Vacuum Forming's Role in Advanced Healthcare Solutions Production
JUL 30, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Vacuum Forming in Healthcare: Evolution and Objectives
Vacuum forming has played a pivotal role in the evolution of healthcare solutions, transforming the production of medical devices and equipment over the past several decades. This technology, which originated in the 1940s, has steadily advanced to meet the increasingly complex demands of the healthcare industry.
Initially, vacuum forming was primarily used for simple, disposable medical items such as trays and packaging. As the technique refined, it found applications in producing more sophisticated components for medical equipment, including casings for diagnostic devices and custom-fitted orthopedic supports. The evolution of vacuum forming in healthcare has been driven by the need for cost-effective, sterile, and customizable solutions.
The objectives of vacuum forming in healthcare have expanded significantly. Today, it aims to create lightweight yet durable medical products, reduce manufacturing costs, and enable rapid prototyping for innovative medical designs. A key goal is to produce components that meet stringent regulatory requirements while maintaining the flexibility to adapt to diverse patient needs.
One of the most significant developments has been the integration of vacuum forming with advanced materials science. This synergy has led to the creation of medical-grade plastics that offer enhanced biocompatibility, chemical resistance, and sterilization capabilities. These advancements have opened new possibilities for producing complex anatomical models, surgical guides, and patient-specific implants.
The technology's evolution also aligns with the broader trend towards personalized medicine. Vacuum forming now plays a crucial role in creating custom-fit prosthetics, orthotics, and dental aligners. Its ability to produce precise, patient-specific solutions has significantly improved treatment outcomes and patient comfort.
Looking ahead, the objectives for vacuum forming in healthcare are becoming increasingly ambitious. There is a growing focus on developing sustainable practices, including the use of biodegradable materials and more energy-efficient forming processes. Additionally, the integration of smart materials and embedded sensors in vacuum-formed products is emerging as a promising frontier, potentially enabling real-time monitoring of patient health indicators.
As healthcare continues to advance, vacuum forming is expected to evolve further, with objectives centered on enhancing precision, expanding material capabilities, and improving integration with digital health technologies. The ultimate goal remains to provide innovative, cost-effective solutions that improve patient care and treatment outcomes across a wide spectrum of medical applications.
Initially, vacuum forming was primarily used for simple, disposable medical items such as trays and packaging. As the technique refined, it found applications in producing more sophisticated components for medical equipment, including casings for diagnostic devices and custom-fitted orthopedic supports. The evolution of vacuum forming in healthcare has been driven by the need for cost-effective, sterile, and customizable solutions.
The objectives of vacuum forming in healthcare have expanded significantly. Today, it aims to create lightweight yet durable medical products, reduce manufacturing costs, and enable rapid prototyping for innovative medical designs. A key goal is to produce components that meet stringent regulatory requirements while maintaining the flexibility to adapt to diverse patient needs.
One of the most significant developments has been the integration of vacuum forming with advanced materials science. This synergy has led to the creation of medical-grade plastics that offer enhanced biocompatibility, chemical resistance, and sterilization capabilities. These advancements have opened new possibilities for producing complex anatomical models, surgical guides, and patient-specific implants.
The technology's evolution also aligns with the broader trend towards personalized medicine. Vacuum forming now plays a crucial role in creating custom-fit prosthetics, orthotics, and dental aligners. Its ability to produce precise, patient-specific solutions has significantly improved treatment outcomes and patient comfort.
Looking ahead, the objectives for vacuum forming in healthcare are becoming increasingly ambitious. There is a growing focus on developing sustainable practices, including the use of biodegradable materials and more energy-efficient forming processes. Additionally, the integration of smart materials and embedded sensors in vacuum-formed products is emerging as a promising frontier, potentially enabling real-time monitoring of patient health indicators.
As healthcare continues to advance, vacuum forming is expected to evolve further, with objectives centered on enhancing precision, expanding material capabilities, and improving integration with digital health technologies. The ultimate goal remains to provide innovative, cost-effective solutions that improve patient care and treatment outcomes across a wide spectrum of medical applications.
Market Demand Analysis for Medical Device Manufacturing
The market demand for medical device manufacturing using vacuum forming technology has been experiencing significant growth in recent years. This surge is primarily driven by the increasing need for cost-effective, high-quality healthcare solutions across the globe. Vacuum forming offers a versatile and efficient method for producing a wide range of medical devices, from disposable components to complex diagnostic equipment housings.
In the United States, the medical device manufacturing market is projected to reach $208 billion by 2023, with a compound annual growth rate (CAGR) of 5.6% from 2018 to 2023. A substantial portion of this growth can be attributed to the adoption of advanced manufacturing techniques, including vacuum forming. The technology's ability to produce lightweight, durable, and sterile components aligns well with the stringent requirements of the healthcare industry.
Europe also presents a robust market for vacuum-formed medical devices, with Germany, France, and the UK leading the way. The European medical device market is expected to grow at a CAGR of 4.7% from 2020 to 2025, reaching a value of $171 billion. The region's focus on innovative healthcare solutions and stringent quality standards creates a favorable environment for vacuum forming applications in medical device production.
Emerging markets, particularly in Asia-Pacific, are showing rapid growth in demand for medical devices manufactured using vacuum forming. Countries like China and India are investing heavily in healthcare infrastructure, driving the need for cost-effective medical equipment. The Asia-Pacific medical device market is forecasted to grow at a CAGR of 8.7% from 2020 to 2025, presenting significant opportunities for vacuum forming technology.
Key factors contributing to the increasing market demand include the rising prevalence of chronic diseases, aging populations in developed countries, and the need for personalized medical solutions. Vacuum forming's ability to produce customized components quickly and efficiently makes it an attractive option for manufacturers looking to meet these evolving needs.
The COVID-19 pandemic has further accelerated the demand for medical devices, particularly those related to respiratory care and personal protective equipment (PPE). Vacuum forming has played a crucial role in rapidly scaling up production of face shields, ventilator components, and diagnostic equipment housings, demonstrating its versatility and importance in addressing urgent healthcare needs.
As the healthcare industry continues to evolve, the demand for vacuum-formed medical devices is expected to grow further. Emerging trends such as wearable medical devices, point-of-care diagnostics, and telemedicine solutions are likely to create new opportunities for vacuum forming applications in the medical device manufacturing sector.
In the United States, the medical device manufacturing market is projected to reach $208 billion by 2023, with a compound annual growth rate (CAGR) of 5.6% from 2018 to 2023. A substantial portion of this growth can be attributed to the adoption of advanced manufacturing techniques, including vacuum forming. The technology's ability to produce lightweight, durable, and sterile components aligns well with the stringent requirements of the healthcare industry.
Europe also presents a robust market for vacuum-formed medical devices, with Germany, France, and the UK leading the way. The European medical device market is expected to grow at a CAGR of 4.7% from 2020 to 2025, reaching a value of $171 billion. The region's focus on innovative healthcare solutions and stringent quality standards creates a favorable environment for vacuum forming applications in medical device production.
Emerging markets, particularly in Asia-Pacific, are showing rapid growth in demand for medical devices manufactured using vacuum forming. Countries like China and India are investing heavily in healthcare infrastructure, driving the need for cost-effective medical equipment. The Asia-Pacific medical device market is forecasted to grow at a CAGR of 8.7% from 2020 to 2025, presenting significant opportunities for vacuum forming technology.
Key factors contributing to the increasing market demand include the rising prevalence of chronic diseases, aging populations in developed countries, and the need for personalized medical solutions. Vacuum forming's ability to produce customized components quickly and efficiently makes it an attractive option for manufacturers looking to meet these evolving needs.
The COVID-19 pandemic has further accelerated the demand for medical devices, particularly those related to respiratory care and personal protective equipment (PPE). Vacuum forming has played a crucial role in rapidly scaling up production of face shields, ventilator components, and diagnostic equipment housings, demonstrating its versatility and importance in addressing urgent healthcare needs.
As the healthcare industry continues to evolve, the demand for vacuum-formed medical devices is expected to grow further. Emerging trends such as wearable medical devices, point-of-care diagnostics, and telemedicine solutions are likely to create new opportunities for vacuum forming applications in the medical device manufacturing sector.
Current Challenges in Healthcare Vacuum Forming
Vacuum forming in healthcare faces several significant challenges that hinder its full potential in producing advanced medical solutions. One of the primary issues is the limited material selection suitable for medical applications. While vacuum forming is versatile, finding materials that meet stringent medical standards for biocompatibility, sterilization resistance, and durability remains a hurdle.
The complexity of medical device designs poses another challenge. As healthcare solutions become more sophisticated, vacuum forming struggles to create intricate shapes and fine details required for certain medical components. This limitation often necessitates additional post-processing steps, increasing production time and costs.
Maintaining consistent quality across large production runs is also a concern. Vacuum forming can sometimes result in thickness variations or surface imperfections, which are particularly problematic in medical applications where precision is crucial. Ensuring uniformity and meeting tight tolerances consistently is an ongoing challenge for manufacturers.
Sterilization compatibility is another critical issue. Many medical devices require sterilization processes that can potentially degrade or deform vacuum-formed parts. Finding materials and forming techniques that can withstand various sterilization methods without compromising functionality or safety is a persistent challenge in the industry.
Regulatory compliance adds another layer of complexity. With stringent regulations governing medical device production, vacuum forming processes must meet exacting standards for material traceability, process validation, and quality control. This often requires significant investment in documentation and quality management systems.
The demand for customization in healthcare solutions also presents challenges for vacuum forming. While the technique is suitable for mass production, adapting it for small-batch or personalized medical devices can be less cost-effective and time-efficient compared to other manufacturing methods.
Lastly, the integration of smart technologies into medical devices is pushing the boundaries of what traditional vacuum forming can achieve. Incorporating electronic components or sensors into vacuum-formed parts requires innovative approaches and often hybrid manufacturing techniques, which are still in developmental stages for many applications.
The complexity of medical device designs poses another challenge. As healthcare solutions become more sophisticated, vacuum forming struggles to create intricate shapes and fine details required for certain medical components. This limitation often necessitates additional post-processing steps, increasing production time and costs.
Maintaining consistent quality across large production runs is also a concern. Vacuum forming can sometimes result in thickness variations or surface imperfections, which are particularly problematic in medical applications where precision is crucial. Ensuring uniformity and meeting tight tolerances consistently is an ongoing challenge for manufacturers.
Sterilization compatibility is another critical issue. Many medical devices require sterilization processes that can potentially degrade or deform vacuum-formed parts. Finding materials and forming techniques that can withstand various sterilization methods without compromising functionality or safety is a persistent challenge in the industry.
Regulatory compliance adds another layer of complexity. With stringent regulations governing medical device production, vacuum forming processes must meet exacting standards for material traceability, process validation, and quality control. This often requires significant investment in documentation and quality management systems.
The demand for customization in healthcare solutions also presents challenges for vacuum forming. While the technique is suitable for mass production, adapting it for small-batch or personalized medical devices can be less cost-effective and time-efficient compared to other manufacturing methods.
Lastly, the integration of smart technologies into medical devices is pushing the boundaries of what traditional vacuum forming can achieve. Incorporating electronic components or sensors into vacuum-formed parts requires innovative approaches and often hybrid manufacturing techniques, which are still in developmental stages for many applications.
Existing Vacuum Forming Solutions for Healthcare
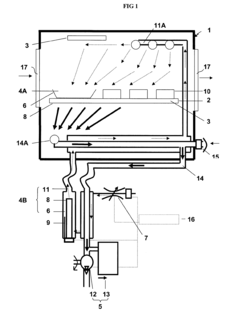
01 Vacuum forming process and apparatus
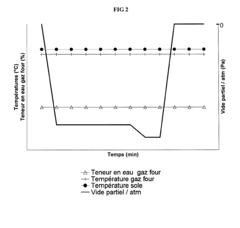
Vacuum forming is a thermoforming process where a plastic sheet is heated to a forming temperature, stretched onto a single-surface mold, and forced against the mold by vacuum. This process is widely used in manufacturing various plastic products. The apparatus typically includes a heating element, a mold, and a vacuum system.- Vacuum forming process improvements: Advancements in vacuum forming techniques to enhance efficiency and product quality. This includes optimizing the heating process, improving mold design, and refining the vacuum application method to achieve better formed products with reduced material waste and cycle times.
- Material innovations for vacuum forming: Development of new materials and composites specifically designed for vacuum forming applications. These materials offer improved formability, durability, and surface finish, expanding the range of products that can be manufactured using vacuum forming techniques.
- Automation and control systems in vacuum forming: Integration of advanced automation and control systems in vacuum forming equipment. This includes the use of sensors, programmable logic controllers, and machine learning algorithms to optimize process parameters, reduce human error, and increase overall production efficiency.
- Specialized vacuum forming applications: Adaptation of vacuum forming techniques for specific industries or product types. This includes customized solutions for automotive parts, medical devices, packaging, and aerospace components, addressing unique challenges and requirements in each field.
- Sustainable vacuum forming practices: Implementation of environmentally friendly approaches in vacuum forming processes. This includes the use of recycled materials, energy-efficient equipment, and waste reduction strategies to minimize the environmental impact of vacuum forming manufacturing.
02 Improvements in mold design for vacuum forming
Advancements in mold design for vacuum forming focus on enhancing the quality of formed products and increasing efficiency. These improvements may include optimized air venting systems, temperature control mechanisms, and innovative mold materials that allow for better heat distribution and faster cooling.Expand Specific Solutions03 Control systems for vacuum forming machines
Modern vacuum forming machines incorporate sophisticated control systems to improve precision and automation. These systems may include programmable logic controllers (PLCs), touch screen interfaces, and sensors for monitoring various parameters such as temperature, pressure, and material thickness.Expand Specific Solutions04 Material innovations for vacuum forming
Research in vacuum forming materials focuses on developing new plastic formulations with improved properties such as better heat resistance, increased flexibility, or enhanced surface finish. These innovations aim to expand the range of applications for vacuum-formed products and improve their overall quality.Expand Specific Solutions05 Energy-efficient vacuum forming techniques
Efforts to reduce energy consumption in vacuum forming processes include the development of more efficient heating systems, improved insulation methods, and optimized cycle times. These techniques aim to decrease production costs and minimize environmental impact while maintaining product quality.Expand Specific Solutions
Key Players in Medical Vacuum Forming Industry
The vacuum forming industry for advanced healthcare solutions is in a growth phase, driven by increasing demand for customized medical devices and packaging. The market size is expanding, with projections indicating significant growth in the coming years. Technologically, vacuum forming is evolving rapidly, with companies like Tokyo Electron Ltd. and Corning, Inc. leading innovations in materials and processes. Anhui KingPower Equipment & Mould Manufacture Co. Ltd. and ULVAC, Inc. are advancing equipment capabilities, while Arsenal Medical, Inc. and QIAGEN GmbH are applying vacuum forming techniques to develop novel healthcare products. The competitive landscape is diverse, with both established players and innovative startups contributing to technological advancements in this field.
Corning, Inc.
Technical Solution: Corning has applied vacuum forming techniques to the production of advanced glass and ceramic components for healthcare applications. Their process involves using high-temperature vacuum forming to create complex shapes and structures in glass materials[1]. This technology allows for the production of specialized labware, microfluidic devices, and cell culture vessels with precise optical and surface properties[2]. Corning's vacuum forming process also incorporates ion-exchange strengthening techniques, resulting in more durable and chemically resistant products[3]. The company has developed methods for integrating sensors and electrodes into vacuum-formed glass components, enabling advanced in vitro diagnostic platforms[4].
Strengths: Excellent optical properties, high chemical resistance, ability to integrate sensors. Weaknesses: Higher energy requirements for processing, potential limitations in product size.
Arsenal Medical, Inc.
Technical Solution: Arsenal Medical has developed advanced vacuum forming techniques for healthcare solutions production. Their approach involves using biodegradable polymers to create intricate medical devices and implants[1]. The company utilizes a proprietary vacuum forming process that allows for precise control over material thickness and shape, resulting in highly customized medical products[2]. This technology enables the production of complex geometries and internal structures that are crucial for tissue engineering and drug delivery systems[3]. Arsenal Medical's vacuum forming process also incorporates antimicrobial additives during fabrication, enhancing the sterility and safety of the final products[4].
Strengths: Highly customizable products, ability to create complex internal structures, enhanced sterility. Weaknesses: Potentially higher production costs, limited to specific biodegradable materials.
Innovations in Materials and Processes
A vacuum forming machine in which vacuum is automatically initiated upon delivery of the sheet to the machine
PatentInactiveGB2248579A
Innovation
- A simplified vacuum forming machine design featuring a holder that moves from a heating position to a forming position automatically operates the valve, creating a vacuum to shape the plastic sheet, with a spring-biased valve plate and guide means for easy operation and reduced manual intervention.
Anlage und Verfahren zur Behandlung von Lebensmittelprodukten, wie beispielsweise Wabenprodukte, insbesondere im Hinblick auf eine Expansion dieser Produkte
PatentActiveEP2572583A1
Innovation
- An installation with a direct communication between the vaporized fluid reserve and the treatment enclosure, allowing for continuous steam regulation and vacuum management without pressure fluctuations, using gas suction to control steam production and reduce heating needs, and featuring a simplified architecture by eliminating the need for separate steam supply and vacuum pumps.
Regulatory Compliance in Medical Device Production
Regulatory compliance is a critical aspect of medical device production, particularly in the context of vacuum forming's role in advanced healthcare solutions. The regulatory landscape for medical devices is complex and constantly evolving, with stringent requirements set by various governing bodies worldwide.
In the United States, the Food and Drug Administration (FDA) is the primary regulatory agency overseeing medical device production. The FDA classifies medical devices into three categories based on their risk level and intended use. Class I devices are low-risk and subject to general controls, while Class II devices require special controls and premarket notification. Class III devices, which are high-risk, must undergo premarket approval.
For vacuum-formed medical devices, manufacturers must adhere to the FDA's Quality System Regulation (QSR), which outlines good manufacturing practices. This includes maintaining proper documentation, implementing quality control measures, and ensuring traceability throughout the production process. The QSR also mandates risk management procedures to identify and mitigate potential hazards associated with the use of vacuum-formed medical products.
In the European Union, medical devices must comply with the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR). These regulations emphasize post-market surveillance, clinical evaluation, and unique device identification. Manufacturers using vacuum forming techniques for medical devices must obtain CE marking to demonstrate compliance with EU safety, health, and environmental protection standards.
International standards such as ISO 13485 for quality management systems in medical devices are widely recognized and often required for regulatory compliance. This standard provides a framework for consistent quality control in the production of vacuum-formed healthcare solutions, ensuring that products meet both regulatory requirements and customer expectations.
Material selection is a crucial aspect of regulatory compliance in vacuum forming for medical devices. Materials must be biocompatible, sterilizable, and meet specific performance criteria. Manufacturers must provide extensive documentation on material properties, processing conditions, and any potential leachables or extractables that could affect patient safety.
Validation and verification processes are essential components of regulatory compliance. For vacuum-formed medical devices, this includes validating the forming process, ensuring consistent product quality, and verifying that the final product meets all specified requirements. These processes must be thoroughly documented and may be subject to regulatory inspections.
As the healthcare industry continues to advance, regulatory bodies are increasingly focusing on cybersecurity and data protection for connected medical devices. While this may not directly impact the vacuum forming process, it is an important consideration for manufacturers integrating formed components into smart medical devices or systems.
In the United States, the Food and Drug Administration (FDA) is the primary regulatory agency overseeing medical device production. The FDA classifies medical devices into three categories based on their risk level and intended use. Class I devices are low-risk and subject to general controls, while Class II devices require special controls and premarket notification. Class III devices, which are high-risk, must undergo premarket approval.
For vacuum-formed medical devices, manufacturers must adhere to the FDA's Quality System Regulation (QSR), which outlines good manufacturing practices. This includes maintaining proper documentation, implementing quality control measures, and ensuring traceability throughout the production process. The QSR also mandates risk management procedures to identify and mitigate potential hazards associated with the use of vacuum-formed medical products.
In the European Union, medical devices must comply with the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR). These regulations emphasize post-market surveillance, clinical evaluation, and unique device identification. Manufacturers using vacuum forming techniques for medical devices must obtain CE marking to demonstrate compliance with EU safety, health, and environmental protection standards.
International standards such as ISO 13485 for quality management systems in medical devices are widely recognized and often required for regulatory compliance. This standard provides a framework for consistent quality control in the production of vacuum-formed healthcare solutions, ensuring that products meet both regulatory requirements and customer expectations.
Material selection is a crucial aspect of regulatory compliance in vacuum forming for medical devices. Materials must be biocompatible, sterilizable, and meet specific performance criteria. Manufacturers must provide extensive documentation on material properties, processing conditions, and any potential leachables or extractables that could affect patient safety.
Validation and verification processes are essential components of regulatory compliance. For vacuum-formed medical devices, this includes validating the forming process, ensuring consistent product quality, and verifying that the final product meets all specified requirements. These processes must be thoroughly documented and may be subject to regulatory inspections.
As the healthcare industry continues to advance, regulatory bodies are increasingly focusing on cybersecurity and data protection for connected medical devices. While this may not directly impact the vacuum forming process, it is an important consideration for manufacturers integrating formed components into smart medical devices or systems.
Sustainability in Healthcare Vacuum Forming
Sustainability in healthcare vacuum forming is becoming increasingly crucial as the industry seeks to balance the demand for advanced medical solutions with environmental responsibility. The process of vacuum forming, while efficient for producing complex medical components, traditionally involves materials and practices that can have significant environmental impacts.
To address these concerns, manufacturers are exploring eco-friendly alternatives to conventional plastics used in vacuum forming. Biodegradable and compostable materials derived from renewable sources are being developed and tested for their suitability in medical applications. These materials aim to maintain the necessary properties for healthcare products while reducing the environmental footprint of their production and disposal.
Energy efficiency is another key focus in sustainable vacuum forming practices. Advanced machinery with improved thermal management and precision control systems are being introduced to minimize energy consumption during the forming process. Additionally, manufacturers are implementing heat recovery systems to capture and reuse thermal energy, further reducing overall energy requirements.
Waste reduction strategies are also being employed throughout the vacuum forming lifecycle. This includes optimizing mold designs to minimize material waste, implementing closed-loop recycling systems for production scrap, and developing more efficient packaging solutions to reduce transportation-related emissions. Some facilities are even exploring on-site recycling capabilities to process end-of-life medical products, creating a more circular economy within the healthcare sector.
Water conservation is becoming an integral part of sustainable vacuum forming practices. New cooling systems and cleaning processes are being developed to minimize water usage without compromising product quality or hygiene standards. Some manufacturers are implementing water recycling and treatment systems to further reduce their environmental impact.
The adoption of lean manufacturing principles in vacuum forming operations is contributing to sustainability efforts by reducing overproduction and optimizing resource utilization. This approach not only minimizes waste but also improves overall operational efficiency, leading to reduced energy consumption and lower carbon emissions per unit produced.
As the healthcare industry continues to prioritize sustainability, vacuum forming manufacturers are collaborating with healthcare providers and environmental experts to develop innovative solutions. This includes designing products for easier disassembly and recycling, as well as exploring new business models that emphasize product longevity and reusability where appropriate.
To address these concerns, manufacturers are exploring eco-friendly alternatives to conventional plastics used in vacuum forming. Biodegradable and compostable materials derived from renewable sources are being developed and tested for their suitability in medical applications. These materials aim to maintain the necessary properties for healthcare products while reducing the environmental footprint of their production and disposal.
Energy efficiency is another key focus in sustainable vacuum forming practices. Advanced machinery with improved thermal management and precision control systems are being introduced to minimize energy consumption during the forming process. Additionally, manufacturers are implementing heat recovery systems to capture and reuse thermal energy, further reducing overall energy requirements.
Waste reduction strategies are also being employed throughout the vacuum forming lifecycle. This includes optimizing mold designs to minimize material waste, implementing closed-loop recycling systems for production scrap, and developing more efficient packaging solutions to reduce transportation-related emissions. Some facilities are even exploring on-site recycling capabilities to process end-of-life medical products, creating a more circular economy within the healthcare sector.
Water conservation is becoming an integral part of sustainable vacuum forming practices. New cooling systems and cleaning processes are being developed to minimize water usage without compromising product quality or hygiene standards. Some manufacturers are implementing water recycling and treatment systems to further reduce their environmental impact.
The adoption of lean manufacturing principles in vacuum forming operations is contributing to sustainability efforts by reducing overproduction and optimizing resource utilization. This approach not only minimizes waste but also improves overall operational efficiency, leading to reduced energy consumption and lower carbon emissions per unit produced.
As the healthcare industry continues to prioritize sustainability, vacuum forming manufacturers are collaborating with healthcare providers and environmental experts to develop innovative solutions. This includes designing products for easier disassembly and recycling, as well as exploring new business models that emphasize product longevity and reusability where appropriate.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!