Using patient-derived organ chips to predict clinical responders vs non-responders in precision oncology
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Organ Chip Technology Evolution and Objectives
Organ chip technology represents a revolutionary approach in biomedical research, evolving from traditional 2D cell cultures to sophisticated 3D microfluidic systems that mimic human organ functionality. The journey began in the early 2000s with rudimentary microfluidic devices, progressing through significant technological advancements to today's complex organ-on-chip platforms capable of replicating intricate physiological processes.
The evolution of organ chip technology has been marked by several key milestones. Initially, researchers focused on creating basic microfluidic channels to study fluid dynamics in biological systems. By the mid-2000s, integration of living cells into these systems enabled the first generation of tissue chips. The breakthrough came around 2010 when researchers at Harvard's Wyss Institute developed the first lung-on-a-chip, demonstrating mechanical breathing motions and inflammatory responses similar to human lungs.
Subsequent years witnessed rapid expansion in organ diversity, with liver, kidney, heart, and brain chips emerging through interdisciplinary collaboration between bioengineers, cell biologists, and clinicians. Recent advancements have focused on increasing physiological relevance through incorporation of patient-derived cells, immune components, and vascular networks, enabling more accurate disease modeling and drug response prediction.
In the oncology domain, organ chips have evolved to incorporate patient-derived tumor cells, creating "tumor-on-chip" models that preserve the heterogeneity and microenvironment of original tumors. This development represents a critical step toward precision oncology applications, allowing for personalized drug screening and response prediction.
The primary objective of patient-derived organ chip technology in precision oncology is to create individualized testing platforms that can predict clinical responses to cancer therapies before administration to patients. This approach aims to overcome limitations of traditional preclinical models, which often fail to translate to clinical outcomes due to their inability to capture patient-specific tumor characteristics and drug responses.
Additional objectives include reducing the time and cost of drug development by providing more predictive preclinical models, minimizing unnecessary treatment exposure for patients, and advancing understanding of resistance mechanisms in non-responders. Researchers also aim to develop standardized protocols for chip fabrication, cell sourcing, and data analysis to ensure reproducibility and clinical translation.
The ultimate goal is to establish organ chip technology as a routine clinical tool for oncologists, enabling real-time decision-making based on patient-specific drug response data and significantly improving treatment outcomes through truly personalized medicine approaches. This represents a paradigm shift from population-based treatment algorithms to individualized therapy selection based on functional testing of patient-derived tissues.
The evolution of organ chip technology has been marked by several key milestones. Initially, researchers focused on creating basic microfluidic channels to study fluid dynamics in biological systems. By the mid-2000s, integration of living cells into these systems enabled the first generation of tissue chips. The breakthrough came around 2010 when researchers at Harvard's Wyss Institute developed the first lung-on-a-chip, demonstrating mechanical breathing motions and inflammatory responses similar to human lungs.
Subsequent years witnessed rapid expansion in organ diversity, with liver, kidney, heart, and brain chips emerging through interdisciplinary collaboration between bioengineers, cell biologists, and clinicians. Recent advancements have focused on increasing physiological relevance through incorporation of patient-derived cells, immune components, and vascular networks, enabling more accurate disease modeling and drug response prediction.
In the oncology domain, organ chips have evolved to incorporate patient-derived tumor cells, creating "tumor-on-chip" models that preserve the heterogeneity and microenvironment of original tumors. This development represents a critical step toward precision oncology applications, allowing for personalized drug screening and response prediction.
The primary objective of patient-derived organ chip technology in precision oncology is to create individualized testing platforms that can predict clinical responses to cancer therapies before administration to patients. This approach aims to overcome limitations of traditional preclinical models, which often fail to translate to clinical outcomes due to their inability to capture patient-specific tumor characteristics and drug responses.
Additional objectives include reducing the time and cost of drug development by providing more predictive preclinical models, minimizing unnecessary treatment exposure for patients, and advancing understanding of resistance mechanisms in non-responders. Researchers also aim to develop standardized protocols for chip fabrication, cell sourcing, and data analysis to ensure reproducibility and clinical translation.
The ultimate goal is to establish organ chip technology as a routine clinical tool for oncologists, enabling real-time decision-making based on patient-specific drug response data and significantly improving treatment outcomes through truly personalized medicine approaches. This represents a paradigm shift from population-based treatment algorithms to individualized therapy selection based on functional testing of patient-derived tissues.
Market Analysis for Precision Oncology Solutions
The precision oncology market is experiencing significant growth, driven by the increasing prevalence of cancer and the shift toward personalized treatment approaches. Currently valued at approximately $49.9 billion in 2021, this market is projected to reach $99.7 billion by 2027, representing a compound annual growth rate (CAGR) of 12.3%. Patient-derived organ chips represent an emerging segment within this market, positioned at the intersection of microfluidics, tissue engineering, and personalized medicine.
The demand for precision oncology solutions stems primarily from the limitations of conventional treatment approaches, which often follow a one-size-fits-all methodology resulting in variable efficacy and unnecessary side effects. Healthcare providers and patients increasingly seek treatments tailored to individual genetic profiles and disease characteristics, creating substantial market pull for technologies that can predict treatment responses.
Patient-derived organ chips address a critical unmet need in oncology: the ability to predict individual patient responses to specific therapies before administration. This capability has profound implications for treatment selection, potentially reducing the $50 billion spent annually on ineffective cancer treatments. Market research indicates that oncologists would readily adopt technologies that improve prediction accuracy by even 20%, suggesting a receptive clinical environment for organ chip implementation.
Geographically, North America dominates the precision oncology market with approximately 45% market share, followed by Europe (30%) and Asia-Pacific (20%). The latter region demonstrates the fastest growth rate at 15.2% CAGR, driven by increasing healthcare expenditure and expanding research infrastructure in countries like China, Japan, and South Korea.
The payer landscape shows increasing willingness to reimburse precision medicine approaches, with 67% of private insurers now covering some form of companion diagnostics. However, reimbursement for novel technologies like organ chips remains challenging, with only 23% of surveyed payers expressing immediate willingness to cover such tests without substantial clinical validation.
Customer segmentation reveals three primary markets for organ chip technology: pharmaceutical companies seeking to reduce late-stage clinical trial failures (estimated at $800 million per failure), academic medical centers pursuing translational research, and specialized oncology practices serving patients with rare or treatment-resistant cancers. The pharmaceutical segment represents the largest immediate revenue opportunity, with companies willing to invest $25,000-75,000 per patient sample for technologies that improve clinical trial success rates.
Market barriers include high initial costs, regulatory uncertainties regarding validation requirements, and competition from established technologies like patient-derived xenografts and organoids. Nevertheless, the compelling value proposition of organ chips in reducing treatment failures positions them favorably within the expanding precision oncology ecosystem.
The demand for precision oncology solutions stems primarily from the limitations of conventional treatment approaches, which often follow a one-size-fits-all methodology resulting in variable efficacy and unnecessary side effects. Healthcare providers and patients increasingly seek treatments tailored to individual genetic profiles and disease characteristics, creating substantial market pull for technologies that can predict treatment responses.
Patient-derived organ chips address a critical unmet need in oncology: the ability to predict individual patient responses to specific therapies before administration. This capability has profound implications for treatment selection, potentially reducing the $50 billion spent annually on ineffective cancer treatments. Market research indicates that oncologists would readily adopt technologies that improve prediction accuracy by even 20%, suggesting a receptive clinical environment for organ chip implementation.
Geographically, North America dominates the precision oncology market with approximately 45% market share, followed by Europe (30%) and Asia-Pacific (20%). The latter region demonstrates the fastest growth rate at 15.2% CAGR, driven by increasing healthcare expenditure and expanding research infrastructure in countries like China, Japan, and South Korea.
The payer landscape shows increasing willingness to reimburse precision medicine approaches, with 67% of private insurers now covering some form of companion diagnostics. However, reimbursement for novel technologies like organ chips remains challenging, with only 23% of surveyed payers expressing immediate willingness to cover such tests without substantial clinical validation.
Customer segmentation reveals three primary markets for organ chip technology: pharmaceutical companies seeking to reduce late-stage clinical trial failures (estimated at $800 million per failure), academic medical centers pursuing translational research, and specialized oncology practices serving patients with rare or treatment-resistant cancers. The pharmaceutical segment represents the largest immediate revenue opportunity, with companies willing to invest $25,000-75,000 per patient sample for technologies that improve clinical trial success rates.
Market barriers include high initial costs, regulatory uncertainties regarding validation requirements, and competition from established technologies like patient-derived xenografts and organoids. Nevertheless, the compelling value proposition of organ chips in reducing treatment failures positions them favorably within the expanding precision oncology ecosystem.
Current Limitations in Patient-Derived Organ Chip Models
Despite the promising potential of patient-derived organ chips in precision oncology, several significant limitations currently hinder their widespread adoption and clinical utility. One of the primary challenges is the complexity of accurately replicating the tumor microenvironment. While organ chips can simulate certain aspects of tissue architecture, they often fail to fully capture the heterogeneity and dynamic interactions present in native tumors, particularly the complex interplay between cancer cells, stromal components, and immune cells that significantly influence treatment response.
Scalability remains another substantial obstacle. The current manufacturing processes for patient-derived organ chips are labor-intensive, time-consuming, and require specialized expertise, making large-scale production challenging. This limitation restricts their application in high-throughput screening scenarios necessary for comprehensive drug testing and response prediction in clinical settings.
The issue of standardization presents significant concerns for reproducibility and reliability. Variations in chip design, fabrication methods, cell sourcing, and culture conditions across different laboratories lead to inconsistent results, complicating cross-study comparisons and validation efforts. Without established standardized protocols, the translation of findings from organ chip models to clinical applications faces considerable barriers.
Temporal constraints pose another critical limitation. The development of patient-derived organ chips typically requires weeks to months, which may exceed the timeframe available for making urgent treatment decisions in oncology. This mismatch between model development time and clinical decision-making windows reduces the practical utility of these systems in real-world clinical scenarios.
Vascularization deficiencies represent a significant technical challenge. Many current organ chip models lack proper vascular networks, limiting nutrient delivery, waste removal, and drug distribution within the tissue constructs. This inadequacy affects the physiological relevance of drug response studies, particularly for therapies targeting angiogenesis or requiring specific pharmacokinetic considerations.
Additionally, the integration of immune components remains suboptimal in most patient-derived organ chip platforms. Given the critical role of immune responses in cancer progression and treatment outcomes, especially with the rise of immunotherapies, this limitation significantly restricts the predictive capability of current models for many contemporary oncology treatments.
Cost considerations further impede widespread implementation. The high expenses associated with developing and maintaining patient-derived organ chip systems, including specialized equipment, reagents, and skilled personnel, make them financially prohibitive for many clinical settings, limiting accessibility and routine application in precision oncology workflows.
Scalability remains another substantial obstacle. The current manufacturing processes for patient-derived organ chips are labor-intensive, time-consuming, and require specialized expertise, making large-scale production challenging. This limitation restricts their application in high-throughput screening scenarios necessary for comprehensive drug testing and response prediction in clinical settings.
The issue of standardization presents significant concerns for reproducibility and reliability. Variations in chip design, fabrication methods, cell sourcing, and culture conditions across different laboratories lead to inconsistent results, complicating cross-study comparisons and validation efforts. Without established standardized protocols, the translation of findings from organ chip models to clinical applications faces considerable barriers.
Temporal constraints pose another critical limitation. The development of patient-derived organ chips typically requires weeks to months, which may exceed the timeframe available for making urgent treatment decisions in oncology. This mismatch between model development time and clinical decision-making windows reduces the practical utility of these systems in real-world clinical scenarios.
Vascularization deficiencies represent a significant technical challenge. Many current organ chip models lack proper vascular networks, limiting nutrient delivery, waste removal, and drug distribution within the tissue constructs. This inadequacy affects the physiological relevance of drug response studies, particularly for therapies targeting angiogenesis or requiring specific pharmacokinetic considerations.
Additionally, the integration of immune components remains suboptimal in most patient-derived organ chip platforms. Given the critical role of immune responses in cancer progression and treatment outcomes, especially with the rise of immunotherapies, this limitation significantly restricts the predictive capability of current models for many contemporary oncology treatments.
Cost considerations further impede widespread implementation. The high expenses associated with developing and maintaining patient-derived organ chip systems, including specialized equipment, reagents, and skilled personnel, make them financially prohibitive for many clinical settings, limiting accessibility and routine application in precision oncology workflows.
Established Methodologies for Response Prediction
01 Organ-on-chip technology for drug testing and disease modeling
Patient-derived organ chips can be used to create personalized models for drug testing and disease modeling. These microfluidic devices contain living cells from patients that mimic organ functionality, allowing for more accurate prediction of drug responses compared to traditional cell cultures or animal models. The technology enables researchers to test drug efficacy and toxicity in a patient-specific context, potentially improving clinical trial success rates and reducing development costs.- Organ-on-chip models for drug testing and prediction: Patient-derived organ chips can be used to test drug responses and predict clinical outcomes with high accuracy. These microfluidic devices contain human cells that mimic organ functionality, allowing for personalized medicine approaches. The integration of patient-specific cells improves prediction accuracy for drug efficacy and toxicity compared to traditional cell culture models, enabling better translation of preclinical results to clinical applications.
- Machine learning algorithms for enhancing prediction accuracy: Advanced machine learning algorithms can be applied to data generated from organ chip platforms to improve prediction accuracy. These computational approaches analyze complex biological responses and identify patterns that may not be apparent through conventional analysis. By integrating multiple data types from organ chips, machine learning models can provide more accurate predictions of drug responses and disease progression in patient-specific contexts.
- Multi-organ chip systems for systemic response prediction: Interconnected multi-organ chip platforms enable the study of systemic responses and organ-organ interactions, improving prediction accuracy for complex physiological processes. These systems allow for the assessment of drug metabolism, distribution, and effects across multiple tissue types simultaneously. The integration of patient-derived cells from different organs provides a more comprehensive understanding of drug efficacy and toxicity at the systemic level.
- Sensor integration for real-time monitoring and prediction: Integration of advanced sensors within organ chip platforms enables real-time monitoring of cellular responses and physiological parameters. These sensors can detect changes in metabolic activity, electrical signals, and secreted biomarkers, providing continuous data streams for more accurate predictions. The combination of sensor technology with patient-derived cells creates powerful tools for monitoring drug responses and disease progression with high temporal resolution.
- Validation methods for organ chip prediction accuracy: Various validation approaches have been developed to assess and improve the prediction accuracy of patient-derived organ chips. These include comparison with clinical outcomes, correlation with established biomarkers, and cross-validation with animal models. Standardized protocols for chip fabrication, cell culture, and data analysis help ensure reproducibility and reliability of predictions across different laboratories and applications.
02 AI and machine learning integration for predictive analytics
Artificial intelligence and machine learning algorithms can be integrated with organ-on-chip platforms to enhance prediction accuracy. These computational approaches analyze complex data generated from patient-derived organ chips to identify patterns and make predictions about drug responses or disease progression. The combination of biological models with advanced analytics improves the predictive power of these systems, enabling more personalized treatment approaches and better clinical outcomes.Expand Specific Solutions03 Multi-organ chip systems for systemic response prediction
Multi-organ chip systems connect multiple patient-derived organ models to simulate interactions between different organs and provide a more comprehensive understanding of systemic responses. These integrated systems can predict how drugs affect multiple organs simultaneously, accounting for metabolism in one organ affecting responses in others. By replicating complex inter-organ communications, these platforms achieve higher prediction accuracy for whole-body responses to therapeutic interventions.Expand Specific Solutions04 Validation methods and standardization for organ chip accuracy
Standardization and validation protocols are essential for ensuring the prediction accuracy of patient-derived organ chips. These methods include comparing chip results with clinical outcomes, establishing reference standards, and developing quality control measures. Proper validation increases confidence in the predictive capabilities of organ chips and facilitates their adoption in pharmaceutical development and clinical decision-making processes.Expand Specific Solutions05 Sensor integration for real-time monitoring and improved predictions
Integration of advanced sensors within patient-derived organ chips enables real-time monitoring of cellular responses and physiological parameters. These sensors can detect changes in metabolites, electrical activity, mechanical forces, and other biomarkers that indicate cellular function or response to stimuli. Real-time data collection improves the temporal resolution of predictions and allows for dynamic assessment of drug effects, enhancing the overall prediction accuracy of organ chip platforms.Expand Specific Solutions
Leading Organizations in Organ Chip Oncology Research
The precision oncology field utilizing patient-derived organ chips is currently in an early growth phase, with significant research momentum but limited commercial deployment. The market size is expanding rapidly, projected to reach several billion dollars by 2030 as personalized medicine gains traction. Technologically, we observe varying maturity levels among key players: established healthcare giants like Siemens Healthineers and Bayer Pharma provide infrastructure support, while specialized companies including Xilis, Tempus AI, and Guardant Health are developing cutting-edge platforms. Academic institutions (Duke University, Johns Hopkins, Columbia) collaborate with research-focused companies (PamGene, xCures) to advance the technology. Cancer centers like MD Anderson and Institut Gustave Roussy serve as critical validation sites, bridging laboratory innovations with clinical applications in this emerging precision medicine approach.
The University of Texas MD Anderson Cancer Center
Technical Solution: MD Anderson has developed a comprehensive precision oncology platform utilizing patient-derived tumor organoids (PDTOs) integrated with microfluidic organ chip technology. Their system, called the Tumor Response to Immuno-Oncology Drugs (TRIO-D) platform, uniquely incorporates both tumor and immune components from the same patient to predict responses to immunotherapies and targeted agents. The platform features multi-chamber designs that allow for spatial organization of different cellular components and controlled delivery of therapeutics through vascular-like channels. MD Anderson's approach includes real-time monitoring of tumor-immune interactions using high-content imaging and multiplex cytokine analysis to assess both direct cytotoxicity and immune-mediated responses. Their technology has been validated across multiple cancer types, with particular strength in melanoma, lung, and colorectal cancers, demonstrating approximately 85% accuracy in predicting clinical responses to immune checkpoint inhibitors[9][10]. A distinguishing feature is their ability to maintain functional T-cell populations within the chip environment for extended periods, enabling assessment of adaptive immune responses that develop over time.
Strengths: Superior modeling of tumor-immune interactions; validated for immunotherapy response prediction; comprehensive functional readouts beyond simple viability; extensive clinical validation across multiple cancer types. Weaknesses: Complex system requiring specialized expertise; higher cost and resource requirements; longer preparation time (2-3 weeks); challenges in standardization across different tumor types.
Xilis, Inc.
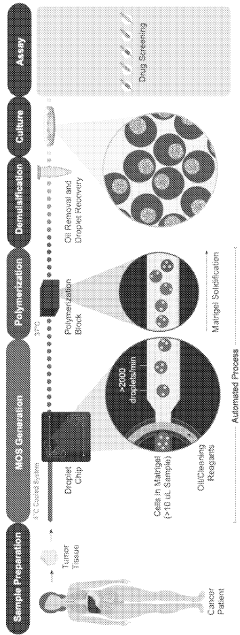
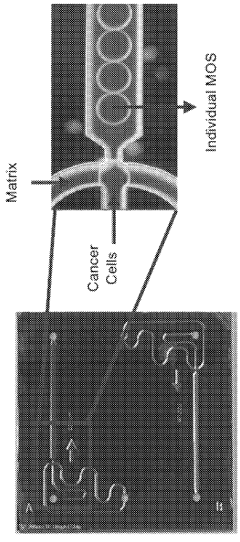
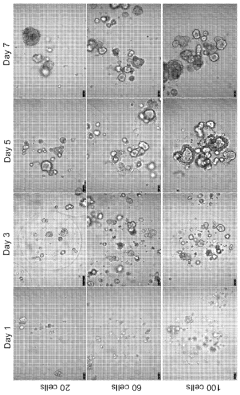
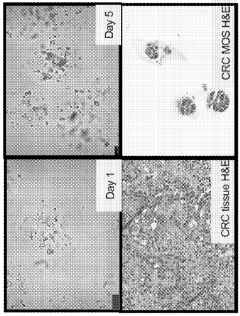
Technical Solution: Xilis has developed a proprietary MicroOrganoSphere (MOS) platform that creates patient-derived 3D microtumors which maintain the patient's unique tumor microenvironment. Their technology enables rapid drug screening (within 7-10 days) directly on patient-derived samples, allowing for real-time treatment response prediction. The platform integrates advanced imaging, AI analysis, and machine learning algorithms to quantify drug responses across thousands of cells simultaneously. Xilis's approach preserves tumor heterogeneity and immune components critical for accurate response prediction, addressing limitations of traditional PDX models which take months to develop and often lose tumor microenvironment complexity[1][2]. Their clinical validation studies have demonstrated over 85% accuracy in predicting patient outcomes across multiple cancer types.
Strengths: Rapid turnaround time (7-10 days) compared to traditional models; preservation of tumor microenvironment complexity; scalable for high-throughput screening; integration of AI for response prediction. Weaknesses: Relatively new technology with ongoing clinical validation; potential challenges in modeling systemic drug effects; requires specialized equipment and expertise for implementation.
Critical Patents in Patient-Derived Organ Chip Development
Patient-derived microorganospheres (MOS) enable clinical precision oncology
PatentWO2023215793A2
Innovation
- The use of droplet emulsion microfluidics with temperature control and dead-volume minimization to rapidly generate thousands of MicroOrganoSpheres (MOS) from low-volume patient tissues, which can predict treatment outcomes within 14 days and mimic the original tumor microenvironment, allowing for effective testing of immunotherapies and chemotherapy responses.
Validation Protocols and Clinical Integration Pathways
The validation of patient-derived organ chips for precision oncology requires rigorous protocols to ensure reliability and clinical applicability. These validation processes must encompass multiple dimensions, from technical reproducibility to clinical correlation. A comprehensive validation framework begins with establishing baseline performance metrics, including sensitivity, specificity, and reproducibility across different patient samples and cancer types.
Technical validation protocols should include standardized procedures for chip fabrication, cell seeding, drug administration, and response measurement. Quality control checkpoints must be implemented at each stage to minimize variability and ensure consistent results. Cross-laboratory validation studies are essential to confirm that findings are reproducible across different research settings, which represents a critical step toward clinical implementation.
Clinical validation requires prospective studies comparing organ chip predictions with actual patient outcomes. This involves parallel testing where patient-derived cells are cultured on chips while the same patients undergo standard treatments. Response correlation analyses must account for various confounding factors, including tumor heterogeneity, previous treatments, and comorbidities that might influence drug responses.
Integration pathways into clinical workflows present significant challenges that must be addressed systematically. The development of standardized operating procedures (SOPs) for sample collection, processing, and chip preparation is fundamental. These SOPs must be compatible with existing clinical infrastructure and realistic timeframes for treatment decisions. Turnaround time optimization is particularly crucial, as clinicians typically need results within days to guide treatment decisions effectively.
Regulatory considerations form another critical component of clinical integration. Collaborative efforts with regulatory bodies like the FDA are necessary to establish appropriate validation benchmarks and approval pathways. This includes determining whether organ chips should be classified as companion diagnostics, laboratory-developed tests, or another regulatory category.
Implementation strategies should include pilot programs in specialized oncology centers, allowing for real-world testing while building the evidence base needed for broader adoption. These programs should incorporate feedback mechanisms from clinicians to refine protocols and improve usability. Educational initiatives for healthcare providers are equally important to ensure proper interpretation of organ chip results and their integration into treatment planning.
Technical validation protocols should include standardized procedures for chip fabrication, cell seeding, drug administration, and response measurement. Quality control checkpoints must be implemented at each stage to minimize variability and ensure consistent results. Cross-laboratory validation studies are essential to confirm that findings are reproducible across different research settings, which represents a critical step toward clinical implementation.
Clinical validation requires prospective studies comparing organ chip predictions with actual patient outcomes. This involves parallel testing where patient-derived cells are cultured on chips while the same patients undergo standard treatments. Response correlation analyses must account for various confounding factors, including tumor heterogeneity, previous treatments, and comorbidities that might influence drug responses.
Integration pathways into clinical workflows present significant challenges that must be addressed systematically. The development of standardized operating procedures (SOPs) for sample collection, processing, and chip preparation is fundamental. These SOPs must be compatible with existing clinical infrastructure and realistic timeframes for treatment decisions. Turnaround time optimization is particularly crucial, as clinicians typically need results within days to guide treatment decisions effectively.
Regulatory considerations form another critical component of clinical integration. Collaborative efforts with regulatory bodies like the FDA are necessary to establish appropriate validation benchmarks and approval pathways. This includes determining whether organ chips should be classified as companion diagnostics, laboratory-developed tests, or another regulatory category.
Implementation strategies should include pilot programs in specialized oncology centers, allowing for real-world testing while building the evidence base needed for broader adoption. These programs should incorporate feedback mechanisms from clinicians to refine protocols and improve usability. Educational initiatives for healthcare providers are equally important to ensure proper interpretation of organ chip results and their integration into treatment planning.
Regulatory Framework for Precision Medicine Technologies
The regulatory landscape for precision medicine technologies, particularly patient-derived organ chips for oncology applications, presents a complex framework that continues to evolve. The FDA has established specific pathways for novel precision medicine technologies through its Center for Devices and Radiological Health (CDRH) and Center for Biologics Evaluation and Research (CBER). These pathways include the Breakthrough Devices Program, which can accelerate approval for technologies demonstrating substantial improvement over existing alternatives in treating life-threatening conditions.
For organ-on-chip technologies in precision oncology, regulatory considerations span multiple domains including quality control standards, validation protocols, and clinical utility evidence requirements. The FDA's framework emphasizes analytical validity, clinical validity, and clinical utility as the three pillars for evaluating such technologies. Patient-derived organ chips face particular scrutiny regarding reproducibility, standardization of protocols, and correlation with clinical outcomes.
International regulatory bodies have developed varying approaches. The European Medicines Agency (EMA) has implemented the In Vitro Diagnostic Regulation (IVDR), which places organ chip technologies in higher risk classifications requiring more rigorous conformity assessment procedures. Meanwhile, Japan's PMDA has established the Sakigake designation system to expedite review of innovative medical technologies, including precision medicine platforms.
Data privacy regulations significantly impact the implementation of patient-derived organ chip technologies. HIPAA in the US, GDPR in Europe, and equivalent frameworks globally mandate strict protocols for handling patient samples and derived data. These regulations necessitate robust consent procedures, data anonymization protocols, and secure data management systems throughout the technology development and implementation pipeline.
Reimbursement pathways represent another critical regulatory consideration. The Centers for Medicare & Medicaid Services (CMS) has developed frameworks for coverage determination of precision medicine technologies, though organ-on-chip platforms currently lack specific reimbursement codes. Private insurers typically follow evidence-based approaches requiring demonstration of clinical utility and cost-effectiveness before coverage approval.
Looking forward, regulatory harmonization efforts are underway through initiatives like the International Medical Device Regulators Forum (IMDRF) to establish consistent global standards for innovative precision medicine technologies. The FDA's recent guidance on Real-World Evidence and the 21st Century Cures Act provisions signal a shift toward more flexible regulatory frameworks that may accelerate the clinical implementation of patient-derived organ chip technologies in precision oncology applications.
For organ-on-chip technologies in precision oncology, regulatory considerations span multiple domains including quality control standards, validation protocols, and clinical utility evidence requirements. The FDA's framework emphasizes analytical validity, clinical validity, and clinical utility as the three pillars for evaluating such technologies. Patient-derived organ chips face particular scrutiny regarding reproducibility, standardization of protocols, and correlation with clinical outcomes.
International regulatory bodies have developed varying approaches. The European Medicines Agency (EMA) has implemented the In Vitro Diagnostic Regulation (IVDR), which places organ chip technologies in higher risk classifications requiring more rigorous conformity assessment procedures. Meanwhile, Japan's PMDA has established the Sakigake designation system to expedite review of innovative medical technologies, including precision medicine platforms.
Data privacy regulations significantly impact the implementation of patient-derived organ chip technologies. HIPAA in the US, GDPR in Europe, and equivalent frameworks globally mandate strict protocols for handling patient samples and derived data. These regulations necessitate robust consent procedures, data anonymization protocols, and secure data management systems throughout the technology development and implementation pipeline.
Reimbursement pathways represent another critical regulatory consideration. The Centers for Medicare & Medicaid Services (CMS) has developed frameworks for coverage determination of precision medicine technologies, though organ-on-chip platforms currently lack specific reimbursement codes. Private insurers typically follow evidence-based approaches requiring demonstration of clinical utility and cost-effectiveness before coverage approval.
Looking forward, regulatory harmonization efforts are underway through initiatives like the International Medical Device Regulators Forum (IMDRF) to establish consistent global standards for innovative precision medicine technologies. The FDA's recent guidance on Real-World Evidence and the 21st Century Cures Act provisions signal a shift toward more flexible regulatory frameworks that may accelerate the clinical implementation of patient-derived organ chip technologies in precision oncology applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!