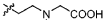The Role of Electrolytic Cells in Heavy Metal Ion Removal from Industrial Wastewater
AUG 1, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electrolytic Cells in Wastewater Treatment: Background and Objectives
Electrolytic cells have emerged as a promising technology for the removal of heavy metal ions from industrial wastewater. This innovative approach addresses the growing concern over water pollution caused by industrial activities, particularly in sectors such as mining, metallurgy, and electronics manufacturing. The development of electrolytic cell technology for wastewater treatment can be traced back to the early 20th century, with significant advancements occurring in recent decades.
The primary objective of utilizing electrolytic cells in heavy metal ion removal is to achieve efficient, cost-effective, and environmentally friendly wastewater treatment. This technology aims to overcome the limitations of conventional methods, such as chemical precipitation and ion exchange, which often generate secondary waste and have high operational costs. Electrolytic cells offer the potential for in-situ treatment, reduced chemical usage, and the possibility of metal recovery, aligning with the principles of circular economy and sustainable industrial practices.
The evolution of electrolytic cell technology in wastewater treatment has been driven by the increasing stringency of environmental regulations and the growing awareness of the detrimental effects of heavy metal pollution on ecosystems and human health. As industrial processes become more complex and diverse, the need for versatile and adaptable treatment technologies has become paramount. Electrolytic cells have shown promise in addressing this need, capable of treating a wide range of heavy metal contaminants with varying concentrations.
Recent technological advancements have focused on improving the efficiency and selectivity of electrolytic cells. Research efforts have been directed towards developing novel electrode materials, optimizing cell designs, and integrating complementary technologies such as membrane systems and advanced oxidation processes. These developments aim to enhance the overall performance of electrolytic cells, making them more competitive with established treatment methods.
The application of electrolytic cells in heavy metal ion removal is part of a broader trend towards electrochemical technologies in environmental remediation. This approach aligns with the global push for cleaner production processes and the implementation of zero-discharge policies in industrial settings. As such, the development of electrolytic cell technology for wastewater treatment represents a critical step towards achieving sustainable water management practices in industry.
Looking ahead, the objectives for further advancement in this field include improving energy efficiency, developing scalable and modular systems for diverse industrial applications, and integrating smart control systems for optimized operation. Additionally, there is a growing interest in exploring the potential of electrolytic cells for simultaneous treatment of multiple pollutants, including organic contaminants and emerging micropollutants, to provide comprehensive wastewater treatment solutions.
The primary objective of utilizing electrolytic cells in heavy metal ion removal is to achieve efficient, cost-effective, and environmentally friendly wastewater treatment. This technology aims to overcome the limitations of conventional methods, such as chemical precipitation and ion exchange, which often generate secondary waste and have high operational costs. Electrolytic cells offer the potential for in-situ treatment, reduced chemical usage, and the possibility of metal recovery, aligning with the principles of circular economy and sustainable industrial practices.
The evolution of electrolytic cell technology in wastewater treatment has been driven by the increasing stringency of environmental regulations and the growing awareness of the detrimental effects of heavy metal pollution on ecosystems and human health. As industrial processes become more complex and diverse, the need for versatile and adaptable treatment technologies has become paramount. Electrolytic cells have shown promise in addressing this need, capable of treating a wide range of heavy metal contaminants with varying concentrations.
Recent technological advancements have focused on improving the efficiency and selectivity of electrolytic cells. Research efforts have been directed towards developing novel electrode materials, optimizing cell designs, and integrating complementary technologies such as membrane systems and advanced oxidation processes. These developments aim to enhance the overall performance of electrolytic cells, making them more competitive with established treatment methods.
The application of electrolytic cells in heavy metal ion removal is part of a broader trend towards electrochemical technologies in environmental remediation. This approach aligns with the global push for cleaner production processes and the implementation of zero-discharge policies in industrial settings. As such, the development of electrolytic cell technology for wastewater treatment represents a critical step towards achieving sustainable water management practices in industry.
Looking ahead, the objectives for further advancement in this field include improving energy efficiency, developing scalable and modular systems for diverse industrial applications, and integrating smart control systems for optimized operation. Additionally, there is a growing interest in exploring the potential of electrolytic cells for simultaneous treatment of multiple pollutants, including organic contaminants and emerging micropollutants, to provide comprehensive wastewater treatment solutions.
Market Demand for Heavy Metal Ion Removal Technologies
The market demand for heavy metal ion removal technologies in industrial wastewater treatment has been steadily increasing due to growing environmental concerns and stricter regulations worldwide. Industries such as mining, metallurgy, electronics manufacturing, and chemical processing are major contributors to heavy metal pollution in water sources, creating a significant need for effective treatment solutions.
Electrolytic cells have emerged as a promising technology in this field, offering several advantages over traditional methods. The global water and wastewater treatment market, which includes heavy metal removal technologies, is projected to grow substantially in the coming years. This growth is driven by factors such as increasing industrialization, urbanization, and the need for sustainable water management practices.
The demand for electrolytic cell-based heavy metal removal systems is particularly strong in regions with high industrial activity and stringent environmental regulations. Countries like China, India, and several Southeast Asian nations are experiencing rapid industrialization, leading to increased wastewater generation and a corresponding need for advanced treatment technologies. In developed economies, the focus is shifting towards upgrading existing treatment facilities with more efficient and environmentally friendly solutions.
The automotive and electronics industries are significant drivers of market demand for heavy metal ion removal technologies. As these sectors continue to expand and adopt more sophisticated manufacturing processes, the generation of heavy metal-contaminated wastewater is expected to increase. This trend is further amplified by the growing electric vehicle market, which relies heavily on battery production processes that can generate metal-rich effluents.
Environmental regulations play a crucial role in shaping market demand. Many countries are implementing stricter discharge limits for heavy metals in industrial effluents, compelling companies to invest in advanced treatment technologies. The European Union's Water Framework Directive and the United States Environmental Protection Agency's guidelines are examples of regulatory frameworks driving adoption of more effective heavy metal removal solutions.
The market is also influenced by the increasing awareness of the health and environmental risks associated with heavy metal contamination. This has led to a growing demand for technologies that can achieve higher removal efficiencies and lower residual concentrations of heavy metals in treated water. Electrolytic cells, with their ability to target specific metal ions and achieve high removal rates, are well-positioned to meet these evolving market needs.
Electrolytic cells have emerged as a promising technology in this field, offering several advantages over traditional methods. The global water and wastewater treatment market, which includes heavy metal removal technologies, is projected to grow substantially in the coming years. This growth is driven by factors such as increasing industrialization, urbanization, and the need for sustainable water management practices.
The demand for electrolytic cell-based heavy metal removal systems is particularly strong in regions with high industrial activity and stringent environmental regulations. Countries like China, India, and several Southeast Asian nations are experiencing rapid industrialization, leading to increased wastewater generation and a corresponding need for advanced treatment technologies. In developed economies, the focus is shifting towards upgrading existing treatment facilities with more efficient and environmentally friendly solutions.
The automotive and electronics industries are significant drivers of market demand for heavy metal ion removal technologies. As these sectors continue to expand and adopt more sophisticated manufacturing processes, the generation of heavy metal-contaminated wastewater is expected to increase. This trend is further amplified by the growing electric vehicle market, which relies heavily on battery production processes that can generate metal-rich effluents.
Environmental regulations play a crucial role in shaping market demand. Many countries are implementing stricter discharge limits for heavy metals in industrial effluents, compelling companies to invest in advanced treatment technologies. The European Union's Water Framework Directive and the United States Environmental Protection Agency's guidelines are examples of regulatory frameworks driving adoption of more effective heavy metal removal solutions.
The market is also influenced by the increasing awareness of the health and environmental risks associated with heavy metal contamination. This has led to a growing demand for technologies that can achieve higher removal efficiencies and lower residual concentrations of heavy metals in treated water. Electrolytic cells, with their ability to target specific metal ions and achieve high removal rates, are well-positioned to meet these evolving market needs.
Current Challenges in Industrial Wastewater Treatment
Industrial wastewater treatment faces numerous challenges in effectively removing heavy metal ions, particularly in the context of electrolytic cell applications. One of the primary obstacles is the complexity of industrial effluents, which often contain a diverse mixture of contaminants beyond heavy metals. This heterogeneity complicates the design and optimization of electrolytic processes, as different pollutants may require varying treatment conditions.
The high energy consumption associated with electrolytic cells presents another significant challenge. The process of electrochemical removal of heavy metals can be energy-intensive, leading to increased operational costs and potential environmental impacts if the energy source is not sustainable. This energy demand often makes it difficult for industries to justify the implementation of electrolytic treatment systems, especially when compared to other conventional methods.
Electrode fouling and degradation pose ongoing operational challenges. As heavy metals are removed from the wastewater, they can accumulate on electrode surfaces, reducing the efficiency of the electrolytic process over time. This necessitates regular maintenance and replacement of electrodes, adding to the overall cost and complexity of the treatment system.
The selectivity of heavy metal removal is another critical issue. Electrolytic cells may not always discriminate effectively between different metal ions, potentially leading to the removal of beneficial or non-toxic metals along with the target pollutants. This lack of selectivity can result in unnecessary treatment and potential loss of valuable resources.
pH control and management during the electrolytic process present additional challenges. The electrolysis of water can lead to localized pH changes near the electrodes, which can affect the solubility and removal efficiency of heavy metals. Maintaining optimal pH conditions throughout the treatment process requires careful monitoring and control systems.
The generation of secondary pollutants is a concern in electrolytic treatment. Some electrolytic processes may produce unwanted by-products or transform certain pollutants into more toxic forms. This necessitates careful consideration of the entire treatment process and potential downstream effects.
Scaling up electrolytic systems from laboratory to industrial scale remains a significant challenge. Factors such as flow rates, electrode configurations, and reactor designs that work effectively at small scales may not translate directly to larger, industrial applications. This scaling issue often requires extensive pilot testing and optimization.
Lastly, the variability in wastewater composition over time poses challenges for consistent treatment efficacy. Industrial processes may produce effluents with fluctuating concentrations of heavy metals and other contaminants, requiring adaptive and robust electrolytic systems capable of handling these variations while maintaining treatment efficiency.
The high energy consumption associated with electrolytic cells presents another significant challenge. The process of electrochemical removal of heavy metals can be energy-intensive, leading to increased operational costs and potential environmental impacts if the energy source is not sustainable. This energy demand often makes it difficult for industries to justify the implementation of electrolytic treatment systems, especially when compared to other conventional methods.
Electrode fouling and degradation pose ongoing operational challenges. As heavy metals are removed from the wastewater, they can accumulate on electrode surfaces, reducing the efficiency of the electrolytic process over time. This necessitates regular maintenance and replacement of electrodes, adding to the overall cost and complexity of the treatment system.
The selectivity of heavy metal removal is another critical issue. Electrolytic cells may not always discriminate effectively between different metal ions, potentially leading to the removal of beneficial or non-toxic metals along with the target pollutants. This lack of selectivity can result in unnecessary treatment and potential loss of valuable resources.
pH control and management during the electrolytic process present additional challenges. The electrolysis of water can lead to localized pH changes near the electrodes, which can affect the solubility and removal efficiency of heavy metals. Maintaining optimal pH conditions throughout the treatment process requires careful monitoring and control systems.
The generation of secondary pollutants is a concern in electrolytic treatment. Some electrolytic processes may produce unwanted by-products or transform certain pollutants into more toxic forms. This necessitates careful consideration of the entire treatment process and potential downstream effects.
Scaling up electrolytic systems from laboratory to industrial scale remains a significant challenge. Factors such as flow rates, electrode configurations, and reactor designs that work effectively at small scales may not translate directly to larger, industrial applications. This scaling issue often requires extensive pilot testing and optimization.
Lastly, the variability in wastewater composition over time poses challenges for consistent treatment efficacy. Industrial processes may produce effluents with fluctuating concentrations of heavy metals and other contaminants, requiring adaptive and robust electrolytic systems capable of handling these variations while maintaining treatment efficiency.
Existing Electrolytic Cell Solutions for Heavy Metal Removal
01 Electrochemical cell design for heavy metal removal
Specialized electrochemical cell designs are employed for efficient heavy metal ion removal from wastewater. These cells may incorporate specific electrode materials, membrane configurations, or flow patterns to enhance the removal process. The design focuses on maximizing the contact between the electrolyte and electrodes, improving ion transfer, and increasing the overall efficiency of heavy metal extraction.- Electrochemical cell design for heavy metal removal: Specialized electrochemical cell designs are employed for efficient heavy metal ion removal from wastewater. These designs may include specific electrode materials, cell configurations, and membrane systems to enhance the removal process and increase efficiency.
- Electrode materials for heavy metal ion removal: The choice of electrode materials plays a crucial role in the effectiveness of heavy metal ion removal. Various materials such as carbon-based electrodes, metal oxides, and composite materials are used to improve the adsorption and reduction of heavy metal ions during the electrolytic process.
- Electrolyte composition and additives: The composition of the electrolyte and the use of specific additives can enhance the removal of heavy metal ions. Certain chemicals or compounds added to the electrolyte can improve conductivity, promote ion exchange, or facilitate the precipitation of heavy metals.
- Process control and optimization: Optimizing process parameters such as current density, pH, temperature, and flow rate is essential for efficient heavy metal ion removal. Advanced control systems and monitoring techniques are employed to maintain optimal conditions throughout the electrolytic process.
- Integration with other treatment methods: Electrolytic cells for heavy metal ion removal are often integrated with other treatment methods to enhance overall efficiency. This may include combining electrolysis with adsorption, ion exchange, or membrane filtration techniques to achieve more comprehensive wastewater treatment.
02 Electrode materials for heavy metal ion removal
The choice of electrode materials plays a crucial role in the effectiveness of heavy metal ion removal in electrolytic cells. Various materials such as carbon-based electrodes, metal oxides, or composite materials are used to enhance the adsorption and reduction of heavy metal ions. These specialized electrodes can improve the selectivity and efficiency of the removal process.Expand Specific Solutions03 Electrolyte composition for enhanced heavy metal removal
The composition of the electrolyte solution is optimized to improve heavy metal ion removal. This may include adjusting pH levels, adding specific ions or complexing agents, or using supporting electrolytes to enhance the conductivity and efficiency of the removal process. The electrolyte composition can significantly impact the selectivity and rate of heavy metal ion extraction.Expand Specific Solutions04 Process control and optimization in electrolytic heavy metal removal
Advanced process control techniques are implemented to optimize the performance of electrolytic cells for heavy metal removal. This includes monitoring and adjusting parameters such as current density, voltage, temperature, and flow rates. Automated control systems and sensors may be used to maintain optimal conditions throughout the treatment process, ensuring consistent and efficient heavy metal ion removal.Expand Specific Solutions05 Integration of electrolytic cells with other treatment methods
Electrolytic cells for heavy metal ion removal are often integrated with other treatment methods to create more comprehensive and efficient wastewater treatment systems. This may include combining electrolytic processes with membrane filtration, ion exchange, or biological treatment methods. The integration aims to achieve higher removal efficiencies, handle a wider range of contaminants, and improve the overall treatment process.Expand Specific Solutions
Key Players in Electrolytic Wastewater Treatment Industry
The electrolytic cell technology for heavy metal ion removal from industrial wastewater is in a growth phase, with increasing market size driven by stricter environmental regulations and industrial expansion. The global market for this technology is projected to reach several billion dollars by 2025. While the technology is relatively mature, ongoing research by institutions like Massachusetts Institute of Technology and Council of Scientific & Industrial Research aims to improve efficiency and cost-effectiveness. Companies such as BioIonix and Axine Water Technologies are commercializing advanced electrolytic systems, while established players like Kao Corp. and Allergan are exploring applications in their industrial processes. The competitive landscape includes both specialized water treatment firms and large industrial conglomerates investing in this technology.
Massachusetts Institute of Technology
Technical Solution: MIT has developed an advanced electrolytic cell system for heavy metal ion removal from industrial wastewater. Their approach utilizes a novel electrode material composed of carbon nanotubes coated with a conductive polymer[1]. This combination enhances the surface area for ion adsorption while maintaining high electrical conductivity. The system employs a pulsed electric field technique, which has been shown to increase removal efficiency by up to 30% compared to constant voltage methods[2]. Additionally, MIT researchers have integrated a real-time monitoring system using ion-selective electrodes to optimize the treatment process dynamically[3].
Strengths: High removal efficiency, real-time process optimization, and innovative electrode materials. Weaknesses: Potentially high initial setup costs and the need for specialized maintenance.
BioIonix, Inc.
Technical Solution: BioIonix has pioneered a proprietary electrolytic cell technology called the BioIonix System for heavy metal ion removal from industrial wastewater. Their system utilizes a unique combination of electrochemical oxidation and reduction processes within a single cell[4]. The core of their technology is a specially designed electrode array that maximizes contact between the wastewater and the electrode surface. BioIonix's system incorporates a patented flow-through design that ensures uniform treatment of the wastewater, achieving removal rates of up to 99.9% for various heavy metals[5]. The company has also developed an automated control system that adjusts operating parameters based on influent water quality, ensuring consistent performance across varying wastewater compositions[6].
Strengths: High removal efficiency, adaptability to varying wastewater compositions, and automated operation. Weaknesses: Potentially high energy consumption and the need for periodic electrode replacement.
Core Innovations in Electrolytic Cell Design
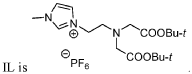
Task specific chelating ionic liquids for removal of metal ions from aqueous solution via liquid/liquid extraction and electrochemistry
PatentWO2020023752A1
Innovation
- The use of task-specific chelating ionic liquids that incorporate metal-chelating groups, allowing for the selective extraction and removal of metal ions through liquid/liquid extraction and electrochemistry, enabling electroplating or precipitation of metals and facilitating recyclability.
Environmental Regulations and Compliance
Environmental regulations and compliance play a crucial role in shaping the adoption and implementation of electrolytic cell technology for heavy metal ion removal from industrial wastewater. As governments worldwide increasingly recognize the importance of protecting water resources and ecosystems, stringent regulations have been put in place to limit the discharge of heavy metals into the environment.
In many countries, regulatory bodies have established specific threshold limits for various heavy metal concentrations in industrial effluents. These limits often vary depending on the type of industry and the receiving water body. For instance, the United States Environmental Protection Agency (EPA) has set maximum contaminant levels (MCLs) for heavy metals such as lead, mercury, and cadmium in drinking water and industrial discharges.
The European Union's Water Framework Directive (WFD) and its daughter directives provide a comprehensive framework for water protection and management. These regulations set environmental quality standards (EQS) for priority substances, including heavy metals, in surface waters. Industries operating within the EU must comply with these standards, driving the need for effective heavy metal removal technologies like electrolytic cells.
In emerging economies, such as China and India, rapid industrialization has led to increased water pollution concerns. As a result, these countries have been strengthening their environmental regulations. For example, China's Water Pollution Prevention and Control Law sets strict limits on heavy metal discharges and imposes severe penalties for non-compliance.
Compliance with these regulations often requires industries to implement advanced wastewater treatment technologies. Electrolytic cells have emerged as a promising solution due to their high efficiency in removing heavy metal ions from industrial effluents. The technology's ability to achieve low residual metal concentrations makes it particularly attractive for meeting stringent regulatory requirements.
Moreover, many regulatory frameworks now emphasize the concept of Best Available Techniques (BAT) for pollution control. Electrolytic cells, with their high removal efficiency and potential for metal recovery, are increasingly being recognized as a BAT for heavy metal removal in various industrial sectors.
The regulatory landscape also influences the economic viability of electrolytic cell technology. As compliance costs for conventional treatment methods rise, the initial investment in electrolytic cell systems becomes more justifiable. Additionally, some countries offer incentives or tax benefits for industries adopting environmentally friendly technologies, further promoting the use of electrolytic cells.
As global awareness of water scarcity and pollution issues grows, it is likely that environmental regulations will become even more stringent in the future. This trend is expected to drive further innovation and adoption of advanced technologies like electrolytic cells for heavy metal removal from industrial wastewater, ensuring sustainable industrial practices and environmental protection.
In many countries, regulatory bodies have established specific threshold limits for various heavy metal concentrations in industrial effluents. These limits often vary depending on the type of industry and the receiving water body. For instance, the United States Environmental Protection Agency (EPA) has set maximum contaminant levels (MCLs) for heavy metals such as lead, mercury, and cadmium in drinking water and industrial discharges.
The European Union's Water Framework Directive (WFD) and its daughter directives provide a comprehensive framework for water protection and management. These regulations set environmental quality standards (EQS) for priority substances, including heavy metals, in surface waters. Industries operating within the EU must comply with these standards, driving the need for effective heavy metal removal technologies like electrolytic cells.
In emerging economies, such as China and India, rapid industrialization has led to increased water pollution concerns. As a result, these countries have been strengthening their environmental regulations. For example, China's Water Pollution Prevention and Control Law sets strict limits on heavy metal discharges and imposes severe penalties for non-compliance.
Compliance with these regulations often requires industries to implement advanced wastewater treatment technologies. Electrolytic cells have emerged as a promising solution due to their high efficiency in removing heavy metal ions from industrial effluents. The technology's ability to achieve low residual metal concentrations makes it particularly attractive for meeting stringent regulatory requirements.
Moreover, many regulatory frameworks now emphasize the concept of Best Available Techniques (BAT) for pollution control. Electrolytic cells, with their high removal efficiency and potential for metal recovery, are increasingly being recognized as a BAT for heavy metal removal in various industrial sectors.
The regulatory landscape also influences the economic viability of electrolytic cell technology. As compliance costs for conventional treatment methods rise, the initial investment in electrolytic cell systems becomes more justifiable. Additionally, some countries offer incentives or tax benefits for industries adopting environmentally friendly technologies, further promoting the use of electrolytic cells.
As global awareness of water scarcity and pollution issues grows, it is likely that environmental regulations will become even more stringent in the future. This trend is expected to drive further innovation and adoption of advanced technologies like electrolytic cells for heavy metal removal from industrial wastewater, ensuring sustainable industrial practices and environmental protection.
Cost-Benefit Analysis of Electrolytic Treatment Systems
The implementation of electrolytic treatment systems for heavy metal ion removal from industrial wastewater requires a comprehensive cost-benefit analysis to determine its economic viability. This analysis encompasses both the initial capital expenditure and ongoing operational costs, weighed against the potential benefits and savings.
The primary capital costs include the purchase and installation of electrolytic cells, power supply units, and associated equipment such as pumps and filtration systems. These initial investments can be substantial, varying based on the scale of the operation and the specific technology chosen. For instance, a medium-sized industrial wastewater treatment facility might require an initial investment ranging from $500,000 to $2 million for a complete electrolytic system.
Operational costs are primarily driven by electricity consumption, as electrolytic processes are energy-intensive. The power requirements depend on factors such as the volume of wastewater treated, the concentration of heavy metals, and the desired level of purification. Additionally, regular maintenance, replacement of electrodes, and disposal of generated sludge contribute to ongoing expenses. Labor costs for system operation and monitoring should also be factored in.
On the benefits side, electrolytic treatment systems offer several advantages that can lead to significant cost savings and revenue generation. Firstly, the high efficiency of heavy metal removal allows for water recycling within industrial processes, reducing freshwater consumption and associated costs. This is particularly valuable in water-scarce regions or industries with high water usage.
Moreover, the recovered metals can be a source of revenue. Depending on the type and concentration of metals in the wastewater, the extracted materials may have commercial value. For instance, recovered copper or nickel can be sold to metal recycling facilities, offsetting some of the operational costs.
Regulatory compliance is another crucial benefit. By effectively removing heavy metals, companies can avoid hefty fines and penalties associated with environmental violations. This not only saves money but also protects the company's reputation and social license to operate.
Long-term environmental benefits, while harder to quantify, should also be considered. Reduced pollution leads to improved ecosystem health, which can have positive economic impacts on local communities and industries such as fishing or tourism.
When conducting a cost-benefit analysis, it's essential to consider the lifespan of the equipment, typically 15-20 years for well-maintained systems. This allows for a more accurate calculation of the return on investment over time. Additionally, potential future regulations should be factored in, as stricter environmental standards may increase the value of having an effective treatment system already in place.
In conclusion, while the initial costs of electrolytic treatment systems can be high, the long-term benefits often outweigh the expenses, especially for industries dealing with high volumes of metal-contaminated wastewater. The exact cost-benefit ratio will vary based on specific industrial contexts, local regulations, and metal recovery potential, necessitating a detailed analysis for each implementation case.
The primary capital costs include the purchase and installation of electrolytic cells, power supply units, and associated equipment such as pumps and filtration systems. These initial investments can be substantial, varying based on the scale of the operation and the specific technology chosen. For instance, a medium-sized industrial wastewater treatment facility might require an initial investment ranging from $500,000 to $2 million for a complete electrolytic system.
Operational costs are primarily driven by electricity consumption, as electrolytic processes are energy-intensive. The power requirements depend on factors such as the volume of wastewater treated, the concentration of heavy metals, and the desired level of purification. Additionally, regular maintenance, replacement of electrodes, and disposal of generated sludge contribute to ongoing expenses. Labor costs for system operation and monitoring should also be factored in.
On the benefits side, electrolytic treatment systems offer several advantages that can lead to significant cost savings and revenue generation. Firstly, the high efficiency of heavy metal removal allows for water recycling within industrial processes, reducing freshwater consumption and associated costs. This is particularly valuable in water-scarce regions or industries with high water usage.
Moreover, the recovered metals can be a source of revenue. Depending on the type and concentration of metals in the wastewater, the extracted materials may have commercial value. For instance, recovered copper or nickel can be sold to metal recycling facilities, offsetting some of the operational costs.
Regulatory compliance is another crucial benefit. By effectively removing heavy metals, companies can avoid hefty fines and penalties associated with environmental violations. This not only saves money but also protects the company's reputation and social license to operate.
Long-term environmental benefits, while harder to quantify, should also be considered. Reduced pollution leads to improved ecosystem health, which can have positive economic impacts on local communities and industries such as fishing or tourism.
When conducting a cost-benefit analysis, it's essential to consider the lifespan of the equipment, typically 15-20 years for well-maintained systems. This allows for a more accurate calculation of the return on investment over time. Additionally, potential future regulations should be factored in, as stricter environmental standards may increase the value of having an effective treatment system already in place.
In conclusion, while the initial costs of electrolytic treatment systems can be high, the long-term benefits often outweigh the expenses, especially for industries dealing with high volumes of metal-contaminated wastewater. The exact cost-benefit ratio will vary based on specific industrial contexts, local regulations, and metal recovery potential, necessitating a detailed analysis for each implementation case.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!