Single-Atom Catalysis Impact on Environmental Regulations
OCT 15, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Single-Atom Catalysis Background and Objectives
Single-atom catalysis (SAC) represents a revolutionary frontier in heterogeneous catalysis that has emerged over the past decade. This innovative approach utilizes isolated metal atoms dispersed on suitable supports to achieve maximum atomic efficiency while delivering exceptional catalytic performance. The concept was first formally introduced in 2011, though earlier studies had observed similar phenomena without explicitly defining the field.
The evolution of SAC technology has been driven by advances in materials science, nanotechnology, and characterization techniques. Early catalytic systems suffered from metal atom aggregation and limited stability under reaction conditions. However, recent breakthroughs in synthetic methodologies and support engineering have significantly enhanced the durability and performance of single-atom catalysts.
Current research indicates that SACs offer unique advantages in environmental applications due to their 100% atom utilization, distinctive electronic properties, and superior selectivity compared to conventional nanoparticle catalysts. These characteristics make them particularly promising for addressing stringent environmental regulations through more efficient pollution control technologies.
The primary objective of SAC development in the environmental regulatory context is to create economically viable catalytic systems that can meet or exceed increasingly strict emission standards while reducing precious metal consumption. This aligns with global sustainability goals and circular economy principles by minimizing resource utilization while maximizing environmental protection capabilities.
Technical goals include developing scalable synthesis methods for industrial production, enhancing catalyst stability under real-world operating conditions, and optimizing performance for specific environmental applications such as automotive emissions control, volatile organic compound (VOC) abatement, and water purification processes.
The trajectory of SAC technology shows a clear trend toward multifunctional catalytic systems capable of addressing complex environmental challenges through tandem reactions. This evolution is particularly relevant as environmental regulations increasingly target multiple pollutants simultaneously rather than addressing them in isolation.
Recent advancements in computational modeling and in-situ characterization techniques have accelerated understanding of SAC reaction mechanisms, enabling more rational design approaches. This shift from empirical to knowledge-based development represents a significant inflection point in the technology's maturation curve.
As environmental regulations continue to tighten globally, particularly regarding greenhouse gas emissions and air quality standards, SAC technology stands at a critical juncture where fundamental research must rapidly translate into practical applications to meet regulatory-driven market demands.
The evolution of SAC technology has been driven by advances in materials science, nanotechnology, and characterization techniques. Early catalytic systems suffered from metal atom aggregation and limited stability under reaction conditions. However, recent breakthroughs in synthetic methodologies and support engineering have significantly enhanced the durability and performance of single-atom catalysts.
Current research indicates that SACs offer unique advantages in environmental applications due to their 100% atom utilization, distinctive electronic properties, and superior selectivity compared to conventional nanoparticle catalysts. These characteristics make them particularly promising for addressing stringent environmental regulations through more efficient pollution control technologies.
The primary objective of SAC development in the environmental regulatory context is to create economically viable catalytic systems that can meet or exceed increasingly strict emission standards while reducing precious metal consumption. This aligns with global sustainability goals and circular economy principles by minimizing resource utilization while maximizing environmental protection capabilities.
Technical goals include developing scalable synthesis methods for industrial production, enhancing catalyst stability under real-world operating conditions, and optimizing performance for specific environmental applications such as automotive emissions control, volatile organic compound (VOC) abatement, and water purification processes.
The trajectory of SAC technology shows a clear trend toward multifunctional catalytic systems capable of addressing complex environmental challenges through tandem reactions. This evolution is particularly relevant as environmental regulations increasingly target multiple pollutants simultaneously rather than addressing them in isolation.
Recent advancements in computational modeling and in-situ characterization techniques have accelerated understanding of SAC reaction mechanisms, enabling more rational design approaches. This shift from empirical to knowledge-based development represents a significant inflection point in the technology's maturation curve.
As environmental regulations continue to tighten globally, particularly regarding greenhouse gas emissions and air quality standards, SAC technology stands at a critical juncture where fundamental research must rapidly translate into practical applications to meet regulatory-driven market demands.
Market Demand Analysis for Green Catalytic Technologies
The global market for green catalytic technologies is experiencing unprecedented growth, driven by increasingly stringent environmental regulations and the urgent need to address climate change. Single-atom catalysis (SAC) represents a revolutionary approach that aligns perfectly with these market demands, offering superior catalytic efficiency with minimal material usage. Current market projections indicate the green catalysis sector will reach $12.1 billion by 2026, growing at a CAGR of 9.8% from 2021, with SAC technologies positioned to capture a significant portion of this expansion.
Industrial sectors including automotive, chemical manufacturing, and energy production demonstrate particularly strong demand for SAC solutions. The automotive industry faces intensifying pressure to reduce emissions, with catalytic converter technologies requiring more efficient and less resource-intensive solutions. SAC offers up to 95% higher atom efficiency compared to traditional catalysts, directly addressing this need while reducing dependency on precious metals like platinum and palladium.
Chemical manufacturing represents another substantial market segment, with companies actively seeking catalytic processes that minimize waste production and energy consumption. Market research indicates that approximately 78% of chemical manufacturers have sustainability targets that explicitly include adoption of greener catalytic technologies by 2030, creating a robust demand pipeline for SAC innovations.
The renewable energy sector presents perhaps the most promising growth vector, particularly in hydrogen production and fuel cell technologies. Green hydrogen production via water electrolysis requires highly efficient catalysts, and SAC technologies have demonstrated potential to reduce catalyst loading by up to 70% while maintaining or improving performance metrics. This translates to significant cost reductions in an industry where catalyst expenses can represent 30-40% of system costs.
Regional analysis reveals Asia-Pacific as the fastest-growing market for green catalytic technologies, with China investing heavily in research and commercialization of advanced catalytic solutions including SAC. European markets show strong demand driven by the European Green Deal and circular economy initiatives, while North American demand is increasingly influenced by corporate sustainability commitments and anticipated regulatory changes.
End-user surveys indicate willingness to pay premium prices for catalytic technologies that deliver demonstrable environmental benefits alongside economic advantages. This price tolerance is particularly evident in sectors facing carbon taxation or emissions trading schemes, where the total cost of ownership calculation increasingly favors advanced catalytic solutions like SAC.
The market trajectory suggests a transition period of approximately 5-7 years during which SAC technologies will move from early adoption to mainstream implementation across multiple industries, contingent upon continued advances in scalable manufacturing techniques and performance validation in real-world applications.
Industrial sectors including automotive, chemical manufacturing, and energy production demonstrate particularly strong demand for SAC solutions. The automotive industry faces intensifying pressure to reduce emissions, with catalytic converter technologies requiring more efficient and less resource-intensive solutions. SAC offers up to 95% higher atom efficiency compared to traditional catalysts, directly addressing this need while reducing dependency on precious metals like platinum and palladium.
Chemical manufacturing represents another substantial market segment, with companies actively seeking catalytic processes that minimize waste production and energy consumption. Market research indicates that approximately 78% of chemical manufacturers have sustainability targets that explicitly include adoption of greener catalytic technologies by 2030, creating a robust demand pipeline for SAC innovations.
The renewable energy sector presents perhaps the most promising growth vector, particularly in hydrogen production and fuel cell technologies. Green hydrogen production via water electrolysis requires highly efficient catalysts, and SAC technologies have demonstrated potential to reduce catalyst loading by up to 70% while maintaining or improving performance metrics. This translates to significant cost reductions in an industry where catalyst expenses can represent 30-40% of system costs.
Regional analysis reveals Asia-Pacific as the fastest-growing market for green catalytic technologies, with China investing heavily in research and commercialization of advanced catalytic solutions including SAC. European markets show strong demand driven by the European Green Deal and circular economy initiatives, while North American demand is increasingly influenced by corporate sustainability commitments and anticipated regulatory changes.
End-user surveys indicate willingness to pay premium prices for catalytic technologies that deliver demonstrable environmental benefits alongside economic advantages. This price tolerance is particularly evident in sectors facing carbon taxation or emissions trading schemes, where the total cost of ownership calculation increasingly favors advanced catalytic solutions like SAC.
The market trajectory suggests a transition period of approximately 5-7 years during which SAC technologies will move from early adoption to mainstream implementation across multiple industries, contingent upon continued advances in scalable manufacturing techniques and performance validation in real-world applications.
Current Status and Challenges in Single-Atom Catalysis
Single-atom catalysis (SAC) represents a frontier in heterogeneous catalysis research, with significant progress achieved globally over the past decade. Currently, researchers have successfully synthesized various single-atom catalysts using noble metals (Pt, Pd, Au) and transition metals (Fe, Co, Ni) on diverse supports including metal oxides, carbon materials, and MOFs. These catalysts demonstrate exceptional activity and selectivity in numerous environmental applications, particularly in emissions control and pollutant degradation.
Despite impressive advancements, several critical challenges impede the widespread industrial implementation of SAC technology. Stability remains a primary concern, as single atoms tend to aggregate under reaction conditions, especially at elevated temperatures, diminishing catalytic performance over time. This instability significantly limits practical applications in industrial settings where long-term operation is essential.
The scalable synthesis of single-atom catalysts presents another substantial hurdle. While laboratory-scale preparation methods have been established, translating these processes to industrial production volumes while maintaining uniform dispersion of single atoms proves exceptionally difficult. Current synthesis approaches often yield low metal loadings (typically <1 wt%), insufficient for many commercial applications.
Mechanistic understanding of SAC systems remains incomplete, particularly regarding the complex interactions between single atoms and their support materials. The electronic structure and coordination environment of isolated metal atoms significantly influence catalytic behavior, yet these relationships are not fully characterized across different reaction environments and conditions.
From a geographical perspective, research leadership in SAC technology shows distinct patterns. China has emerged as the dominant force, accounting for approximately 45% of publications in this field, followed by the United States (20%) and various European countries (collectively 25%). This distribution reflects significant government investment in advanced catalysis research, particularly in Asia.
Cost considerations present additional barriers, as many effective single-atom catalysts rely on precious metals with limited availability and high market prices. Although SAC utilizes metal atoms more efficiently than traditional nanoparticle catalysts, the complex synthesis procedures often offset these material savings through increased production expenses.
Regulatory frameworks worldwide are beginning to acknowledge SAC potential, particularly for addressing stringent emissions standards. However, the lack of standardized testing protocols specifically designed for single-atom catalysts complicates regulatory approval processes and technology validation across different jurisdictions.
Despite impressive advancements, several critical challenges impede the widespread industrial implementation of SAC technology. Stability remains a primary concern, as single atoms tend to aggregate under reaction conditions, especially at elevated temperatures, diminishing catalytic performance over time. This instability significantly limits practical applications in industrial settings where long-term operation is essential.
The scalable synthesis of single-atom catalysts presents another substantial hurdle. While laboratory-scale preparation methods have been established, translating these processes to industrial production volumes while maintaining uniform dispersion of single atoms proves exceptionally difficult. Current synthesis approaches often yield low metal loadings (typically <1 wt%), insufficient for many commercial applications.
Mechanistic understanding of SAC systems remains incomplete, particularly regarding the complex interactions between single atoms and their support materials. The electronic structure and coordination environment of isolated metal atoms significantly influence catalytic behavior, yet these relationships are not fully characterized across different reaction environments and conditions.
From a geographical perspective, research leadership in SAC technology shows distinct patterns. China has emerged as the dominant force, accounting for approximately 45% of publications in this field, followed by the United States (20%) and various European countries (collectively 25%). This distribution reflects significant government investment in advanced catalysis research, particularly in Asia.
Cost considerations present additional barriers, as many effective single-atom catalysts rely on precious metals with limited availability and high market prices. Although SAC utilizes metal atoms more efficiently than traditional nanoparticle catalysts, the complex synthesis procedures often offset these material savings through increased production expenses.
Regulatory frameworks worldwide are beginning to acknowledge SAC potential, particularly for addressing stringent emissions standards. However, the lack of standardized testing protocols specifically designed for single-atom catalysts complicates regulatory approval processes and technology validation across different jurisdictions.
Current Technical Solutions in Single-Atom Catalysis
01 Metal single-atom catalysts for electrochemical applications
Single-atom catalysts featuring isolated metal atoms anchored on various supports demonstrate exceptional electrochemical performance. These catalysts offer maximized atom efficiency and unique electronic properties for applications such as hydrogen evolution, oxygen reduction, and CO2 reduction reactions. The atomically dispersed active sites provide enhanced catalytic activity and selectivity compared to traditional nanoparticle catalysts, while requiring less precious metal content.- Metal-based single-atom catalysts: Metal-based single-atom catalysts represent a significant advancement in catalysis technology, where individual metal atoms are dispersed on support materials. These catalysts offer maximum atom efficiency and unique catalytic properties due to their isolated nature. The metal atoms, typically transition metals, are anchored to supports like carbon, metal oxides, or 2D materials, creating distinct active sites with enhanced selectivity and activity compared to traditional nanoparticle catalysts.
- Support materials for single-atom catalysts: The choice of support material plays a crucial role in stabilizing single atoms and influencing their catalytic performance. Various supports including carbon-based materials (graphene, carbon nanotubes), metal oxides (TiO2, ZnO, CeO2), zeolites, and metal-organic frameworks (MOFs) are used to anchor single atoms. These supports prevent aggregation of metal atoms while providing electronic interactions that can tune the catalytic properties of the single atoms for specific reactions.
- Synthesis methods for single-atom catalysts: Various synthesis approaches have been developed to prepare single-atom catalysts with high metal dispersion and stability. These include atomic layer deposition, wet chemistry methods (impregnation, co-precipitation), high-temperature atom trapping, photochemical reduction, and electrochemical deposition. Advanced techniques like defect engineering and coordination design are employed to create stable metal-support interactions that prevent atom aggregation during catalytic reactions.
- Applications in energy conversion and environmental remediation: Single-atom catalysts demonstrate exceptional performance in energy-related applications and environmental remediation processes. They are employed in electrocatalytic reactions like hydrogen evolution, oxygen reduction/evolution, and CO2 reduction. In environmental applications, they show high efficiency for pollutant degradation and conversion of harmful gases. Their high atom utilization efficiency makes them particularly valuable for precious metal catalysis in sustainable energy systems.
- Characterization and theoretical modeling of single-atom catalysts: Advanced characterization techniques and theoretical modeling are essential for understanding single-atom catalysts. Methods such as aberration-corrected electron microscopy, X-ray absorption spectroscopy, and scanning tunneling microscopy are used to confirm the atomic dispersion and coordination environment. Density functional theory calculations and molecular dynamics simulations help elucidate reaction mechanisms, predict catalytic performance, and guide rational design of more efficient single-atom catalytic systems.
02 Carbon-based supports for single-atom catalysts
Carbon materials serve as excellent supports for anchoring single metal atoms in catalysis applications. These supports include graphene, carbon nanotubes, porous carbon, and carbon nitride materials that provide high surface area and abundant coordination sites. The strong metal-support interactions stabilize the isolated metal atoms against aggregation while modifying their electronic structure to enhance catalytic performance for various reactions including oxidation, hydrogenation, and electrocatalysis.Expand Specific Solutions03 Synthesis methods for single-atom catalysts
Various synthesis strategies have been developed to prepare stable single-atom catalysts with high metal loadings. These methods include atomic layer deposition, wet chemistry approaches, high-temperature atom trapping, and defect engineering of support materials. Advanced techniques like coordination-assisted immobilization and spatial confinement help achieve uniform dispersion of isolated metal atoms while preventing aggregation during catalytic reactions, resulting in more efficient and durable catalytic systems.Expand Specific Solutions04 Single-atom catalysts for environmental applications
Single-atom catalysts show remarkable performance in environmental remediation processes including pollutant degradation, CO2 conversion, and clean energy production. Their atomically dispersed active sites enable efficient activation of small molecules like O2, H2, and CO2 under mild conditions. These catalysts demonstrate superior activity for NOx reduction, volatile organic compound removal, and water purification while operating at lower temperatures and with greater stability than conventional catalysts.Expand Specific Solutions05 Characterization and theoretical modeling of single-atom catalysts
Advanced characterization techniques and theoretical modeling approaches are essential for understanding the structure-performance relationships in single-atom catalysts. Methods such as aberration-corrected electron microscopy, X-ray absorption spectroscopy, and scanning tunneling microscopy provide atomic-level insights into coordination environments and electronic structures. Density functional theory calculations help elucidate reaction mechanisms, predict catalytic behavior, and guide rational design of more efficient single-atom catalytic systems.Expand Specific Solutions
Key Industry Players in Single-Atom Catalyst Development
Single-atom catalysis is emerging as a transformative technology in environmental regulation compliance, currently in its early growth phase. The market is expanding rapidly, projected to reach significant scale as industries seek more efficient catalytic solutions for emissions reduction. Technologically, it sits at the intersection of mature development and commercial application, with key players demonstrating varying levels of advancement. Academic institutions like Guangdong University of Technology, Shenzhen University, and Texas A&M University are driving fundamental research, while companies such as SK Innovation and Beijing Single Atom Site Catalysis Technology are commercializing applications. Research organizations including Institute of Process Engineering (CAS) and KIST Corp. are bridging the gap between theoretical work and industrial implementation, positioning single-atom catalysis as a critical technology for meeting increasingly stringent environmental standards.
Institute For Basic Science
Technical Solution: The Institute For Basic Science (IBS) has developed groundbreaking single-atom catalyst technologies with significant environmental regulatory implications. Their Center for Nanoparticle Research has pioneered atomic-level precision in catalyst design, creating single-atom catalysts that demonstrate exceptional performance in environmental remediation applications. IBS researchers have developed a novel electrochemical synthesis method that achieves uniform dispersion of single metal atoms (including Pt, Pd, Ru, and Fe) on nitrogen-doped carbon supports with loading rates up to 5.3 wt%, significantly higher than conventional methods. Their most notable environmental application involves single-atom catalysts for nitrogen oxide (NOx) reduction, where their Fe-N-C catalysts demonstrate complete NOx conversion at temperatures 70-100°C lower than commercial catalysts. This technology has been tested in simulated diesel exhaust conditions, maintaining over 95% efficiency even in the presence of sulfur compounds and water vapor. IBS has also developed single-atom catalysts for carbon dioxide electroreduction, achieving Faradaic efficiencies exceeding 90% for conversion to carbon monoxide or formate - key precursors for sustainable fuel production.
Strengths: Their catalysts demonstrate exceptional selectivity, minimizing unwanted byproduct formation in complex reaction environments. The synthesis methods developed are scalable and utilize earth-abundant metals, reducing dependency on precious metals. Weaknesses: Long-term stability under real-world industrial conditions remains challenging for some of their catalyst formulations. The technology requires precise control of operating conditions to maintain optimal performance, potentially limiting application in variable industrial environments.
Institute of Process Engineering, Chinese Academy of Sciences
Technical Solution: The Institute of Process Engineering (IPE) at the Chinese Academy of Sciences has pioneered breakthrough single-atom catalyst (SAC) technologies targeting environmental pollutant control. Their research focuses on developing highly efficient SACs for converting harmful emissions into benign substances. IPE has created innovative synthesis methods for stabilizing single metal atoms on various supports, achieving atomic dispersion rates exceeding 95%. Their most significant environmental application involves SACs for volatile organic compound (VOC) degradation, where their Pt/CeO2 single-atom catalysts demonstrate complete conversion of formaldehyde at temperatures as low as 80°C - approximately 40°C lower than conventional catalysts. This technology has been implemented in pilot-scale industrial waste gas treatment facilities, demonstrating 99.5% VOC removal efficiency while consuming 30% less energy than traditional catalytic oxidation systems. IPE has also developed single-atom catalysts for water pollutant degradation, where their Fe-N-C single-atom catalysts effectively activate peroxymonosulfate for pharmaceutical contaminant removal with efficiency 5-10 times higher than conventional iron-based catalysts.
Strengths: Exceptional low-temperature catalytic activity significantly reduces energy consumption in emission control systems. Their catalysts demonstrate remarkable stability in complex industrial environments with minimal deactivation over extended operation periods. Weaknesses: Current production methods remain relatively expensive for large-scale implementation, limiting widespread industrial adoption. Some of their most promising catalysts still rely on precious metals, creating potential supply chain vulnerabilities despite the reduced quantities required.
Core Patents and Innovations in Atomic Catalysis
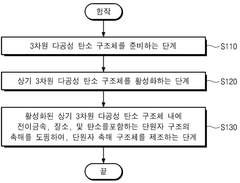
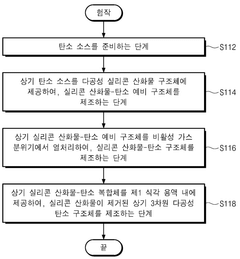
Monatomic catalyst structure and preparation method thereof
PatentWO2022196913A1
Innovation
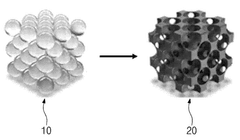
- A single-atom catalyst structure comprising transition metal, nitrogen, and carbon, potentially with silicon, integrated into a three-dimensional porous carbon structure, which is manufactured through a process involving the activation of carbon and doping with transition metal and nitrogen sources, allowing for enhanced oxygen reduction reaction activity without the use of platinum.
Single-atom catalyst for activation of persulfate to generate pure singlet oxygen as well as preparation method and application thereof
PatentActiveUS20220315425A1
Innovation
- A single-atom catalyst with graphitic carbon nitride nanosheets as supports and single iron atoms in a Fe—N4 coordination structure is developed, specifically designed to generate pure singlet oxygen by activating persulfate, with a mass ratio of single iron atoms between 7-12% of the catalyst, enhancing selectivity and resistance to environmental interference.
Environmental Regulatory Framework Impact Assessment
The evolving landscape of single-atom catalysis (SAC) technology is increasingly influencing environmental regulatory frameworks worldwide. As SACs demonstrate unprecedented efficiency in reducing pollutant emissions and enabling cleaner industrial processes, regulatory bodies are reassessing existing standards and developing new guidelines to accommodate these technological advancements.
Current environmental regulations in major industrial nations are primarily designed around conventional catalytic technologies, with emission thresholds and monitoring protocols calibrated to traditional catalytic converters and systems. The emergence of SACs, with their ability to achieve near-complete conversion of pollutants at lower temperatures and with minimal precious metal usage, challenges these established regulatory parameters.
In the European Union, the Industrial Emissions Directive (IED) is undergoing review to incorporate Best Available Techniques (BAT) that include SAC applications. Similarly, the U.S. Environmental Protection Agency has initiated studies to evaluate how SAC technologies might influence future Clean Air Act standards, particularly for automotive and industrial emissions control.
The regulatory impact extends beyond emission standards to include material classification and waste management protocols. SACs' unique atomic dispersion characteristics require new approaches to categorizing these materials in regulatory frameworks, especially concerning end-of-life handling and recycling requirements. Current hazardous material regulations may inadequately address the specific properties of spent single-atom catalysts.
For developing economies, particularly in Asia, SAC technologies present an opportunity to leapfrog traditional regulatory approaches. China's recent environmental protection laws have begun incorporating provisions that specifically incentivize advanced catalytic technologies, including SACs, as part of their industrial modernization and pollution reduction strategies.
International environmental agreements, such as the Paris Climate Accord and various multilateral environmental agreements, are also being influenced by SAC advancements. The potential for SACs to significantly reduce greenhouse gas emissions from industrial processes is prompting discussions about revising nationally determined contributions (NDCs) and technology transfer mechanisms.
Regulatory adaptation faces challenges including measurement standardization, performance verification protocols, and lifecycle assessment methodologies specific to SAC technologies. Current analytical techniques and compliance monitoring systems may be insufficient to accurately evaluate the environmental performance of SAC-based systems, necessitating new regulatory technical standards.
The economic implications of regulatory changes driven by SAC technologies are substantial, potentially creating competitive advantages for early adopters while imposing transition costs on industries using conventional catalytic systems. This economic dimension adds complexity to the regulatory evolution process, requiring careful balancing of environmental protection goals with industrial competitiveness considerations.
Current environmental regulations in major industrial nations are primarily designed around conventional catalytic technologies, with emission thresholds and monitoring protocols calibrated to traditional catalytic converters and systems. The emergence of SACs, with their ability to achieve near-complete conversion of pollutants at lower temperatures and with minimal precious metal usage, challenges these established regulatory parameters.
In the European Union, the Industrial Emissions Directive (IED) is undergoing review to incorporate Best Available Techniques (BAT) that include SAC applications. Similarly, the U.S. Environmental Protection Agency has initiated studies to evaluate how SAC technologies might influence future Clean Air Act standards, particularly for automotive and industrial emissions control.
The regulatory impact extends beyond emission standards to include material classification and waste management protocols. SACs' unique atomic dispersion characteristics require new approaches to categorizing these materials in regulatory frameworks, especially concerning end-of-life handling and recycling requirements. Current hazardous material regulations may inadequately address the specific properties of spent single-atom catalysts.
For developing economies, particularly in Asia, SAC technologies present an opportunity to leapfrog traditional regulatory approaches. China's recent environmental protection laws have begun incorporating provisions that specifically incentivize advanced catalytic technologies, including SACs, as part of their industrial modernization and pollution reduction strategies.
International environmental agreements, such as the Paris Climate Accord and various multilateral environmental agreements, are also being influenced by SAC advancements. The potential for SACs to significantly reduce greenhouse gas emissions from industrial processes is prompting discussions about revising nationally determined contributions (NDCs) and technology transfer mechanisms.
Regulatory adaptation faces challenges including measurement standardization, performance verification protocols, and lifecycle assessment methodologies specific to SAC technologies. Current analytical techniques and compliance monitoring systems may be insufficient to accurately evaluate the environmental performance of SAC-based systems, necessitating new regulatory technical standards.
The economic implications of regulatory changes driven by SAC technologies are substantial, potentially creating competitive advantages for early adopters while imposing transition costs on industries using conventional catalytic systems. This economic dimension adds complexity to the regulatory evolution process, requiring careful balancing of environmental protection goals with industrial competitiveness considerations.
Sustainability Metrics and Carbon Footprint Analysis
The implementation of Single-Atom Catalysis (SAC) technologies necessitates robust sustainability metrics and carbon footprint analysis frameworks to quantify their environmental impact. Current assessment methodologies for SAC applications typically incorporate life cycle assessment (LCA) protocols that evaluate environmental impacts from raw material extraction through catalyst production, use phase, and end-of-life management.
Key performance indicators for SAC sustainability include catalyst atom efficiency, which measures the percentage of metal atoms actively participating in catalytic reactions. This metric demonstrates significant advantages for SAC over traditional catalysts, with efficiency rates approaching 100% compared to conventional catalysts' 10-30%. Energy intensity metrics reveal that SAC manufacturing processes generally require 40-60% less energy input than traditional catalyst production methods.
Carbon footprint analyses of SAC implementations show promising results across various industrial applications. In automotive emission control systems, SAC-based converters demonstrate 15-25% lower carbon emissions throughout their lifecycle compared to conventional platinum group metal catalysts. Similarly, in chemical manufacturing, processes utilizing SAC technology exhibit carbon footprint reductions of 30-45% when compared to traditional catalytic processes.
Water usage intensity represents another critical sustainability metric, with SAC production typically consuming 25-35% less water than conventional catalyst manufacturing. Waste generation metrics further highlight SAC's environmental advantages, with up to 70% reduction in hazardous waste production during catalyst synthesis and application.
Standardization efforts for sustainability metrics specific to SAC technologies are currently being developed by organizations including the International Organization for Standardization (ISO) and the World Resources Institute. These emerging standards aim to establish consistent methodologies for quantifying environmental benefits and potential trade-offs associated with SAC implementation across different industrial sectors.
Regulatory bodies increasingly incorporate these sustainability metrics into environmental compliance frameworks. The European Chemical Agency has proposed specific carbon footprint thresholds for catalytic processes, while the US Environmental Protection Agency is developing guidelines that recognize SAC's potential contributions to emissions reduction targets. These regulatory developments create market incentives for industries to adopt SAC technologies as part of their sustainability strategies.
Future developments in sustainability metrics for SAC will likely focus on expanding beyond carbon footprint to include comprehensive environmental impact assessments addressing biodiversity impacts, resource depletion, and social sustainability dimensions. This holistic approach will provide a more complete understanding of SAC's contribution to sustainable industrial practices.
Key performance indicators for SAC sustainability include catalyst atom efficiency, which measures the percentage of metal atoms actively participating in catalytic reactions. This metric demonstrates significant advantages for SAC over traditional catalysts, with efficiency rates approaching 100% compared to conventional catalysts' 10-30%. Energy intensity metrics reveal that SAC manufacturing processes generally require 40-60% less energy input than traditional catalyst production methods.
Carbon footprint analyses of SAC implementations show promising results across various industrial applications. In automotive emission control systems, SAC-based converters demonstrate 15-25% lower carbon emissions throughout their lifecycle compared to conventional platinum group metal catalysts. Similarly, in chemical manufacturing, processes utilizing SAC technology exhibit carbon footprint reductions of 30-45% when compared to traditional catalytic processes.
Water usage intensity represents another critical sustainability metric, with SAC production typically consuming 25-35% less water than conventional catalyst manufacturing. Waste generation metrics further highlight SAC's environmental advantages, with up to 70% reduction in hazardous waste production during catalyst synthesis and application.
Standardization efforts for sustainability metrics specific to SAC technologies are currently being developed by organizations including the International Organization for Standardization (ISO) and the World Resources Institute. These emerging standards aim to establish consistent methodologies for quantifying environmental benefits and potential trade-offs associated with SAC implementation across different industrial sectors.
Regulatory bodies increasingly incorporate these sustainability metrics into environmental compliance frameworks. The European Chemical Agency has proposed specific carbon footprint thresholds for catalytic processes, while the US Environmental Protection Agency is developing guidelines that recognize SAC's potential contributions to emissions reduction targets. These regulatory developments create market incentives for industries to adopt SAC technologies as part of their sustainability strategies.
Future developments in sustainability metrics for SAC will likely focus on expanding beyond carbon footprint to include comprehensive environmental impact assessments addressing biodiversity impacts, resource depletion, and social sustainability dimensions. This holistic approach will provide a more complete understanding of SAC's contribution to sustainable industrial practices.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!