Wearable-style flexible electrodes for chronic electrophysiology recordings in cardiac and neural chips
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Flexible Electrode Technology Background and Objectives
Flexible electrode technology has evolved significantly over the past two decades, transitioning from rigid implantable devices to highly conformable interfaces capable of long-term integration with biological tissues. The development trajectory began with traditional metal electrodes that, while effective for short-term recordings, caused tissue damage and signal degradation during chronic applications. This limitation prompted research into materials and fabrication techniques that could maintain electrical performance while minimizing mechanical mismatch with soft tissues.
The emergence of flexible electronics in the early 2000s provided the foundation for next-generation bioelectronic interfaces. Initial breakthroughs came through the application of thin-film technologies and the incorporation of elastomeric substrates, allowing devices to bend and stretch without compromising functionality. By 2010, researchers had demonstrated proof-of-concept flexible electrode arrays for neural recording, though these early designs still faced challenges in long-term stability and biocompatibility.
Recent advances in materials science have accelerated progress, with the introduction of conducting polymers, carbon-based nanomaterials, and hybrid composites specifically engineered for bio-integration. These materials offer unique combinations of electrical conductivity, mechanical flexibility, and biological compatibility that were previously unattainable with conventional electrode materials.
The primary technical objective in this field is to develop wearable-style flexible electrodes capable of maintaining stable electrical interfaces with cardiac and neural tissues over extended periods—months to years—without degradation in signal quality or tissue health. This requires addressing several interconnected challenges: achieving ultra-low electrode impedance, minimizing foreign body response, ensuring mechanical durability under repeated deformation, and developing hermetic packaging solutions that protect electronic components while maintaining flexibility.
Another critical objective is miniaturization and system integration, enabling wireless operation and reduced form factors that minimize invasiveness while maximizing coverage of target tissues. This includes the development of flexible multiplexing architectures and low-power signal processing capabilities directly on the flexible substrate.
The technology aims to bridge fundamental neuroscience research with clinical applications, potentially revolutionizing treatments for neurological disorders, cardiac arrhythmias, and enabling advanced brain-machine interfaces. The ultimate goal is to create "invisible" electronic systems that can seamlessly integrate with biological systems, providing continuous monitoring and therapeutic capabilities without the limitations of conventional rigid electronics.
The emergence of flexible electronics in the early 2000s provided the foundation for next-generation bioelectronic interfaces. Initial breakthroughs came through the application of thin-film technologies and the incorporation of elastomeric substrates, allowing devices to bend and stretch without compromising functionality. By 2010, researchers had demonstrated proof-of-concept flexible electrode arrays for neural recording, though these early designs still faced challenges in long-term stability and biocompatibility.
Recent advances in materials science have accelerated progress, with the introduction of conducting polymers, carbon-based nanomaterials, and hybrid composites specifically engineered for bio-integration. These materials offer unique combinations of electrical conductivity, mechanical flexibility, and biological compatibility that were previously unattainable with conventional electrode materials.
The primary technical objective in this field is to develop wearable-style flexible electrodes capable of maintaining stable electrical interfaces with cardiac and neural tissues over extended periods—months to years—without degradation in signal quality or tissue health. This requires addressing several interconnected challenges: achieving ultra-low electrode impedance, minimizing foreign body response, ensuring mechanical durability under repeated deformation, and developing hermetic packaging solutions that protect electronic components while maintaining flexibility.
Another critical objective is miniaturization and system integration, enabling wireless operation and reduced form factors that minimize invasiveness while maximizing coverage of target tissues. This includes the development of flexible multiplexing architectures and low-power signal processing capabilities directly on the flexible substrate.
The technology aims to bridge fundamental neuroscience research with clinical applications, potentially revolutionizing treatments for neurological disorders, cardiac arrhythmias, and enabling advanced brain-machine interfaces. The ultimate goal is to create "invisible" electronic systems that can seamlessly integrate with biological systems, providing continuous monitoring and therapeutic capabilities without the limitations of conventional rigid electronics.
Market Analysis for Chronic Electrophysiology Recording Solutions
The global market for chronic electrophysiology recording solutions is experiencing robust growth, driven by increasing prevalence of cardiac and neurological disorders. The market size for wearable medical devices was valued at approximately $21.3 billion in 2022, with projections to reach $196.6 billion by 2030, growing at a CAGR of 28.5%. Within this broader category, flexible electrode technologies for chronic monitoring represent a rapidly expanding segment.
Healthcare expenditure on cardiac and neurological conditions continues to rise globally, with cardiac diseases remaining the leading cause of mortality worldwide. The economic burden of these conditions has created strong demand for continuous monitoring solutions that can provide early detection and intervention, potentially reducing hospitalization costs and improving patient outcomes.
The aging global population serves as a significant market driver, with individuals over 65 years representing the demographic most affected by cardiac arrhythmias and neurodegenerative disorders. This demographic is projected to double by 2050, creating sustained demand growth for chronic monitoring technologies.
Geographically, North America currently dominates the market with approximately 40% share, followed by Europe at 30% and Asia-Pacific at 20%. However, the Asia-Pacific region is expected to witness the fastest growth rate due to improving healthcare infrastructure, increasing healthcare expenditure, and rising awareness about preventive healthcare.
The reimbursement landscape has evolved favorably for chronic monitoring solutions, with many insurance providers now covering long-term cardiac and neural monitoring devices. This shift has significantly expanded market accessibility, particularly in developed economies.
Consumer demand patterns indicate growing preference for non-invasive, comfortable monitoring solutions that integrate seamlessly into daily life. This trend aligns perfectly with the development trajectory of wearable-style flexible electrodes, which offer improved patient comfort during extended monitoring periods.
Regulatory pathways for chronic monitoring devices have become more streamlined in recent years, with the FDA and European regulatory bodies establishing clearer approval processes for wearable medical technologies. This regulatory clarity has accelerated time-to-market for innovative solutions.
Market segmentation reveals distinct categories including hospital-based monitoring systems, ambulatory monitoring devices, and consumer-grade health tracking solutions. The ambulatory and consumer segments are experiencing the most rapid growth, reflecting the shift toward decentralized healthcare delivery models.
Investment in this sector has seen significant uptick, with venture capital funding for flexible bioelectronics startups reaching $1.2 billion in 2022, a 35% increase from the previous year. This investment surge indicates strong confidence in the market's growth potential and technological viability.
Healthcare expenditure on cardiac and neurological conditions continues to rise globally, with cardiac diseases remaining the leading cause of mortality worldwide. The economic burden of these conditions has created strong demand for continuous monitoring solutions that can provide early detection and intervention, potentially reducing hospitalization costs and improving patient outcomes.
The aging global population serves as a significant market driver, with individuals over 65 years representing the demographic most affected by cardiac arrhythmias and neurodegenerative disorders. This demographic is projected to double by 2050, creating sustained demand growth for chronic monitoring technologies.
Geographically, North America currently dominates the market with approximately 40% share, followed by Europe at 30% and Asia-Pacific at 20%. However, the Asia-Pacific region is expected to witness the fastest growth rate due to improving healthcare infrastructure, increasing healthcare expenditure, and rising awareness about preventive healthcare.
The reimbursement landscape has evolved favorably for chronic monitoring solutions, with many insurance providers now covering long-term cardiac and neural monitoring devices. This shift has significantly expanded market accessibility, particularly in developed economies.
Consumer demand patterns indicate growing preference for non-invasive, comfortable monitoring solutions that integrate seamlessly into daily life. This trend aligns perfectly with the development trajectory of wearable-style flexible electrodes, which offer improved patient comfort during extended monitoring periods.
Regulatory pathways for chronic monitoring devices have become more streamlined in recent years, with the FDA and European regulatory bodies establishing clearer approval processes for wearable medical technologies. This regulatory clarity has accelerated time-to-market for innovative solutions.
Market segmentation reveals distinct categories including hospital-based monitoring systems, ambulatory monitoring devices, and consumer-grade health tracking solutions. The ambulatory and consumer segments are experiencing the most rapid growth, reflecting the shift toward decentralized healthcare delivery models.
Investment in this sector has seen significant uptick, with venture capital funding for flexible bioelectronics startups reaching $1.2 billion in 2022, a 35% increase from the previous year. This investment surge indicates strong confidence in the market's growth potential and technological viability.
Current Challenges in Wearable Electrode Development
Despite significant advancements in wearable electrode technology, several critical challenges persist in developing effective flexible electrodes for chronic electrophysiology recordings in cardiac and neural applications. The foremost challenge remains achieving long-term biocompatibility while maintaining signal integrity. Current electrode materials often trigger foreign body responses when implanted for extended periods, leading to inflammation, fibrosis, and signal degradation over time. This immune response not only compromises data quality but also poses safety risks to patients.
Signal stability presents another major hurdle, particularly for chronic recordings. Environmental factors such as moisture, temperature fluctuations, and mechanical deformation during body movement can significantly alter electrode properties. These variations introduce noise and artifacts that complicate signal interpretation, especially problematic for applications requiring precise monitoring of cardiac rhythms or neural activity patterns.
Mechanical durability represents a significant technical barrier. Flexible electrodes must withstand repeated bending, stretching, and compression while maintaining electrical performance. Current materials often face trade-offs between flexibility and durability, with many electrodes showing performance deterioration after extended wear periods. This limitation severely restricts the practical deployment of wearable cardiac and neural monitoring systems in real-world settings.
Power management remains problematic for chronic recording applications. While flexible electrodes themselves may be passive components, the associated recording systems require power sources that are similarly flexible and long-lasting. Current battery technologies struggle to meet these requirements without adding substantial bulk or requiring frequent recharging, limiting the true wearability of these systems.
Manufacturing scalability presents additional challenges. Many promising electrode designs rely on complex fabrication processes that are difficult to scale for mass production. Techniques such as photolithography, laser patterning, and specialized deposition methods often require expensive equipment and precise control, increasing production costs and limiting widespread adoption.
Data processing and transmission capabilities also face limitations. The enormous volume of electrophysiological data generated during chronic recordings requires sophisticated on-board processing to extract meaningful information while minimizing power consumption. Current systems often struggle to balance these competing demands, particularly when wireless transmission is required.
Regulatory hurdles further complicate development efforts. The novel materials and designs used in flexible electrodes often lack established safety profiles, requiring extensive testing and validation before clinical deployment. This regulatory pathway can significantly delay the introduction of innovative electrode technologies into medical practice.
Signal stability presents another major hurdle, particularly for chronic recordings. Environmental factors such as moisture, temperature fluctuations, and mechanical deformation during body movement can significantly alter electrode properties. These variations introduce noise and artifacts that complicate signal interpretation, especially problematic for applications requiring precise monitoring of cardiac rhythms or neural activity patterns.
Mechanical durability represents a significant technical barrier. Flexible electrodes must withstand repeated bending, stretching, and compression while maintaining electrical performance. Current materials often face trade-offs between flexibility and durability, with many electrodes showing performance deterioration after extended wear periods. This limitation severely restricts the practical deployment of wearable cardiac and neural monitoring systems in real-world settings.
Power management remains problematic for chronic recording applications. While flexible electrodes themselves may be passive components, the associated recording systems require power sources that are similarly flexible and long-lasting. Current battery technologies struggle to meet these requirements without adding substantial bulk or requiring frequent recharging, limiting the true wearability of these systems.
Manufacturing scalability presents additional challenges. Many promising electrode designs rely on complex fabrication processes that are difficult to scale for mass production. Techniques such as photolithography, laser patterning, and specialized deposition methods often require expensive equipment and precise control, increasing production costs and limiting widespread adoption.
Data processing and transmission capabilities also face limitations. The enormous volume of electrophysiological data generated during chronic recordings requires sophisticated on-board processing to extract meaningful information while minimizing power consumption. Current systems often struggle to balance these competing demands, particularly when wireless transmission is required.
Regulatory hurdles further complicate development efforts. The novel materials and designs used in flexible electrodes often lack established safety profiles, requiring extensive testing and validation before clinical deployment. This regulatory pathway can significantly delay the introduction of innovative electrode technologies into medical practice.
Current Flexible Electrode Design Approaches
01 Flexible electrode materials for chronic recordings
Flexible materials such as polymers and thin-film metals are used to create electrodes that can conform to tissue surfaces, reducing mechanical mismatch between the electrode and neural tissue. These materials minimize tissue damage and inflammatory responses during long-term implantation, which is crucial for chronic electrophysiology recordings. Common flexible substrates include polyimide, parylene-C, and silicone elastomers that provide durability while maintaining flexibility for movement with the target tissue.- Flexible electrode materials for chronic recordings: Flexible materials such as conductive polymers, thin metal films, and carbon-based composites are used to create electrodes that can conform to tissue surfaces while maintaining electrical conductivity. These materials reduce mechanical mismatch between electrodes and neural tissue, minimizing inflammation and scar formation during long-term implantation. The flexibility allows the electrodes to move with the tissue, maintaining stable contact points for chronic electrophysiological recordings while reducing tissue damage.
- Implantable electrode arrays for long-term neural monitoring: Specialized electrode arrays designed for implantation can record neural activity over extended periods. These arrays incorporate multiple recording sites on flexible substrates that can be positioned across different brain regions. Advanced designs include wireless transmission capabilities, eliminating the need for transcutaneous connections that increase infection risk. The arrays are engineered to minimize foreign body response while maintaining signal quality during chronic recordings.
- Biocompatible coatings for chronic electrode stability: Specialized coatings are applied to electrodes to improve biocompatibility and recording stability during chronic implantation. These coatings include anti-inflammatory agents, cell-adhesion molecules, and drug-eluting compounds that mitigate tissue response. Some coatings incorporate hydrogels that match tissue mechanical properties and can deliver anti-fibrotic agents locally. These approaches extend electrode functional lifespan by preventing signal degradation caused by glial scarring and electrode encapsulation.
- Miniaturized wireless recording systems: Compact wireless systems enable chronic electrophysiological recordings without tethering subjects to recording equipment. These systems integrate flexible electrodes with miniaturized amplifiers, processors, and transmission components. Power solutions include inductive charging, energy harvesting, or long-life batteries. The wireless approach eliminates movement artifacts and allows for recordings in naturalistic environments, providing more representative neural data during chronic studies.
- Advanced signal processing for chronic recordings: Specialized algorithms and processing techniques are developed to maintain signal quality during chronic recordings. These methods address common challenges such as baseline drift, signal attenuation, and increasing noise over time. Adaptive filtering techniques compensate for electrode impedance changes, while machine learning approaches can extract meaningful signals even as recording quality degrades. Real-time processing capabilities allow for closed-loop applications where stimulation parameters adjust based on ongoing neural activity.
02 Microelectrode array designs for long-term neural interfaces
Advanced microelectrode array designs incorporate multiple recording sites on flexible substrates to capture signals from various neural populations simultaneously. These arrays can be customized in terms of electrode density, spacing, and configuration to target specific brain regions. The designs often include features that minimize tissue damage during insertion and stabilize the position of recording sites to maintain signal quality over extended periods, which is essential for chronic electrophysiology studies.Expand Specific Solutions03 Biocompatible coatings and surface modifications
Electrodes for chronic recordings are often coated with biocompatible materials to improve integration with surrounding tissue and reduce foreign body responses. These coatings can include hydrogels, conducting polymers like PEDOT:PSS, or biomolecules that promote cell adhesion while maintaining electrical properties. Surface modifications may also incorporate anti-inflammatory agents or growth factors to create a more favorable interface between the electrode and neural tissue, enhancing long-term recording stability.Expand Specific Solutions04 Wireless and telemetric recording systems
Wireless technology enables chronic electrophysiology recordings without tethered connections that restrict movement and increase infection risk. These systems incorporate miniaturized electronics for signal amplification, processing, and wireless transmission, allowing subjects to move freely during long-term monitoring. Power management strategies, including inductive charging or energy harvesting techniques, extend the operational lifetime of implanted devices for continuous chronic recordings in research or clinical applications.Expand Specific Solutions05 Implantation techniques and stabilization methods
Specialized implantation techniques have been developed to minimize tissue damage when placing flexible electrodes for chronic recordings. These include shuttle devices, dissolvable carriers, or injection methods that facilitate accurate positioning while preserving electrode integrity. Post-implantation stabilization methods such as bioresorbable anchors, microfabricated barbs, or tissue-integrating structures help maintain electrode position despite brain micromotion or body movement, ensuring consistent recording quality over extended periods.Expand Specific Solutions
Leading Companies in Cardiac and Neural Monitoring
The wearable-style flexible electrodes for chronic electrophysiology recordings market is currently in a growth phase, characterized by increasing adoption in cardiac and neural monitoring applications. The global market size is estimated to reach $3.5 billion by 2027, driven by rising prevalence of cardiovascular diseases and neurological disorders. Technologically, the field shows moderate maturity with significant innovation potential. Leading players include established medical device companies like Medtronic and ZOLL Medical, which focus on clinical applications, while Verily Life Sciences and Bardy Diagnostics are advancing novel monitoring solutions with enhanced comfort and data analytics. Academic institutions such as Zhejiang University and Tokyo Institute of Technology are contributing fundamental research, while specialized firms like VivaQuant and Cogwear are developing niche applications with improved signal processing capabilities.
Medtronic, Inc.
Technical Solution: Medtronic has developed advanced flexible electrode technology for chronic cardiac monitoring through their Reveal LINQ™ insertable cardiac monitoring system. Their approach utilizes ultra-thin, conformable electrodes with specialized coatings that minimize tissue reaction while maintaining signal quality over extended periods. The electrodes incorporate a proprietary hydrogel interface that improves electrode-tissue contact while reducing impedance fluctuations. Medtronic's technology employs microfabrication techniques to create serpentine-structured electrodes that can withstand mechanical deformation while maintaining electrical performance. Their system integrates wireless data transmission capabilities, allowing for continuous remote monitoring without compromising patient mobility. Recent innovations include electrodes with anti-inflammatory drug-eluting capabilities to further reduce tissue reaction and extend functional longevity in chronic implantation scenarios.
Strengths: Industry-leading biocompatibility with proven long-term implantation safety record; extensive clinical validation across diverse patient populations; sophisticated signal processing algorithms that compensate for motion artifacts. Weaknesses: Higher manufacturing costs compared to conventional electrodes; limited flexibility in some designs that may restrict application in highly dynamic anatomical locations.
Verily Life Sciences LLC
Technical Solution: Verily has pioneered flexible electrode technology through their Study Watch platform, which incorporates multiple sensing modalities for chronic physiological monitoring. Their approach utilizes ultra-thin, stretchable electrode arrays fabricated on elastomeric substrates that conform to skin contours while maintaining reliable electrical contact. The company has developed proprietary nanomaterial composites that enhance conductivity while preserving mechanical flexibility, allowing for continuous wear without signal degradation. Verily's electrodes feature multilayer designs with specialized interface layers that optimize signal transduction while minimizing motion artifacts. Their technology incorporates advanced microfluidic channels that can deliver electrolyte solutions to maintain electrode hydration during extended wear periods. The electrodes are integrated with custom low-power acquisition circuits that enable continuous high-fidelity recording while maximizing battery life in wearable form factors.
Strengths: Exceptional signal-to-noise ratio in ambulatory conditions; sophisticated data analytics platform that extracts clinically relevant features from raw signals; seamless integration with cloud infrastructure for real-time monitoring. Weaknesses: Higher production costs compared to traditional electrodes; potential for skin irritation during extended wear in some patient populations.
Key Patents in Wearable Electrophysiology Recording
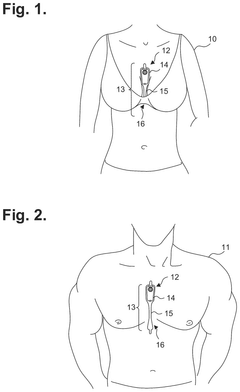
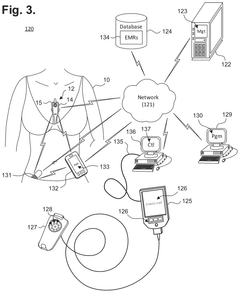
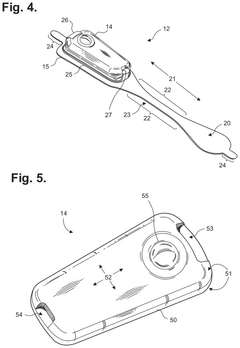
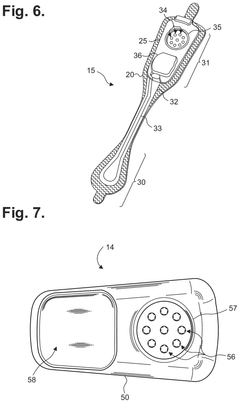
Extended wear electrocardiography patch using interlaced wire electrodes
PatentPendingHK1227272A
Innovation
- A lightweight, flexible extended wear ECG patch with interlaced wire electrodes and a reusable monitor recorder that snaps into a receptacle, providing improved adhesion and comfort by using a non-irritating adhesive and flexible backing, allowing for long-term monitoring without constant reapplication, and reducing manufacturing complexity and costs.
Electrocardiography patch
PatentActiveUS20250160722A1
Innovation
- A wearable electrocardiography patch with a flexible extended wear electrode patch and a removable reusable monitor recorder that can be wirelessly interfaced with other devices, allowing for long-term ECG recording and integration of wider-ranging physiological data.
Biocompatibility and Long-term Tissue Interface Considerations
Biocompatibility represents a critical consideration in the development of wearable-style flexible electrodes for chronic electrophysiology recordings. The long-term implantation of these devices necessitates materials that minimize foreign body responses while maintaining functional integrity. Current research indicates that materials such as PEDOT:PSS, platinum-silicone composites, and certain carbon-based nanomaterials demonstrate superior biocompatibility profiles compared to traditional rigid metal electrodes.
The tissue-electrode interface undergoes significant changes during chronic implantation, characterized by initial acute inflammation followed by potential fibrotic encapsulation. Studies have shown that flexible electrodes with elastic moduli closer to biological tissues (approximately 1-100 kPa) significantly reduce this foreign body response compared to rigid alternatives. This mechanical matching minimizes micromotion-induced trauma and subsequent inflammatory cascades that can compromise signal quality over time.
Surface modifications have emerged as effective strategies for enhancing long-term biocompatibility. Anti-inflammatory drug elution systems incorporated into electrode substrates have demonstrated promising results in mitigating chronic inflammation. Additionally, biomimetic coatings such as phosphorylcholine-based polymers and decellularized extracellular matrix components have shown potential in reducing protein adsorption and subsequent immune recognition.
Degradation mechanisms present substantial challenges for chronic recording applications. Hydrolytic breakdown of polymeric substrates and oxidative stress at the electrode-tissue interface can compromise device performance over time. Recent innovations include self-healing polymers and antioxidant-releasing coatings that actively counteract these degradative processes, potentially extending functional lifetimes from months to years.
The vascularization of surrounding tissue plays a dual role in biocompatibility considerations. While excessive angiogenesis can disrupt electrode positioning, controlled neovascularization may enhance nutrient delivery and waste removal at the implant site. Emerging research explores angiogenic factor gradients to optimize this balance for sustained electrode function in both cardiac and neural applications.
Sterilization protocols significantly impact long-term biocompatibility, with traditional methods like ethylene oxide and gamma irradiation potentially compromising the mechanical and electrical properties of flexible materials. Novel approaches including supercritical CO2 sterilization and cold atmospheric plasma treatment offer promising alternatives that preserve material integrity while ensuring sterility.
Regulatory frameworks increasingly emphasize the importance of long-term biocompatibility testing beyond the standard ISO 10993 protocols. Comprehensive evaluation now includes chronic in vivo models that assess not only tissue response but also sustained recording quality over extended timeframes, reflecting the intended clinical use of these chronic electrophysiology platforms.
The tissue-electrode interface undergoes significant changes during chronic implantation, characterized by initial acute inflammation followed by potential fibrotic encapsulation. Studies have shown that flexible electrodes with elastic moduli closer to biological tissues (approximately 1-100 kPa) significantly reduce this foreign body response compared to rigid alternatives. This mechanical matching minimizes micromotion-induced trauma and subsequent inflammatory cascades that can compromise signal quality over time.
Surface modifications have emerged as effective strategies for enhancing long-term biocompatibility. Anti-inflammatory drug elution systems incorporated into electrode substrates have demonstrated promising results in mitigating chronic inflammation. Additionally, biomimetic coatings such as phosphorylcholine-based polymers and decellularized extracellular matrix components have shown potential in reducing protein adsorption and subsequent immune recognition.
Degradation mechanisms present substantial challenges for chronic recording applications. Hydrolytic breakdown of polymeric substrates and oxidative stress at the electrode-tissue interface can compromise device performance over time. Recent innovations include self-healing polymers and antioxidant-releasing coatings that actively counteract these degradative processes, potentially extending functional lifetimes from months to years.
The vascularization of surrounding tissue plays a dual role in biocompatibility considerations. While excessive angiogenesis can disrupt electrode positioning, controlled neovascularization may enhance nutrient delivery and waste removal at the implant site. Emerging research explores angiogenic factor gradients to optimize this balance for sustained electrode function in both cardiac and neural applications.
Sterilization protocols significantly impact long-term biocompatibility, with traditional methods like ethylene oxide and gamma irradiation potentially compromising the mechanical and electrical properties of flexible materials. Novel approaches including supercritical CO2 sterilization and cold atmospheric plasma treatment offer promising alternatives that preserve material integrity while ensuring sterility.
Regulatory frameworks increasingly emphasize the importance of long-term biocompatibility testing beyond the standard ISO 10993 protocols. Comprehensive evaluation now includes chronic in vivo models that assess not only tissue response but also sustained recording quality over extended timeframes, reflecting the intended clinical use of these chronic electrophysiology platforms.
Regulatory Pathway for Implantable Monitoring Devices
The regulatory landscape for implantable monitoring devices, particularly those utilizing wearable-style flexible electrodes for chronic electrophysiology recordings, presents a complex pathway requiring careful navigation. In the United States, the Food and Drug Administration (FDA) classifies these devices primarily under Class III, necessitating a Premarket Approval (PMA) process due to their implantable nature and direct interaction with critical organs like the heart and brain.
For cardiac and neural monitoring devices, manufacturers must first conduct extensive preclinical testing, including biocompatibility assessments, electrical safety evaluations, and long-term implantation studies. These tests must demonstrate that flexible electrode materials maintain integrity without degradation or causing adverse tissue reactions during chronic use.
Clinical trials for these devices typically follow a phased approach, beginning with small feasibility studies (Early Feasibility Studies or EFS) before progressing to pivotal trials. The FDA's Breakthrough Devices Program offers an accelerated pathway for novel technologies that address unmet medical needs, which may benefit innovative flexible electrode designs that significantly improve chronic monitoring capabilities.
In Europe, the Medical Device Regulation (MDR) framework requires manufacturers to obtain CE marking through Notified Bodies. The classification of neural and cardiac monitoring devices typically falls under Class III, requiring the most stringent conformity assessment procedures and clinical evidence. The MDR places particular emphasis on post-market surveillance, especially relevant for chronic implantable devices where long-term performance data is critical.
Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has established the SAKIGAKE designation system for innovative medical technologies, potentially expediting approval for advanced flexible electrode systems. Similarly, China's National Medical Products Administration (NMPA) has created special review procedures for innovative medical devices.
International standards such as ISO 14708 (implantable medical devices), ISO 10993 (biocompatibility), and IEC 60601 (electrical safety) form the foundation of regulatory compliance across jurisdictions. Manufacturers must demonstrate adherence to these standards while also addressing region-specific requirements.
Post-approval requirements include implementing robust quality management systems, conducting post-market clinical follow-up studies, and maintaining vigilance reporting systems. For chronic monitoring devices, long-term safety monitoring is particularly emphasized by regulatory bodies worldwide, with requirements for extended follow-up periods to capture potential delayed complications.
For cardiac and neural monitoring devices, manufacturers must first conduct extensive preclinical testing, including biocompatibility assessments, electrical safety evaluations, and long-term implantation studies. These tests must demonstrate that flexible electrode materials maintain integrity without degradation or causing adverse tissue reactions during chronic use.
Clinical trials for these devices typically follow a phased approach, beginning with small feasibility studies (Early Feasibility Studies or EFS) before progressing to pivotal trials. The FDA's Breakthrough Devices Program offers an accelerated pathway for novel technologies that address unmet medical needs, which may benefit innovative flexible electrode designs that significantly improve chronic monitoring capabilities.
In Europe, the Medical Device Regulation (MDR) framework requires manufacturers to obtain CE marking through Notified Bodies. The classification of neural and cardiac monitoring devices typically falls under Class III, requiring the most stringent conformity assessment procedures and clinical evidence. The MDR places particular emphasis on post-market surveillance, especially relevant for chronic implantable devices where long-term performance data is critical.
Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has established the SAKIGAKE designation system for innovative medical technologies, potentially expediting approval for advanced flexible electrode systems. Similarly, China's National Medical Products Administration (NMPA) has created special review procedures for innovative medical devices.
International standards such as ISO 14708 (implantable medical devices), ISO 10993 (biocompatibility), and IEC 60601 (electrical safety) form the foundation of regulatory compliance across jurisdictions. Manufacturers must demonstrate adherence to these standards while also addressing region-specific requirements.
Post-approval requirements include implementing robust quality management systems, conducting post-market clinical follow-up studies, and maintaining vigilance reporting systems. For chronic monitoring devices, long-term safety monitoring is particularly emphasized by regulatory bodies worldwide, with requirements for extended follow-up periods to capture potential delayed complications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!