compact power sources. Multi-electron transfer pathways could lead to the development of improved batteries and supercapacitors, meeting the industry's demand for higher energy density and faster charging capabilities.
Emerging applications in biotechnology and pharmaceuticals are also contributing to the industrial demand. Electrolytic cells with enhanced multi-electron transfer capabilities could revolutionize biosensors and drug delivery systems, opening new avenues for medical diagnostics and treatments.
The automotive industry, particularly the electric vehicle (EV) sector, is showing increased interest in this technology. Improved multi-electron transfer pathways could lead to more efficient EV batteries, addressing range anxiety and charging time concerns that currently hinder widespread EV adoption.
As industries worldwide focus on sustainability and energy efficiency, the demand for technologies that can optimize electrochemical processes is expected to grow. Multi-electron transfer pathways in electrolytic cells offer a promising solution to meet these industrial needs, driving research and development efforts across multiple sectors.
Multi-Electron Transfer Challenges
Multi-electron transfer processes in electrolytic cells present significant challenges that hinder the efficiency and applicability of these systems. One of the primary obstacles is the kinetic complexity associated with the transfer of multiple electrons. Unlike single-electron transfers, which are relatively straightforward, multi-electron processes often involve intermediate steps and complex reaction pathways. This complexity can lead to slower reaction rates and increased energy barriers, ultimately reducing the overall efficiency of the electrolytic cell.
Another major challenge is the stability of intermediates formed during multi-electron transfer reactions. These intermediates can be highly reactive and prone to side reactions, potentially leading to undesired products or degradation of the electrolyte or electrode materials. Managing the stability and reactivity of these intermediates is crucial for maintaining the longevity and performance of electrolytic cells.
The coordination of electron and proton transfers poses an additional challenge in multi-electron processes. Many electrochemical reactions involve the coupled transfer of electrons and protons, and synchronizing these transfers across multiple steps can be difficult. Mismatches in the timing or stoichiometry of electron and proton transfers can result in inefficient reactions or the formation of unwanted byproducts.
Electrode surface chemistry plays a critical role in multi-electron transfer reactions. The design and optimization of electrode materials that can facilitate the transfer of multiple electrons while maintaining stability and selectivity is a significant challenge. Factors such as surface area, catalytic activity, and electronic properties of the electrode material must be carefully considered to promote efficient multi-electron transfers.
Furthermore, the management of charge and mass transport in electrolytic cells becomes more complex with multi-electron processes. The movement of reactants to and products from the electrode surface, as well as the transport of electrons through the external circuit and ions through the electrolyte, must be carefully balanced to maintain optimal reaction conditions. This challenge is particularly pronounced in large-scale applications where transport limitations can significantly impact overall system performance.
Lastly, the thermodynamic considerations of multi-electron transfer reactions present unique challenges. The energetics of these processes can be highly dependent on the specific reaction pathway and the stability of intermediates. Optimizing the thermodynamics to favor the desired multi-electron transfer while minimizing competing side reactions requires careful control of reaction conditions and innovative catalyst design.
Another major challenge is the stability of intermediates formed during multi-electron transfer reactions. These intermediates can be highly reactive and prone to side reactions, potentially leading to undesired products or degradation of the electrolyte or electrode materials. Managing the stability and reactivity of these intermediates is crucial for maintaining the longevity and performance of electrolytic cells.
The coordination of electron and proton transfers poses an additional challenge in multi-electron processes. Many electrochemical reactions involve the coupled transfer of electrons and protons, and synchronizing these transfers across multiple steps can be difficult. Mismatches in the timing or stoichiometry of electron and proton transfers can result in inefficient reactions or the formation of unwanted byproducts.
Electrode surface chemistry plays a critical role in multi-electron transfer reactions. The design and optimization of electrode materials that can facilitate the transfer of multiple electrons while maintaining stability and selectivity is a significant challenge. Factors such as surface area, catalytic activity, and electronic properties of the electrode material must be carefully considered to promote efficient multi-electron transfers.
Furthermore, the management of charge and mass transport in electrolytic cells becomes more complex with multi-electron processes. The movement of reactants to and products from the electrode surface, as well as the transport of electrons through the external circuit and ions through the electrolyte, must be carefully balanced to maintain optimal reaction conditions. This challenge is particularly pronounced in large-scale applications where transport limitations can significantly impact overall system performance.
Lastly, the thermodynamic considerations of multi-electron transfer reactions present unique challenges. The energetics of these processes can be highly dependent on the specific reaction pathway and the stability of intermediates. Optimizing the thermodynamics to favor the desired multi-electron transfer while minimizing competing side reactions requires careful control of reaction conditions and innovative catalyst design.
Current Multi-Electron Solutions
01 Electron transfer in photovoltaic devices
Multi-electron transfer pathways play a crucial role in improving the efficiency of photovoltaic devices. These pathways involve the transfer of multiple electrons simultaneously, enhancing charge separation and reducing recombination losses. This approach can lead to increased power conversion efficiency in solar cells and other optoelectronic devices.- Electron transfer in photovoltaic devices: Multi-electron transfer pathways are crucial in photovoltaic devices, enhancing their efficiency and performance. These pathways involve the movement of electrons through various materials and interfaces, optimizing charge separation and collection. Advanced designs incorporate novel materials and structures to facilitate efficient electron transfer, improving overall solar cell performance.
- Electron transfer in electrochemical systems: Multi-electron transfer pathways play a significant role in electrochemical systems, such as batteries and fuel cells. These pathways involve complex redox reactions and charge transfer processes across electrodes and electrolytes. Optimizing these pathways can lead to improved energy storage capacity, faster charging rates, and enhanced overall system efficiency.
- Electron transfer in biological systems: Multi-electron transfer pathways are essential in biological systems, particularly in processes like photosynthesis and cellular respiration. These pathways involve intricate protein complexes and cofactors that facilitate the movement of electrons through various redox centers. Understanding and mimicking these natural processes can lead to advancements in artificial photosynthesis and bio-inspired energy conversion systems.
- Electron transfer in quantum systems: Multi-electron transfer pathways in quantum systems involve the coherent movement of electrons through various quantum states. These processes are relevant in fields such as quantum computing, spintronics, and advanced sensing technologies. Controlling and manipulating these pathways can lead to novel quantum devices with enhanced functionality and performance.
- Electron transfer in catalytic processes: Multi-electron transfer pathways are critical in various catalytic processes, including electrocatalysis and photocatalysis. These pathways involve the transfer of multiple electrons between catalysts, reactants, and products, enabling complex chemical transformations. Optimizing these pathways can lead to more efficient and selective catalytic systems for applications in energy conversion, environmental remediation, and chemical synthesis.
02 Electron transfer in electrochemical systems
Multi-electron transfer pathways are essential in electrochemical systems, such as batteries and fuel cells. These pathways enable more efficient energy storage and conversion processes by facilitating the transfer of multiple electrons in a single step. This can lead to improved performance, higher energy density, and faster charging/discharging rates in electrochemical devices.Expand Specific Solutions03 Electron transfer in biological systems
Multi-electron transfer pathways are crucial in biological systems, particularly in processes such as photosynthesis and cellular respiration. These pathways involve complex protein structures that facilitate the transfer of multiple electrons, enabling efficient energy conversion and storage in living organisms. Understanding these pathways can lead to advancements in biomimetic technologies and artificial photosynthesis.Expand Specific Solutions04 Electron transfer in catalytic reactions
Multi-electron transfer pathways play a significant role in catalytic reactions, particularly in redox processes. These pathways can enhance the efficiency and selectivity of catalytic reactions by enabling the simultaneous transfer of multiple electrons. This approach is particularly important in the development of new catalysts for energy conversion, environmental remediation, and chemical synthesis applications.Expand Specific Solutions05 Electron transfer in quantum systems
Multi-electron transfer pathways are studied in quantum systems to understand and manipulate electron behavior at the nanoscale. These pathways involve quantum mechanical effects and can be utilized in the development of quantum computing devices, single-electron transistors, and other advanced electronic systems. Research in this area focuses on controlling and optimizing electron transfer processes for improved device performance and functionality.Expand Specific Solutions
Key Industry Players
The multi-electron transfer pathways in electrolytic cells represent a complex and evolving technological landscape. The industry is in a growth phase, with increasing market size driven by the rising demand for clean energy solutions. The technology's maturity is advancing rapidly, with companies like Robert Bosch GmbH, Intelligent Energy Ltd., and Palo Alto Research Center LLC leading research efforts. Major automotive players such as GM Global Technology Operations LLC and Hyundai Motor Co., Ltd. are also investing heavily in this field, indicating its potential for widespread application in the transportation sector. The involvement of diverse players, from established corporations to innovative startups like Electric Hydrogen Co., suggests a competitive and dynamic market environment poised for significant breakthroughs in efficiency and scalability.
Robert Bosch GmbH
Technical Solution: Robert Bosch GmbH has developed advanced electrolytic cell technologies focusing on multi-electron transfer pathways. Their approach involves the use of novel catalyst materials and electrode designs to facilitate efficient multi-electron transfer processes. The company has implemented a nanostructured electrode architecture that increases the active surface area and promotes faster electron transfer kinetics[1]. Additionally, they have developed proprietary electrolyte formulations that enhance the stability and conductivity of the electrolytic system, allowing for improved multi-electron transfer efficiency[3]. Bosch's technology also incorporates advanced control systems that optimize the electron transfer pathways based on real-time monitoring of cell conditions[5].
Strengths: High efficiency due to optimized electron transfer, scalable technology suitable for various applications. Weaknesses: Potentially high production costs, may require specialized maintenance.
Palo Alto Research Center LLC
Technical Solution: Palo Alto Research Center (PARC) has pioneered innovative approaches to multi-electron transfer in electrolytic cells. Their technology focuses on the development of novel electrode materials with tailored electronic structures to facilitate multi-electron transfer processes. PARC has engineered composite electrodes incorporating graphene-based materials and metal nanoparticles, which exhibit enhanced catalytic activity and electron transfer capabilities[2]. They have also developed advanced computational models to simulate and optimize multi-electron transfer pathways, leading to more efficient electrolytic cell designs[4]. Furthermore, PARC's research includes the integration of redox mediators to promote faster and more efficient multi-electron transfer between electrodes and reactants[6].
Strengths: Cutting-edge materials science, strong computational modeling capabilities. Weaknesses: Technology may be in early stages of development, potential scalability challenges.
Innovative Transfer Mechanisms
Multi-electron beam source with a cut off circuit and image device using the same
PatentInactiveUS5627436A
Innovation
- A multi-electron beam source with a matrix-like arrangement of electron-emitting elements, incorporating modulation or focusing electrodes that apply a cutoff voltage to prevent electron beam emission during spike-like voltage occurrences, effectively cutting off unwanted beams and maintaining image contrast.
Multi-electron beam image acquisition apparatus, and multi-electron beam image acquisition method
PatentPendingUS20230077403A1
Innovation
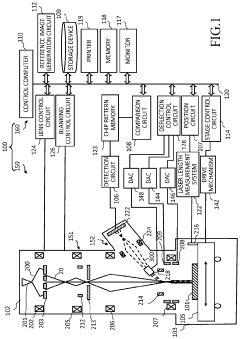
- A multi-electron beam image acquisition apparatus and method that includes a beam separator configured to form a perpendicular electric and magnetic field at a position conjugate to the image plane of primary electron beams, separating and refracting secondary electron beams in a converging direction to prevent spreading and aberration, and directing them to a multi-detector for accurate detection.
Electrochemical Efficiency Metrics
Electrochemical efficiency metrics play a crucial role in evaluating the performance of multi-electron transfer pathways in electrolytic cells. These metrics provide quantitative measures to assess the effectiveness of electron transfer processes and the overall efficiency of electrochemical systems.
One of the primary metrics used in this context is the Faradaic efficiency, which quantifies the percentage of electrical charge utilized for the desired electrochemical reaction. In multi-electron transfer systems, this metric becomes particularly important as it helps identify the extent to which electrons are directed towards the intended reaction pathway versus competing side reactions.
Another key metric is the energy efficiency, which relates the useful energy output to the total energy input in the electrolytic cell. This metric is essential for understanding the overall performance of the system and its potential for practical applications. In multi-electron transfer pathways, energy efficiency can be significantly impacted by the complexity of the electron transfer processes and the presence of rate-limiting steps.
The current efficiency is also a vital metric, measuring the ratio of the actual current used for the desired reaction to the total current applied. This metric is particularly relevant in multi-electron transfer systems, where current can be diverted to undesired side reactions or lost through other mechanisms.
Overpotential is another critical metric that quantifies the additional potential required to drive an electrochemical reaction beyond its thermodynamic equilibrium potential. In multi-electron transfer pathways, minimizing overpotential is crucial for improving overall system efficiency and reducing energy losses.
The turnover frequency (TOF) and turnover number (TON) are metrics that provide insights into the catalytic activity and stability of the electrodes involved in multi-electron transfer processes. These metrics are particularly useful for comparing different catalyst systems and optimizing electrode materials for enhanced performance.
Mass transport limitations can significantly impact the efficiency of multi-electron transfer pathways. Metrics such as the mass transfer coefficient and limiting current density help quantify these effects and guide the design of more efficient electrolytic cell configurations.
Lastly, the charge transfer resistance, derived from electrochemical impedance spectroscopy, offers valuable information about the kinetics of electron transfer at the electrode-electrolyte interface. This metric is crucial for understanding and optimizing the fundamental processes governing multi-electron transfer pathways in electrolytic cells.
One of the primary metrics used in this context is the Faradaic efficiency, which quantifies the percentage of electrical charge utilized for the desired electrochemical reaction. In multi-electron transfer systems, this metric becomes particularly important as it helps identify the extent to which electrons are directed towards the intended reaction pathway versus competing side reactions.
Another key metric is the energy efficiency, which relates the useful energy output to the total energy input in the electrolytic cell. This metric is essential for understanding the overall performance of the system and its potential for practical applications. In multi-electron transfer pathways, energy efficiency can be significantly impacted by the complexity of the electron transfer processes and the presence of rate-limiting steps.
The current efficiency is also a vital metric, measuring the ratio of the actual current used for the desired reaction to the total current applied. This metric is particularly relevant in multi-electron transfer systems, where current can be diverted to undesired side reactions or lost through other mechanisms.
Overpotential is another critical metric that quantifies the additional potential required to drive an electrochemical reaction beyond its thermodynamic equilibrium potential. In multi-electron transfer pathways, minimizing overpotential is crucial for improving overall system efficiency and reducing energy losses.
The turnover frequency (TOF) and turnover number (TON) are metrics that provide insights into the catalytic activity and stability of the electrodes involved in multi-electron transfer processes. These metrics are particularly useful for comparing different catalyst systems and optimizing electrode materials for enhanced performance.
Mass transport limitations can significantly impact the efficiency of multi-electron transfer pathways. Metrics such as the mass transfer coefficient and limiting current density help quantify these effects and guide the design of more efficient electrolytic cell configurations.
Lastly, the charge transfer resistance, derived from electrochemical impedance spectroscopy, offers valuable information about the kinetics of electron transfer at the electrode-electrolyte interface. This metric is crucial for understanding and optimizing the fundamental processes governing multi-electron transfer pathways in electrolytic cells.
Environmental Impact Assessment
The environmental impact of multi-electron transfer pathways in electrolytic cells is a critical consideration in the development and implementation of these technologies. These pathways have the potential to significantly reduce energy consumption and improve the efficiency of electrochemical processes, leading to a decrease in overall environmental footprint.
One of the primary environmental benefits of multi-electron transfer pathways is the reduction in energy requirements for electrolytic processes. By enabling the transfer of multiple electrons simultaneously, these pathways can lower the overpotential needed for reactions, resulting in decreased electricity consumption. This reduction in energy demand translates to lower greenhouse gas emissions associated with power generation, particularly in regions where fossil fuels remain a significant source of electricity.
Furthermore, the increased efficiency of multi-electron transfer pathways can lead to a reduction in the use of raw materials and resources. By improving the yield and selectivity of electrochemical reactions, these pathways minimize waste production and decrease the need for extensive purification processes. This not only conserves valuable resources but also reduces the environmental impact associated with waste disposal and treatment.
The implementation of multi-electron transfer pathways in electrolytic cells can also contribute to the development of more sustainable industrial processes. For instance, in the production of chemicals and materials, these pathways can enable the use of renewable feedstocks and promote the principles of green chemistry. This shift towards more environmentally friendly production methods can help mitigate the negative impacts of traditional chemical manufacturing on ecosystems and human health.
However, it is essential to consider potential environmental challenges associated with the adoption of multi-electron transfer technologies. The development and production of advanced catalysts and electrode materials required for these pathways may involve the use of rare or precious metals, which can have their own environmental implications in terms of mining and processing. Additionally, the disposal or recycling of these materials at the end of their lifecycle must be carefully managed to prevent environmental contamination.
In conclusion, the role of multi-electron transfer pathways in electrolytic cells presents significant opportunities for environmental improvement through reduced energy consumption, increased resource efficiency, and the promotion of sustainable industrial practices. However, a comprehensive life cycle assessment is necessary to fully understand and optimize the environmental impact of these technologies, ensuring that their implementation aligns with broader sustainability goals.
One of the primary environmental benefits of multi-electron transfer pathways is the reduction in energy requirements for electrolytic processes. By enabling the transfer of multiple electrons simultaneously, these pathways can lower the overpotential needed for reactions, resulting in decreased electricity consumption. This reduction in energy demand translates to lower greenhouse gas emissions associated with power generation, particularly in regions where fossil fuels remain a significant source of electricity.
Furthermore, the increased efficiency of multi-electron transfer pathways can lead to a reduction in the use of raw materials and resources. By improving the yield and selectivity of electrochemical reactions, these pathways minimize waste production and decrease the need for extensive purification processes. This not only conserves valuable resources but also reduces the environmental impact associated with waste disposal and treatment.
The implementation of multi-electron transfer pathways in electrolytic cells can also contribute to the development of more sustainable industrial processes. For instance, in the production of chemicals and materials, these pathways can enable the use of renewable feedstocks and promote the principles of green chemistry. This shift towards more environmentally friendly production methods can help mitigate the negative impacts of traditional chemical manufacturing on ecosystems and human health.
However, it is essential to consider potential environmental challenges associated with the adoption of multi-electron transfer technologies. The development and production of advanced catalysts and electrode materials required for these pathways may involve the use of rare or precious metals, which can have their own environmental implications in terms of mining and processing. Additionally, the disposal or recycling of these materials at the end of their lifecycle must be carefully managed to prevent environmental contamination.
In conclusion, the role of multi-electron transfer pathways in electrolytic cells presents significant opportunities for environmental improvement through reduced energy consumption, increased resource efficiency, and the promotion of sustainable industrial practices. However, a comprehensive life cycle assessment is necessary to fully understand and optimize the environmental impact of these technologies, ensuring that their implementation aligns with broader sustainability goals.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!