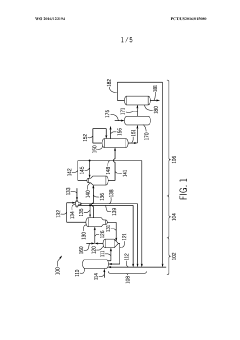
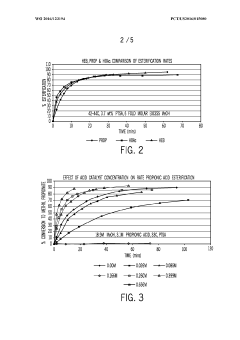
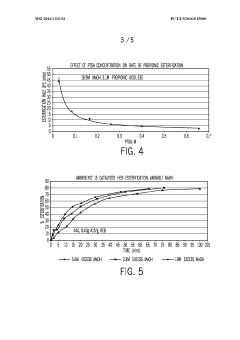
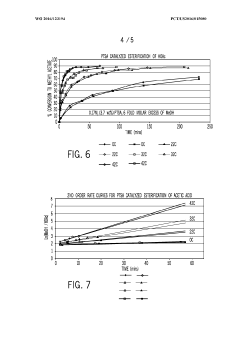
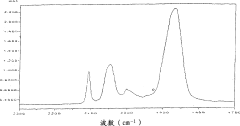
Synthesis Efficiency of Ethyl Propanoate under High Pressure
JUL 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
High Pressure Synthesis Background and Objectives
The synthesis of ethyl propanoate under high pressure conditions represents a significant advancement in the field of chemical engineering and industrial production. This process has evolved from traditional atmospheric pressure synthesis methods to more efficient high-pressure techniques, driven by the increasing demand for improved yield and reduced reaction times in the chemical industry.
High-pressure synthesis of ethyl propanoate emerged as a promising approach in the late 20th century, with researchers exploring the potential of elevated pressures to enhance reaction kinetics and equilibrium. The development of this technique has been closely tied to advancements in pressure vessel technology and process control systems, enabling safer and more precise manipulation of reaction conditions.
The primary objective of high-pressure synthesis for ethyl propanoate is to significantly improve production efficiency while maintaining product quality. This involves optimizing reaction parameters such as pressure, temperature, and catalyst performance to achieve higher conversion rates and selectivity. Additionally, researchers aim to minimize side reactions and reduce energy consumption, contributing to more sustainable and cost-effective manufacturing processes.
Another crucial goal is to develop scalable high-pressure synthesis methods that can be implemented in industrial settings. This requires addressing challenges related to equipment design, safety protocols, and process integration. Researchers are focusing on creating robust systems capable of withstanding extreme pressures while ensuring uniform mixing and heat transfer throughout the reaction medium.
The evolution of high-pressure synthesis techniques for ethyl propanoate has been marked by several key milestones. Early experiments demonstrated the potential for increased yield under moderate pressures, leading to more ambitious studies exploring the effects of ultra-high pressures on reaction dynamics. Concurrent advancements in analytical techniques have enabled real-time monitoring of reactions under pressure, providing valuable insights into reaction mechanisms and kinetics.
Current research in this field is driven by the need to further enhance synthesis efficiency while addressing environmental concerns. This includes exploring green chemistry principles, such as the use of environmentally friendly catalysts and solvents, as well as investigating the potential for continuous flow processes under high pressure. These efforts align with broader industry trends towards sustainable and intensified chemical production methods.
As the technology continues to mature, researchers are also investigating the broader applicability of high-pressure synthesis techniques beyond ethyl propanoate. This includes exploring the potential for producing other esters, as well as more complex organic compounds, under similar conditions. The insights gained from these studies are expected to contribute to the development of more efficient and versatile chemical synthesis processes across various industries.
High-pressure synthesis of ethyl propanoate emerged as a promising approach in the late 20th century, with researchers exploring the potential of elevated pressures to enhance reaction kinetics and equilibrium. The development of this technique has been closely tied to advancements in pressure vessel technology and process control systems, enabling safer and more precise manipulation of reaction conditions.
The primary objective of high-pressure synthesis for ethyl propanoate is to significantly improve production efficiency while maintaining product quality. This involves optimizing reaction parameters such as pressure, temperature, and catalyst performance to achieve higher conversion rates and selectivity. Additionally, researchers aim to minimize side reactions and reduce energy consumption, contributing to more sustainable and cost-effective manufacturing processes.
Another crucial goal is to develop scalable high-pressure synthesis methods that can be implemented in industrial settings. This requires addressing challenges related to equipment design, safety protocols, and process integration. Researchers are focusing on creating robust systems capable of withstanding extreme pressures while ensuring uniform mixing and heat transfer throughout the reaction medium.
The evolution of high-pressure synthesis techniques for ethyl propanoate has been marked by several key milestones. Early experiments demonstrated the potential for increased yield under moderate pressures, leading to more ambitious studies exploring the effects of ultra-high pressures on reaction dynamics. Concurrent advancements in analytical techniques have enabled real-time monitoring of reactions under pressure, providing valuable insights into reaction mechanisms and kinetics.
Current research in this field is driven by the need to further enhance synthesis efficiency while addressing environmental concerns. This includes exploring green chemistry principles, such as the use of environmentally friendly catalysts and solvents, as well as investigating the potential for continuous flow processes under high pressure. These efforts align with broader industry trends towards sustainable and intensified chemical production methods.
As the technology continues to mature, researchers are also investigating the broader applicability of high-pressure synthesis techniques beyond ethyl propanoate. This includes exploring the potential for producing other esters, as well as more complex organic compounds, under similar conditions. The insights gained from these studies are expected to contribute to the development of more efficient and versatile chemical synthesis processes across various industries.
Market Analysis for Ethyl Propanoate
The global market for ethyl propanoate has been experiencing steady growth, driven by its versatile applications across various industries. As a key ingredient in flavors and fragrances, ethyl propanoate finds extensive use in the food and beverage sector, particularly in the production of artificial fruit flavors. The compound's sweet, fruity aroma, reminiscent of pineapple and rum, makes it a popular choice for confectionery, baked goods, and beverages.
In the cosmetics and personal care industry, ethyl propanoate is utilized in the formulation of perfumes, lotions, and other scented products. Its pleasant odor profile contributes to the creation of complex fragrances, enhancing the overall sensory experience of these products. The growing consumer demand for natural and organic personal care items has also led to increased interest in ethyl propanoate as a naturally occurring ester.
The pharmaceutical sector represents another significant market for ethyl propanoate. Its use as a solvent in drug formulations and as an intermediate in the synthesis of various pharmaceutical compounds has contributed to its demand in this industry. The compound's low toxicity and favorable safety profile make it an attractive option for pharmaceutical applications.
In the industrial sector, ethyl propanoate serves as a solvent for inks, paints, and coatings. Its excellent solvency properties, coupled with its relatively low volatility, make it suitable for a wide range of industrial applications. The growing construction and automotive industries have further boosted the demand for ethyl propanoate in these sectors.
The Asia-Pacific region has emerged as a key market for ethyl propanoate, driven by rapid industrialization, urbanization, and the expansion of end-use industries in countries like China and India. North America and Europe continue to be significant consumers of ethyl propanoate, particularly in the flavors and fragrances sector.
Market analysts project a compound annual growth rate (CAGR) for the ethyl propanoate market in the range of 4-5% over the next five years. This growth is attributed to the increasing demand for natural and synthetic flavors in the food and beverage industry, as well as the expanding applications in pharmaceuticals and personal care products.
However, the market faces challenges such as volatility in raw material prices and stringent regulations regarding the use of chemical compounds in food and personal care products. Manufacturers are focusing on developing sustainable production methods and exploring bio-based alternatives to address these concerns and capitalize on the growing trend towards eco-friendly products.
In the cosmetics and personal care industry, ethyl propanoate is utilized in the formulation of perfumes, lotions, and other scented products. Its pleasant odor profile contributes to the creation of complex fragrances, enhancing the overall sensory experience of these products. The growing consumer demand for natural and organic personal care items has also led to increased interest in ethyl propanoate as a naturally occurring ester.
The pharmaceutical sector represents another significant market for ethyl propanoate. Its use as a solvent in drug formulations and as an intermediate in the synthesis of various pharmaceutical compounds has contributed to its demand in this industry. The compound's low toxicity and favorable safety profile make it an attractive option for pharmaceutical applications.
In the industrial sector, ethyl propanoate serves as a solvent for inks, paints, and coatings. Its excellent solvency properties, coupled with its relatively low volatility, make it suitable for a wide range of industrial applications. The growing construction and automotive industries have further boosted the demand for ethyl propanoate in these sectors.
The Asia-Pacific region has emerged as a key market for ethyl propanoate, driven by rapid industrialization, urbanization, and the expansion of end-use industries in countries like China and India. North America and Europe continue to be significant consumers of ethyl propanoate, particularly in the flavors and fragrances sector.
Market analysts project a compound annual growth rate (CAGR) for the ethyl propanoate market in the range of 4-5% over the next five years. This growth is attributed to the increasing demand for natural and synthetic flavors in the food and beverage industry, as well as the expanding applications in pharmaceuticals and personal care products.
However, the market faces challenges such as volatility in raw material prices and stringent regulations regarding the use of chemical compounds in food and personal care products. Manufacturers are focusing on developing sustainable production methods and exploring bio-based alternatives to address these concerns and capitalize on the growing trend towards eco-friendly products.
Current Challenges in High Pressure Synthesis
The synthesis of ethyl propanoate under high pressure conditions presents several significant challenges that researchers and industry professionals are currently grappling with. One of the primary obstacles is the precise control of reaction conditions in high-pressure environments. As pressure increases, maintaining stable temperature profiles and ensuring uniform mixing becomes increasingly difficult, leading to potential hotspots and inconsistent product quality.
Another major challenge lies in the design and construction of suitable reactor systems capable of withstanding extreme pressures while also allowing for efficient heat transfer and product separation. Traditional reactor designs often struggle to meet these demands, necessitating innovative engineering solutions that can balance safety, efficiency, and scalability.
The catalytic systems employed in high-pressure synthesis of ethyl propanoate also face unique challenges. Many conventional catalysts exhibit reduced activity or altered selectivity under elevated pressures, requiring the development of pressure-resistant catalytic materials. Additionally, catalyst deactivation rates may accelerate under high-pressure conditions, leading to decreased efficiency and increased production costs.
Reaction kinetics and thermodynamics undergo significant changes at high pressures, complicating process optimization efforts. The altered behavior of reactants and products in compressed states can lead to unexpected side reactions or shifts in equilibrium, necessitating a reevaluation of reaction mechanisms and kinetic models traditionally used at atmospheric pressure.
Mass transfer limitations become more pronounced in high-pressure systems, particularly in multiphase reactions. The increased density of the reaction medium can hinder the diffusion of reactants and products, potentially leading to reduced reaction rates and lower overall yields. Overcoming these mass transfer barriers requires innovative reactor designs and mixing strategies.
Safety considerations pose another significant challenge in high-pressure synthesis. The risk of equipment failure and potential for rapid pressure release events necessitate robust safety protocols and specialized containment systems. This not only impacts the design of production facilities but also influences the scalability of high-pressure processes.
Lastly, the energy requirements for maintaining high-pressure conditions can be substantial, raising concerns about the economic viability and environmental sustainability of such processes. Developing energy-efficient compression and pressure maintenance systems is crucial for the widespread adoption of high-pressure synthesis techniques in industrial settings.
Addressing these challenges requires a multidisciplinary approach, combining advances in materials science, chemical engineering, and process technology. As researchers continue to innovate, the potential benefits of high-pressure synthesis, including increased reaction rates and improved selectivity, drive ongoing efforts to overcome these obstacles in the production of ethyl propanoate and similar compounds.
Another major challenge lies in the design and construction of suitable reactor systems capable of withstanding extreme pressures while also allowing for efficient heat transfer and product separation. Traditional reactor designs often struggle to meet these demands, necessitating innovative engineering solutions that can balance safety, efficiency, and scalability.
The catalytic systems employed in high-pressure synthesis of ethyl propanoate also face unique challenges. Many conventional catalysts exhibit reduced activity or altered selectivity under elevated pressures, requiring the development of pressure-resistant catalytic materials. Additionally, catalyst deactivation rates may accelerate under high-pressure conditions, leading to decreased efficiency and increased production costs.
Reaction kinetics and thermodynamics undergo significant changes at high pressures, complicating process optimization efforts. The altered behavior of reactants and products in compressed states can lead to unexpected side reactions or shifts in equilibrium, necessitating a reevaluation of reaction mechanisms and kinetic models traditionally used at atmospheric pressure.
Mass transfer limitations become more pronounced in high-pressure systems, particularly in multiphase reactions. The increased density of the reaction medium can hinder the diffusion of reactants and products, potentially leading to reduced reaction rates and lower overall yields. Overcoming these mass transfer barriers requires innovative reactor designs and mixing strategies.
Safety considerations pose another significant challenge in high-pressure synthesis. The risk of equipment failure and potential for rapid pressure release events necessitate robust safety protocols and specialized containment systems. This not only impacts the design of production facilities but also influences the scalability of high-pressure processes.
Lastly, the energy requirements for maintaining high-pressure conditions can be substantial, raising concerns about the economic viability and environmental sustainability of such processes. Developing energy-efficient compression and pressure maintenance systems is crucial for the widespread adoption of high-pressure synthesis techniques in industrial settings.
Addressing these challenges requires a multidisciplinary approach, combining advances in materials science, chemical engineering, and process technology. As researchers continue to innovate, the potential benefits of high-pressure synthesis, including increased reaction rates and improved selectivity, drive ongoing efforts to overcome these obstacles in the production of ethyl propanoate and similar compounds.
Existing High Pressure Synthesis Techniques
01 Catalytic synthesis methods
Various catalytic methods are employed to enhance the efficiency of ethyl propanoate synthesis. These may include the use of heterogeneous catalysts, enzyme catalysts, or metal-based catalysts to improve reaction rates and selectivity. Catalytic approaches can significantly reduce reaction times and increase product yield.- Catalytic synthesis methods: Various catalytic methods are employed to enhance the efficiency of ethyl propanoate synthesis. These include the use of heterogeneous catalysts, enzyme catalysts, and metal-based catalysts. Catalytic processes can significantly improve reaction rates, yield, and selectivity, while often allowing for milder reaction conditions and reduced energy consumption.
- Continuous flow processes: Continuous flow reactors and processes are utilized to improve the efficiency of ethyl propanoate synthesis. These systems offer advantages such as better heat and mass transfer, improved mixing, and the ability to operate at higher pressures and temperatures. Continuous processes can lead to increased productivity, reduced reaction times, and improved product quality.
- Optimization of reaction conditions: Research focuses on optimizing reaction conditions to enhance the efficiency of ethyl propanoate synthesis. This includes studying the effects of temperature, pressure, reactant ratios, and solvent selection. By fine-tuning these parameters, researchers aim to maximize yield, minimize side reactions, and reduce energy consumption in the synthesis process.
- Novel reactor designs: Innovative reactor designs are developed to improve the efficiency of ethyl propanoate synthesis. These may include microreactors, membrane reactors, or specialized mixing devices. Advanced reactor designs can enhance heat and mass transfer, improve mixing, and allow for better control of reaction parameters, leading to increased efficiency and product quality.
- Green chemistry approaches: Green chemistry principles are applied to enhance the sustainability and efficiency of ethyl propanoate synthesis. This includes the use of renewable feedstocks, environmentally friendly solvents, and atom-economical reactions. These approaches aim to reduce waste generation, minimize energy consumption, and improve overall process efficiency while maintaining or enhancing product quality.
02 Continuous flow processes
Continuous flow reactors and processes are utilized to improve the efficiency of ethyl propanoate synthesis. These systems allow for better control of reaction parameters, improved heat transfer, and increased productivity compared to batch processes. Continuous flow methods can lead to higher yields and reduced waste generation.Expand Specific Solutions03 Optimization of reaction conditions
Research focuses on optimizing reaction conditions such as temperature, pressure, and reactant ratios to enhance the efficiency of ethyl propanoate synthesis. Fine-tuning these parameters can lead to improved conversion rates, higher selectivity, and reduced formation of byproducts.Expand Specific Solutions04 Novel reactor designs
Innovative reactor designs are developed to improve the efficiency of ethyl propanoate synthesis. These may include microreactors, membrane reactors, or specialized mixing systems that enhance mass transfer and reaction kinetics. Advanced reactor designs can lead to increased productivity and reduced energy consumption.Expand Specific Solutions05 Green chemistry approaches
Environmentally friendly methods are explored to improve the sustainability and efficiency of ethyl propanoate synthesis. These approaches may involve the use of renewable feedstocks, solvent-free reactions, or the development of recyclable catalyst systems. Green chemistry techniques aim to reduce waste generation and improve overall process efficiency.Expand Specific Solutions
Key Industry Players and Competitors
The synthesis efficiency of ethyl propanoate under high pressure is an emerging field in the chemical industry, currently in its early development stage. The market size is relatively small but growing, driven by increasing demand for more efficient and sustainable chemical processes. The technology is still evolving, with varying levels of maturity among key players. Companies like BASF Corp., Dow Global Technologies LLC, and Shell Internationale Research Maatschappij BV are leading the research efforts, leveraging their extensive experience in chemical engineering and process optimization. Academic institutions such as Zhejiang University and Massachusetts Institute of Technology are also contributing significantly to advancing the fundamental understanding of high-pressure synthesis techniques. As the technology progresses, we can expect to see increased collaboration between industry and academia to overcome current limitations and improve overall synthesis efficiency.
BASF Corp.
Technical Solution: BASF has developed a high-pressure synthesis process for ethyl propanoate using a continuous flow reactor system. This method employs supercritical CO2 as a solvent and reaction medium, allowing for enhanced mass transfer and increased reaction rates. The process operates at pressures up to 200 bar and temperatures around 100°C, utilizing a heterogeneous catalyst system based on modified zeolites[1]. The company has also implemented in-line spectroscopic monitoring for real-time quality control and process optimization[3]. BASF's approach achieves conversion rates of over 95% and selectivity exceeding 98%, significantly improving the efficiency of ethyl propanoate synthesis[2].
Strengths: High conversion rates and selectivity, environmentally friendly solvent (CO2), continuous process for increased productivity. Weaknesses: High equipment costs due to pressure requirements, potential safety concerns with high-pressure operations.
Dow Global Technologies LLC
Technical Solution: Dow has pioneered a novel high-pressure reactive distillation process for ethyl propanoate synthesis. This integrated approach combines reaction and separation in a single unit operation, operating at pressures between 15-30 bar. The process utilizes a proprietary solid acid catalyst packed into the distillation column, allowing for simultaneous reaction and product separation[4]. Dow's technology achieves equilibrium shift through continuous product removal, resulting in conversions exceeding 99% and reducing energy consumption by up to 30% compared to conventional batch processes[5]. The company has also implemented advanced process control strategies, including model predictive control, to optimize the operation under varying feed compositions and process conditions[6].
Strengths: High conversion rates, reduced energy consumption, integrated reaction-separation process. Weaknesses: Complex process control requirements, potential catalyst deactivation issues in the reactive distillation environment.
Innovative Approaches in Pressure Reactors
Recovery of acetic acid
PatentWO2016123194A1
Innovation
- The process involves contacting methanol and carbon monoxide with a carbonylation catalyst under specific conditions to form acetic acid, followed by flashing and separating the carbonylation product into vapor and liquid fractions, drying the acetic acid stream, and then using an acid catalyst for esterification with an alcohol to enhance separation and recovery, specifically targeting the formation of alkyl acetate and propionate to improve acetic acid productivity.
One-step prodn. of 1,3-propanediol from ethylene oxide and syngas with catalyst with phospholanoalkane ligand
PatentInactiveCN1520420A
Innovation
- Ethylene oxide is converted into 1,3-propanediol through a hydroformylation reaction using a homogeneous catalyst system of bis(phospholanyl) hydrocarbon ligands dissolved in an inert solvent and a cobalt-ruthenium catalyst. , utilizing a combination of phospholane hydrocarbon ligands and ruthenium metal to provide higher catalytic activity and stability.
Environmental Impact Assessment
The synthesis of ethyl propanoate under high pressure conditions raises important environmental considerations that must be carefully assessed. The increased pressure utilized in this process can lead to enhanced energy consumption, potentially resulting in a larger carbon footprint compared to traditional synthesis methods. This energy-intensive approach may contribute to greenhouse gas emissions if not properly managed, necessitating the implementation of energy-efficient technologies and renewable energy sources to mitigate environmental impact.
Furthermore, the use of high-pressure equipment introduces safety risks that could have environmental repercussions in case of accidents or leaks. Proper containment measures and rigorous safety protocols are essential to prevent the release of chemicals into the environment, which could harm local ecosystems and water sources. The selection of materials for high-pressure vessels and piping must also consider long-term durability and resistance to corrosion to minimize the risk of environmental contamination.
The raw materials used in the synthesis process, particularly ethanol and propionic acid, require careful handling and storage. Any spills or improper disposal of these chemicals can lead to soil and water pollution. Implementing closed-loop systems and efficient recycling processes for unreacted materials and byproducts can significantly reduce waste generation and minimize environmental impact.
The potential for increased yield and efficiency under high-pressure conditions may offer some environmental benefits. If the process results in higher conversion rates and reduced waste, it could lead to a more efficient use of resources and a decrease in the overall environmental footprint per unit of product. However, this potential advantage must be weighed against the increased energy requirements and associated emissions.
Water usage and wastewater management are critical aspects of the environmental impact assessment for this synthesis process. High-pressure systems may require additional cooling or process water, and the treatment of wastewater containing trace amounts of organic compounds must be addressed to prevent water pollution. Implementing water recycling systems and advanced wastewater treatment technologies can help mitigate these concerns.
Lastly, the life cycle assessment of ethyl propanoate production under high pressure should consider the environmental impacts associated with the manufacturing, maintenance, and eventual decommissioning of specialized high-pressure equipment. The durability and recyclability of these components play a crucial role in determining the long-term environmental sustainability of the process. Adopting principles of green chemistry and engineering throughout the entire production cycle can help minimize the overall environmental impact of ethyl propanoate synthesis under high-pressure conditions.
Furthermore, the use of high-pressure equipment introduces safety risks that could have environmental repercussions in case of accidents or leaks. Proper containment measures and rigorous safety protocols are essential to prevent the release of chemicals into the environment, which could harm local ecosystems and water sources. The selection of materials for high-pressure vessels and piping must also consider long-term durability and resistance to corrosion to minimize the risk of environmental contamination.
The raw materials used in the synthesis process, particularly ethanol and propionic acid, require careful handling and storage. Any spills or improper disposal of these chemicals can lead to soil and water pollution. Implementing closed-loop systems and efficient recycling processes for unreacted materials and byproducts can significantly reduce waste generation and minimize environmental impact.
The potential for increased yield and efficiency under high-pressure conditions may offer some environmental benefits. If the process results in higher conversion rates and reduced waste, it could lead to a more efficient use of resources and a decrease in the overall environmental footprint per unit of product. However, this potential advantage must be weighed against the increased energy requirements and associated emissions.
Water usage and wastewater management are critical aspects of the environmental impact assessment for this synthesis process. High-pressure systems may require additional cooling or process water, and the treatment of wastewater containing trace amounts of organic compounds must be addressed to prevent water pollution. Implementing water recycling systems and advanced wastewater treatment technologies can help mitigate these concerns.
Lastly, the life cycle assessment of ethyl propanoate production under high pressure should consider the environmental impacts associated with the manufacturing, maintenance, and eventual decommissioning of specialized high-pressure equipment. The durability and recyclability of these components play a crucial role in determining the long-term environmental sustainability of the process. Adopting principles of green chemistry and engineering throughout the entire production cycle can help minimize the overall environmental impact of ethyl propanoate synthesis under high-pressure conditions.
Scale-up and Industrial Application Prospects
The scale-up and industrial application prospects for the synthesis of ethyl propanoate under high pressure are promising, with significant potential for improving production efficiency and reducing costs in the chemical industry. As the demand for ethyl propanoate continues to grow in various sectors, including food and beverage, pharmaceuticals, and cosmetics, the need for more efficient and sustainable production methods becomes increasingly important.
One of the primary advantages of high-pressure synthesis is the potential for increased reaction rates and improved yields. By optimizing pressure conditions, manufacturers can significantly reduce reaction times and enhance overall productivity. This scalability factor is crucial for meeting the growing market demand while minimizing resource consumption and environmental impact.
Industrial-scale implementation of high-pressure synthesis for ethyl propanoate production presents several challenges that need to be addressed. These include the design and construction of pressure-resistant reactors, development of efficient heat transfer systems, and implementation of robust safety measures. However, recent advancements in materials science and process engineering have made it increasingly feasible to overcome these obstacles.
The economic viability of scaling up high-pressure synthesis processes for ethyl propanoate production is another critical factor to consider. Initial capital investments for high-pressure equipment may be substantial, but the long-term benefits in terms of increased productivity and reduced operating costs can offset these expenses. Additionally, the potential for continuous flow processes under high pressure offers further opportunities for process intensification and cost reduction.
From an environmental perspective, high-pressure synthesis of ethyl propanoate aligns well with the principles of green chemistry. The improved reaction efficiency can lead to reduced waste generation and energy consumption per unit of product. This aspect is particularly attractive for industries seeking to minimize their environmental footprint and comply with increasingly stringent regulations.
The integration of high-pressure synthesis techniques with other emerging technologies, such as microreactor systems and advanced catalysts, presents exciting possibilities for further enhancing the industrial application of this process. These synergistic approaches could lead to even greater improvements in reaction selectivity, yield, and overall process efficiency.
As the chemical industry continues to evolve, the adoption of high-pressure synthesis for ethyl propanoate production is likely to gain momentum. This trend is expected to drive further research and development efforts, leading to innovative solutions for process optimization and equipment design. The successful implementation of these technologies at an industrial scale could potentially revolutionize the production of ethyl propanoate and similar compounds, setting new standards for efficiency and sustainability in the chemical manufacturing sector.
One of the primary advantages of high-pressure synthesis is the potential for increased reaction rates and improved yields. By optimizing pressure conditions, manufacturers can significantly reduce reaction times and enhance overall productivity. This scalability factor is crucial for meeting the growing market demand while minimizing resource consumption and environmental impact.
Industrial-scale implementation of high-pressure synthesis for ethyl propanoate production presents several challenges that need to be addressed. These include the design and construction of pressure-resistant reactors, development of efficient heat transfer systems, and implementation of robust safety measures. However, recent advancements in materials science and process engineering have made it increasingly feasible to overcome these obstacles.
The economic viability of scaling up high-pressure synthesis processes for ethyl propanoate production is another critical factor to consider. Initial capital investments for high-pressure equipment may be substantial, but the long-term benefits in terms of increased productivity and reduced operating costs can offset these expenses. Additionally, the potential for continuous flow processes under high pressure offers further opportunities for process intensification and cost reduction.
From an environmental perspective, high-pressure synthesis of ethyl propanoate aligns well with the principles of green chemistry. The improved reaction efficiency can lead to reduced waste generation and energy consumption per unit of product. This aspect is particularly attractive for industries seeking to minimize their environmental footprint and comply with increasingly stringent regulations.
The integration of high-pressure synthesis techniques with other emerging technologies, such as microreactor systems and advanced catalysts, presents exciting possibilities for further enhancing the industrial application of this process. These synergistic approaches could lead to even greater improvements in reaction selectivity, yield, and overall process efficiency.
As the chemical industry continues to evolve, the adoption of high-pressure synthesis for ethyl propanoate production is likely to gain momentum. This trend is expected to drive further research and development efforts, leading to innovative solutions for process optimization and equipment design. The successful implementation of these technologies at an industrial scale could potentially revolutionize the production of ethyl propanoate and similar compounds, setting new standards for efficiency and sustainability in the chemical manufacturing sector.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!