The Evolution of Hydrochloric Acid in Advanced Chemistry
JUL 2, 20258 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HCl Evolution Background
Hydrochloric acid (HCl) has played a pivotal role in the evolution of advanced chemistry, serving as a cornerstone in numerous chemical processes and applications. Its journey from discovery to widespread industrial use spans centuries, marking significant milestones in the field of chemistry and chemical engineering.
The story of HCl begins in the early alchemical period when it was first isolated by the Arabian alchemist Jabir ibn Hayyan in the 8th century. However, it wasn't until the 17th century that HCl gained prominence in scientific circles. In 1648, Johann Rudolph Glauber produced hydrochloric acid by combining sulfuric acid with table salt. This method, known as the "salt process," became the primary means of HCl production for centuries.
The Industrial Revolution in the 18th and 19th centuries brought about a surge in demand for HCl, particularly in the textile and metal processing industries. This period saw significant advancements in HCl production techniques, including the development of the Leblanc process in 1791, which produced sodium carbonate and hydrochloric acid as a by-product.
The 20th century witnessed a paradigm shift in HCl production with the advent of the chlor-alkali process. This electrolytic method, which produces chlorine, sodium hydroxide, and hydrogen, became the primary source of HCl. The process not only increased production efficiency but also improved the purity of the acid, opening up new avenues for its application in advanced chemistry.
In recent decades, the role of HCl in advanced chemistry has expanded dramatically. Its use in the pharmaceutical industry for drug synthesis, in the electronics sector for semiconductor manufacturing, and in environmental applications for water treatment has underscored its versatility and importance. The development of high-purity HCl grades has been crucial in meeting the stringent requirements of these advanced applications.
The evolution of HCl has also been marked by significant improvements in handling and safety protocols. From the early days of rudimentary glassware to modern corrosion-resistant materials and sophisticated containment systems, the safe management of HCl has been a driving force behind many technological advancements in chemical engineering.
As we look to the future, the evolution of HCl in advanced chemistry continues. Emerging applications in nanotechnology, green chemistry, and materials science are pushing the boundaries of HCl utilization. Researchers are exploring novel synthesis routes, investigating the acid's behavior at the molecular level, and developing innovative applications that promise to further cement HCl's place in the pantheon of essential chemical compounds.
The story of HCl begins in the early alchemical period when it was first isolated by the Arabian alchemist Jabir ibn Hayyan in the 8th century. However, it wasn't until the 17th century that HCl gained prominence in scientific circles. In 1648, Johann Rudolph Glauber produced hydrochloric acid by combining sulfuric acid with table salt. This method, known as the "salt process," became the primary means of HCl production for centuries.
The Industrial Revolution in the 18th and 19th centuries brought about a surge in demand for HCl, particularly in the textile and metal processing industries. This period saw significant advancements in HCl production techniques, including the development of the Leblanc process in 1791, which produced sodium carbonate and hydrochloric acid as a by-product.
The 20th century witnessed a paradigm shift in HCl production with the advent of the chlor-alkali process. This electrolytic method, which produces chlorine, sodium hydroxide, and hydrogen, became the primary source of HCl. The process not only increased production efficiency but also improved the purity of the acid, opening up new avenues for its application in advanced chemistry.
In recent decades, the role of HCl in advanced chemistry has expanded dramatically. Its use in the pharmaceutical industry for drug synthesis, in the electronics sector for semiconductor manufacturing, and in environmental applications for water treatment has underscored its versatility and importance. The development of high-purity HCl grades has been crucial in meeting the stringent requirements of these advanced applications.
The evolution of HCl has also been marked by significant improvements in handling and safety protocols. From the early days of rudimentary glassware to modern corrosion-resistant materials and sophisticated containment systems, the safe management of HCl has been a driving force behind many technological advancements in chemical engineering.
As we look to the future, the evolution of HCl in advanced chemistry continues. Emerging applications in nanotechnology, green chemistry, and materials science are pushing the boundaries of HCl utilization. Researchers are exploring novel synthesis routes, investigating the acid's behavior at the molecular level, and developing innovative applications that promise to further cement HCl's place in the pantheon of essential chemical compounds.
Market Demand Analysis
The market demand for hydrochloric acid in advanced chemistry applications has been steadily growing, driven by its versatile uses across various industries. In the semiconductor sector, high-purity hydrochloric acid is essential for cleaning silicon wafers and etching processes, with the global semiconductor market projected to reach $1 trillion by 2030. This growth directly correlates with increased demand for ultra-pure hydrochloric acid.
The pharmaceutical industry represents another significant market for hydrochloric acid, particularly in drug synthesis and formulation. As the global pharmaceutical market continues to expand, reaching $1.42 trillion in 2021, the demand for pharmaceutical-grade hydrochloric acid is expected to rise proportionally. The acid's role in producing active pharmaceutical ingredients (APIs) and maintaining pH levels in drug formulations underscores its importance in this sector.
In the food industry, hydrochloric acid finds applications in food processing and as a food additive (E507). The global food additives market, valued at $62.6 billion in 2022, is projected to grow at a CAGR of 5.5% from 2023 to 2030, indicating a parallel increase in demand for food-grade hydrochloric acid.
The water treatment sector presents a substantial market for hydrochloric acid, used in pH adjustment and chlorination processes. With growing concerns over water scarcity and quality, the global water treatment chemicals market is expected to reach $56.57 billion by 2030, driving demand for hydrochloric acid in this application.
In the metal processing industry, hydrochloric acid is crucial for pickling and descaling operations. The global metal treatment chemical market, valued at $10.3 billion in 2021, is projected to grow at a CAGR of 4.8% from 2022 to 2030, indicating sustained demand for industrial-grade hydrochloric acid.
The oil and gas industry utilizes hydrochloric acid in well acidizing and oil well completion processes. As global energy demand continues to rise, with oil consumption projected to reach 101.6 million barrels per day by 2023, the demand for hydrochloric acid in this sector remains robust.
Emerging applications in advanced materials, such as graphene production and rare earth element extraction, are opening new markets for high-purity hydrochloric acid. These niche markets, while currently small, show significant growth potential and may drive future demand for specialized grades of hydrochloric acid.
The pharmaceutical industry represents another significant market for hydrochloric acid, particularly in drug synthesis and formulation. As the global pharmaceutical market continues to expand, reaching $1.42 trillion in 2021, the demand for pharmaceutical-grade hydrochloric acid is expected to rise proportionally. The acid's role in producing active pharmaceutical ingredients (APIs) and maintaining pH levels in drug formulations underscores its importance in this sector.
In the food industry, hydrochloric acid finds applications in food processing and as a food additive (E507). The global food additives market, valued at $62.6 billion in 2022, is projected to grow at a CAGR of 5.5% from 2023 to 2030, indicating a parallel increase in demand for food-grade hydrochloric acid.
The water treatment sector presents a substantial market for hydrochloric acid, used in pH adjustment and chlorination processes. With growing concerns over water scarcity and quality, the global water treatment chemicals market is expected to reach $56.57 billion by 2030, driving demand for hydrochloric acid in this application.
In the metal processing industry, hydrochloric acid is crucial for pickling and descaling operations. The global metal treatment chemical market, valued at $10.3 billion in 2021, is projected to grow at a CAGR of 4.8% from 2022 to 2030, indicating sustained demand for industrial-grade hydrochloric acid.
The oil and gas industry utilizes hydrochloric acid in well acidizing and oil well completion processes. As global energy demand continues to rise, with oil consumption projected to reach 101.6 million barrels per day by 2023, the demand for hydrochloric acid in this sector remains robust.
Emerging applications in advanced materials, such as graphene production and rare earth element extraction, are opening new markets for high-purity hydrochloric acid. These niche markets, while currently small, show significant growth potential and may drive future demand for specialized grades of hydrochloric acid.
Current Challenges
The field of advanced chemistry continues to push the boundaries of hydrochloric acid applications, yet several significant challenges persist. One of the primary obstacles is the corrosive nature of hydrochloric acid, which poses substantial risks to both equipment and personnel. Despite advancements in materials science, finding cost-effective, long-lasting containment and transport solutions remains a critical issue.
Environmental concerns also present a major challenge. The production and use of hydrochloric acid can result in harmful emissions and waste products. Developing cleaner, more sustainable manufacturing processes and implementing effective waste management strategies are ongoing challenges that require innovative solutions.
Another significant hurdle is the optimization of hydrochloric acid catalysis in industrial processes. While hydrochloric acid serves as an essential catalyst in numerous chemical reactions, improving its efficiency and selectivity in complex reaction environments continues to be a focus of research. Scientists are grappling with the task of enhancing catalytic performance while minimizing unwanted side reactions and reducing the overall acid consumption.
The purification and concentration of hydrochloric acid also present technical difficulties. Achieving high purity levels for specialized applications, such as semiconductor manufacturing, demands advanced separation and purification techniques. Additionally, the energy-intensive nature of concentration processes poses both economic and environmental challenges.
In the realm of analytical chemistry, the accurate detection and quantification of hydrochloric acid in various matrices remain challenging. Developing sensitive, reliable, and rapid analytical methods for diverse sample types is crucial for quality control, environmental monitoring, and research applications.
The storage and transportation of hydrochloric acid continue to be areas of concern. Ensuring the safety and integrity of storage facilities and transport vehicles, particularly in diverse climatic conditions, requires ongoing innovation in materials and engineering solutions.
Lastly, the search for alternatives to hydrochloric acid in certain applications presents both a challenge and an opportunity. While hydrochloric acid is irreplaceable in many processes, there is a growing need to find less hazardous or more environmentally friendly substitutes where possible. This pursuit necessitates a deep understanding of reaction mechanisms and the development of novel chemical pathways.
Environmental concerns also present a major challenge. The production and use of hydrochloric acid can result in harmful emissions and waste products. Developing cleaner, more sustainable manufacturing processes and implementing effective waste management strategies are ongoing challenges that require innovative solutions.
Another significant hurdle is the optimization of hydrochloric acid catalysis in industrial processes. While hydrochloric acid serves as an essential catalyst in numerous chemical reactions, improving its efficiency and selectivity in complex reaction environments continues to be a focus of research. Scientists are grappling with the task of enhancing catalytic performance while minimizing unwanted side reactions and reducing the overall acid consumption.
The purification and concentration of hydrochloric acid also present technical difficulties. Achieving high purity levels for specialized applications, such as semiconductor manufacturing, demands advanced separation and purification techniques. Additionally, the energy-intensive nature of concentration processes poses both economic and environmental challenges.
In the realm of analytical chemistry, the accurate detection and quantification of hydrochloric acid in various matrices remain challenging. Developing sensitive, reliable, and rapid analytical methods for diverse sample types is crucial for quality control, environmental monitoring, and research applications.
The storage and transportation of hydrochloric acid continue to be areas of concern. Ensuring the safety and integrity of storage facilities and transport vehicles, particularly in diverse climatic conditions, requires ongoing innovation in materials and engineering solutions.
Lastly, the search for alternatives to hydrochloric acid in certain applications presents both a challenge and an opportunity. While hydrochloric acid is irreplaceable in many processes, there is a growing need to find less hazardous or more environmentally friendly substitutes where possible. This pursuit necessitates a deep understanding of reaction mechanisms and the development of novel chemical pathways.
Modern HCl Solutions
01 Production methods of hydrochloric acid
Various methods are employed for the production of hydrochloric acid, including chemical reactions and industrial processes. These methods may involve the use of specific catalysts, reactants, or equipment to efficiently produce hydrochloric acid at different concentrations and purities.- Production and purification of hydrochloric acid: Various methods and systems are employed for the production and purification of hydrochloric acid. These processes may involve chemical reactions, distillation, or other separation techniques to obtain high-quality hydrochloric acid for industrial applications.
- Applications in chemical processing: Hydrochloric acid is widely used in chemical processing industries for various purposes, including as a reagent, catalyst, or pH adjuster. It plays a crucial role in the production of numerous chemicals and materials.
- Waste treatment and recycling: Processes and systems have been developed for the treatment and recycling of hydrochloric acid waste. These methods aim to reduce environmental impact and recover valuable materials from industrial effluents containing hydrochloric acid.
- Corrosion prevention and material handling: Special equipment and materials are designed to handle and store hydrochloric acid safely, preventing corrosion and ensuring worker safety. This includes the development of acid-resistant coatings, containers, and handling systems.
- Analytical and measurement techniques: Various analytical methods and devices have been invented for measuring and monitoring hydrochloric acid concentration, purity, and other properties. These techniques are essential for quality control and process optimization in industries using hydrochloric acid.
02 Purification and concentration of hydrochloric acid
Techniques for purifying and concentrating hydrochloric acid are essential in industrial applications. These processes may involve distillation, membrane separation, or other chemical treatments to remove impurities and achieve desired acid concentrations for specific uses.Expand Specific Solutions03 Applications of hydrochloric acid in chemical processes
Hydrochloric acid is widely used in various chemical processes and industries. It serves as a key reagent in metal processing, pH adjustment, and as a catalyst in organic synthesis reactions. The acid's properties make it valuable in diverse applications across different sectors.Expand Specific Solutions04 Storage and handling of hydrochloric acid
Proper storage and handling of hydrochloric acid are crucial for safety and efficiency. Specialized containers, equipment, and procedures are employed to manage the corrosive nature of the acid and prevent accidents or environmental contamination during transportation and use.Expand Specific Solutions05 Recovery and recycling of hydrochloric acid
Methods for recovering and recycling hydrochloric acid from industrial processes are important for environmental and economic reasons. These techniques may involve absorption, neutralization, or regeneration processes to reclaim the acid for reuse or to minimize waste.Expand Specific Solutions
Key Industry Players
The evolution of hydrochloric acid in advanced chemistry represents a mature field with significant market presence and ongoing technological advancements. The industry is characterized by established players like Covestro Deutschland AG, Industrie De Nora SpA, and Merck Patent GmbH, alongside emerging companies such as WIAB WATER INNOVATION AB and Sunshine Lake Pharma Co., Ltd. The market size is substantial, driven by diverse applications in pharmaceuticals, industrial processes, and water treatment. Technological maturity is evident, with companies like Synthon BV and Bayer CropScience LP contributing to continuous improvements in production methods, purity levels, and application-specific formulations. However, there's still room for innovation, particularly in sustainable production and novel applications in emerging fields.
Covestro Deutschland AG
Technical Solution: Covestro has developed innovative processes for the production and application of hydrochloric acid in advanced chemistry. Their approach focuses on sustainable production methods, including the use of membrane electrolysis technology for chlor-alkali production, which generates high-purity hydrochloric acid as a by-product[1]. They have also implemented a closed-loop recycling system for hydrochloric acid in their polyurethane production, reducing waste and improving resource efficiency[2]. Additionally, Covestro has developed novel catalysts that enable the use of hydrochloric acid in the synthesis of isocyanates, a key component in polyurethane production, with improved yield and selectivity[3].
Strengths: Sustainable production methods, closed-loop recycling, and improved catalytic processes. Weaknesses: Potential high initial investment costs for implementing new technologies and processes.
Industrie De Nora SpA
Technical Solution: De Nora has pioneered advanced electrochemical technologies for the production and utilization of hydrochloric acid. Their innovative approach includes the development of dimensionally stable anodes (DSA) for chlor-alkali electrolysis, which significantly improves the efficiency and purity of hydrochloric acid production[4]. They have also introduced membrane electrolyzers that allow for the direct production of high-purity hydrochloric acid from hydrogen and chlorine gases[5]. Furthermore, De Nora has developed electrochemical processes for the recovery and recycling of hydrochloric acid from industrial waste streams, contributing to circular economy principles in chemical manufacturing[6].
Strengths: Cutting-edge electrochemical technologies, high-purity acid production, and waste recovery solutions. Weaknesses: Reliance on specialized equipment and potentially higher operational costs.
Core HCl Innovations
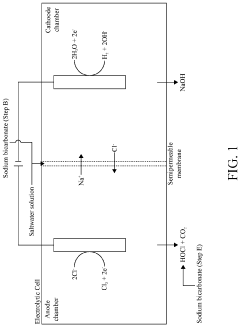
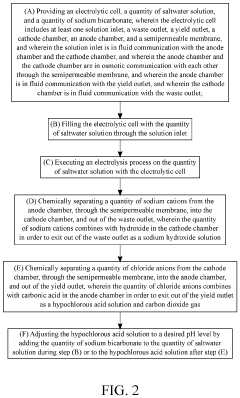
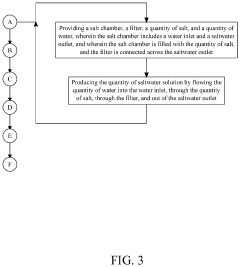
System and Method for Making Hypochlorous Acid Using Saltwater with Sodium Bicarbonate
PatentActiveUS20210395904A1
Innovation
- Incorporating sodium bicarbonate into the saltwater electrolysis process using a semipermeable membrane to separate the solutions, which forms purer hypochlorous acid by reacting chloride ions with carbonic acid, thereby maintaining a higher pH and eliminating strong hydrochloric acid.
Chemical composition, method for manufacturing hypochlorous acid for obtaining said chemical composition and installation to perform said method
PatentPendingUS20240407368A1
Innovation
- A method involving the transformation of sodium or potassic hypochlorite into hypochlorous acid, which also produces chlorine dioxide as a by-product, while minimizing impurities like chlorites, chlorates, and carbonates, resulting in a more pure composition with enhanced biocidal properties by utilizing a controlled process involving reactors and precise control units for pH and total chlorine levels.
Environmental Impact
The environmental impact of hydrochloric acid (HCl) in advanced chemistry has been a subject of increasing concern as its applications have expanded and evolved. The production, use, and disposal of HCl can have significant effects on ecosystems, air quality, and human health if not properly managed.
In industrial processes, the release of HCl emissions into the atmosphere has been a primary environmental concern. These emissions can contribute to acid rain formation, which negatively affects soil, water bodies, and vegetation. Acid rain can lead to the acidification of lakes and streams, damaging aquatic ecosystems and reducing biodiversity. Moreover, it can leach essential nutrients from the soil, impacting plant growth and forest health.
The corrosive nature of HCl poses risks to infrastructure and equipment, potentially leading to accidental releases and subsequent environmental contamination. In cases of improper handling or storage, HCl spills can cause localized soil and water pollution, altering pH levels and potentially harming flora and fauna in the affected areas.
However, advancements in chemistry have led to improved methods for HCl production and handling, mitigating some of these environmental risks. Modern industrial processes have implemented more efficient scrubbing technologies to reduce HCl emissions. Closed-loop systems and recycling techniques have also been developed to minimize waste and prevent environmental release.
In water treatment applications, HCl plays a crucial role in pH adjustment and water purification. While its use can improve water quality for human consumption, careful management is necessary to prevent over-acidification of treated water bodies. Balancing the benefits of water treatment with potential ecological impacts remains an ongoing challenge.
The evolution of HCl in advanced chemistry has also led to the development of alternative synthesis routes and substitutes for certain applications. Green chemistry initiatives have focused on reducing the environmental footprint of HCl production and use. These efforts include exploring bio-based alternatives, developing less hazardous substitutes, and optimizing processes to minimize waste generation.
As environmental regulations have become more stringent, industries using HCl have been compelled to adopt cleaner technologies and improved waste management practices. This has resulted in a gradual reduction of the overall environmental impact of HCl in many advanced chemistry applications. Nonetheless, ongoing research and innovation are essential to further mitigate the environmental risks associated with HCl while maintaining its crucial role in various industrial and scientific processes.
In industrial processes, the release of HCl emissions into the atmosphere has been a primary environmental concern. These emissions can contribute to acid rain formation, which negatively affects soil, water bodies, and vegetation. Acid rain can lead to the acidification of lakes and streams, damaging aquatic ecosystems and reducing biodiversity. Moreover, it can leach essential nutrients from the soil, impacting plant growth and forest health.
The corrosive nature of HCl poses risks to infrastructure and equipment, potentially leading to accidental releases and subsequent environmental contamination. In cases of improper handling or storage, HCl spills can cause localized soil and water pollution, altering pH levels and potentially harming flora and fauna in the affected areas.
However, advancements in chemistry have led to improved methods for HCl production and handling, mitigating some of these environmental risks. Modern industrial processes have implemented more efficient scrubbing technologies to reduce HCl emissions. Closed-loop systems and recycling techniques have also been developed to minimize waste and prevent environmental release.
In water treatment applications, HCl plays a crucial role in pH adjustment and water purification. While its use can improve water quality for human consumption, careful management is necessary to prevent over-acidification of treated water bodies. Balancing the benefits of water treatment with potential ecological impacts remains an ongoing challenge.
The evolution of HCl in advanced chemistry has also led to the development of alternative synthesis routes and substitutes for certain applications. Green chemistry initiatives have focused on reducing the environmental footprint of HCl production and use. These efforts include exploring bio-based alternatives, developing less hazardous substitutes, and optimizing processes to minimize waste generation.
As environmental regulations have become more stringent, industries using HCl have been compelled to adopt cleaner technologies and improved waste management practices. This has resulted in a gradual reduction of the overall environmental impact of HCl in many advanced chemistry applications. Nonetheless, ongoing research and innovation are essential to further mitigate the environmental risks associated with HCl while maintaining its crucial role in various industrial and scientific processes.
Safety Regulations
The evolution of hydrochloric acid in advanced chemistry has necessitated the development of stringent safety regulations to protect workers, the environment, and the public. These regulations have become increasingly comprehensive over time, reflecting the growing understanding of the acid's hazards and the advancement of safety technologies.
In the early stages of industrial hydrochloric acid use, safety measures were often rudimentary, focusing primarily on basic personal protective equipment (PPE) such as gloves and goggles. As the chemical industry expanded, regulatory bodies began to implement more robust safety standards. The Occupational Safety and Health Administration (OSHA) in the United States, for instance, established permissible exposure limits (PELs) for hydrochloric acid in the workplace, setting the foundation for modern safety practices.
The transportation of hydrochloric acid has also been subject to evolving regulations. The United Nations' Recommendations on the Transport of Dangerous Goods classifies hydrochloric acid as a corrosive substance, mandating specific packaging, labeling, and handling requirements. These international guidelines have been adopted and adapted by many countries, ensuring a global standard for the safe movement of the acid.
Environmental concerns have led to the implementation of strict disposal regulations. The Resource Conservation and Recovery Act (RCRA) in the United States categorizes spent hydrochloric acid as a hazardous waste, requiring proper treatment and disposal methods. Similar legislation has been enacted in other countries, emphasizing the need for responsible management of acid waste to prevent environmental contamination.
Recent advancements in safety regulations have focused on risk assessment and prevention. The Process Safety Management (PSM) standard, for example, requires facilities handling large quantities of hydrochloric acid to develop and implement comprehensive safety programs. These programs include hazard analysis, emergency response planning, and employee training, reflecting a shift towards proactive safety management.
The digital age has brought about new regulatory approaches, such as electronic tracking systems for acid shipments and real-time monitoring of storage conditions. These technological solutions enhance compliance with safety regulations and enable faster response times in case of incidents.
As research continues to uncover potential long-term effects of hydrochloric acid exposure, regulations are likely to evolve further. There is an increasing emphasis on minimizing worker exposure through engineering controls and exploring safer alternatives where possible. The future of hydrochloric acid safety regulations will likely see a continued focus on risk reduction, environmental protection, and the integration of advanced monitoring and control technologies.
In the early stages of industrial hydrochloric acid use, safety measures were often rudimentary, focusing primarily on basic personal protective equipment (PPE) such as gloves and goggles. As the chemical industry expanded, regulatory bodies began to implement more robust safety standards. The Occupational Safety and Health Administration (OSHA) in the United States, for instance, established permissible exposure limits (PELs) for hydrochloric acid in the workplace, setting the foundation for modern safety practices.
The transportation of hydrochloric acid has also been subject to evolving regulations. The United Nations' Recommendations on the Transport of Dangerous Goods classifies hydrochloric acid as a corrosive substance, mandating specific packaging, labeling, and handling requirements. These international guidelines have been adopted and adapted by many countries, ensuring a global standard for the safe movement of the acid.
Environmental concerns have led to the implementation of strict disposal regulations. The Resource Conservation and Recovery Act (RCRA) in the United States categorizes spent hydrochloric acid as a hazardous waste, requiring proper treatment and disposal methods. Similar legislation has been enacted in other countries, emphasizing the need for responsible management of acid waste to prevent environmental contamination.
Recent advancements in safety regulations have focused on risk assessment and prevention. The Process Safety Management (PSM) standard, for example, requires facilities handling large quantities of hydrochloric acid to develop and implement comprehensive safety programs. These programs include hazard analysis, emergency response planning, and employee training, reflecting a shift towards proactive safety management.
The digital age has brought about new regulatory approaches, such as electronic tracking systems for acid shipments and real-time monitoring of storage conditions. These technological solutions enhance compliance with safety regulations and enable faster response times in case of incidents.
As research continues to uncover potential long-term effects of hydrochloric acid exposure, regulations are likely to evolve further. There is an increasing emphasis on minimizing worker exposure through engineering controls and exploring safer alternatives where possible. The future of hydrochloric acid safety regulations will likely see a continued focus on risk reduction, environmental protection, and the integration of advanced monitoring and control technologies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!