Why solid oxide electrolysis cells are vital for future energy solutions
OCT 9, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
SOEC Technology Background and Objectives
Solid Oxide Electrolysis Cells (SOECs) represent a transformative technology in the energy sector, with roots dating back to the mid-20th century. The fundamental principle of SOECs—the reverse operation of solid oxide fuel cells—was first conceptualized in the 1960s, but significant research momentum only began building in the early 2000s as global energy challenges intensified. The technology has evolved from laboratory curiosities to pilot-scale demonstrations over the past two decades, marking a steady progression toward commercial viability.
The evolution of SOEC technology has been driven by the growing recognition of its potential to address multiple energy transition challenges simultaneously. Unlike conventional electrolysis technologies, SOECs operate at high temperatures (700-900°C), enabling them to achieve exceptional electrical efficiency by leveraging thermal energy to assist the electrolysis process. This thermodynamic advantage positions SOECs as a critical technology for efficient hydrogen production and carbon-neutral fuel synthesis.
Current technological trends in SOEC development focus on several key areas: materials innovation to enhance durability and reduce costs, system integration with renewable energy sources, and scaling up production capabilities. The convergence of advanced ceramic manufacturing techniques, nanotechnology, and computational modeling has accelerated progress in addressing historical limitations of SOECs, particularly regarding cell degradation and operational stability.
The primary technical objectives for SOEC advancement include achieving cell lifetimes exceeding 40,000 hours under dynamic operation, reducing manufacturing costs below $500/kW, and demonstrating megawatt-scale systems with overall system efficiencies above 80% (electricity-to-hydrogen). These ambitious targets reflect the technology's potential to revolutionize energy storage and sector coupling—the integration of electricity, heat, and chemical production systems.
Beyond hydrogen production, SOECs are increasingly recognized for their versatility in co-electrolysis applications, where steam and carbon dioxide are simultaneously converted to syngas (CO + H₂), the precursor for synthetic fuels and chemicals. This capability positions SOECs as a cornerstone technology for creating circular carbon economies where captured CO₂ becomes a valuable feedstock rather than a waste product.
The strategic importance of SOEC technology extends beyond technical performance metrics to geopolitical considerations. As nations worldwide pursue energy independence and decarbonization strategies, SOECs offer a pathway to leverage domestic renewable resources for producing storable energy carriers and industrial feedstocks, potentially reducing reliance on imported fossil fuels and enhancing energy security.
The evolution of SOEC technology has been driven by the growing recognition of its potential to address multiple energy transition challenges simultaneously. Unlike conventional electrolysis technologies, SOECs operate at high temperatures (700-900°C), enabling them to achieve exceptional electrical efficiency by leveraging thermal energy to assist the electrolysis process. This thermodynamic advantage positions SOECs as a critical technology for efficient hydrogen production and carbon-neutral fuel synthesis.
Current technological trends in SOEC development focus on several key areas: materials innovation to enhance durability and reduce costs, system integration with renewable energy sources, and scaling up production capabilities. The convergence of advanced ceramic manufacturing techniques, nanotechnology, and computational modeling has accelerated progress in addressing historical limitations of SOECs, particularly regarding cell degradation and operational stability.
The primary technical objectives for SOEC advancement include achieving cell lifetimes exceeding 40,000 hours under dynamic operation, reducing manufacturing costs below $500/kW, and demonstrating megawatt-scale systems with overall system efficiencies above 80% (electricity-to-hydrogen). These ambitious targets reflect the technology's potential to revolutionize energy storage and sector coupling—the integration of electricity, heat, and chemical production systems.
Beyond hydrogen production, SOECs are increasingly recognized for their versatility in co-electrolysis applications, where steam and carbon dioxide are simultaneously converted to syngas (CO + H₂), the precursor for synthetic fuels and chemicals. This capability positions SOECs as a cornerstone technology for creating circular carbon economies where captured CO₂ becomes a valuable feedstock rather than a waste product.
The strategic importance of SOEC technology extends beyond technical performance metrics to geopolitical considerations. As nations worldwide pursue energy independence and decarbonization strategies, SOECs offer a pathway to leverage domestic renewable resources for producing storable energy carriers and industrial feedstocks, potentially reducing reliance on imported fossil fuels and enhancing energy security.
Market Demand for Green Hydrogen Production
The global market for green hydrogen production is experiencing unprecedented growth, driven by the urgent need for decarbonization across multiple sectors. Current estimates value the green hydrogen market at approximately $2.5 billion in 2022, with projections indicating a compound annual growth rate exceeding 39% through 2030. This remarkable expansion reflects the increasing recognition of hydrogen as a versatile energy carrier capable of addressing decarbonization challenges in hard-to-abate sectors such as heavy industry, long-haul transportation, and chemical manufacturing.
Solid oxide electrolysis cells (SOECs) are positioned as a critical technology in this burgeoning market due to their superior efficiency in hydrogen production compared to conventional alkaline and PEM electrolyzers. The demand for high-efficiency hydrogen production technologies is particularly acute as industries and governments worldwide commit to ambitious carbon reduction targets, with over 30 countries having established formal hydrogen strategies as of 2023.
The industrial sector represents the largest potential market for SOEC-produced hydrogen, with steel manufacturing, ammonia production, and refining processes collectively accounting for over 90% of current hydrogen consumption. These industries are under mounting pressure to reduce carbon emissions while maintaining production capacity, creating a substantial market opportunity for efficient electrolysis technologies like SOECs.
Transportation applications constitute another rapidly growing market segment, with hydrogen fuel cell vehicles gaining traction in heavy-duty transport applications. Major automotive manufacturers including Toyota, Hyundai, and Daimler have made significant investments in hydrogen mobility solutions, signaling strong industry confidence in hydrogen's role in the future transportation mix.
Energy storage applications represent a third major market driver, with hydrogen increasingly viewed as a solution for long-duration energy storage to complement intermittent renewable energy sources. Grid operators and utilities in regions with high renewable penetration are exploring hydrogen as a seasonal storage medium, creating demand for efficient electrolysis technologies that can utilize excess renewable electricity.
Geographically, Europe leads in green hydrogen initiatives, with the European Union's Hydrogen Strategy targeting 40 GW of electrolyzer capacity by 2030. Asia-Pacific follows closely, with Japan, South Korea, and increasingly China making substantial investments in hydrogen infrastructure. North America is rapidly accelerating its hydrogen ambitions, particularly following the introduction of production tax credits for clean hydrogen in the Inflation Reduction Act.
The market demand for green hydrogen production technologies is further bolstered by declining renewable electricity costs, which constitute the primary operational expense for electrolysis operations. As renewable energy continues to become more affordable, the economic case for green hydrogen production strengthens, creating favorable conditions for advanced electrolysis technologies like SOECs to achieve commercial viability.
Solid oxide electrolysis cells (SOECs) are positioned as a critical technology in this burgeoning market due to their superior efficiency in hydrogen production compared to conventional alkaline and PEM electrolyzers. The demand for high-efficiency hydrogen production technologies is particularly acute as industries and governments worldwide commit to ambitious carbon reduction targets, with over 30 countries having established formal hydrogen strategies as of 2023.
The industrial sector represents the largest potential market for SOEC-produced hydrogen, with steel manufacturing, ammonia production, and refining processes collectively accounting for over 90% of current hydrogen consumption. These industries are under mounting pressure to reduce carbon emissions while maintaining production capacity, creating a substantial market opportunity for efficient electrolysis technologies like SOECs.
Transportation applications constitute another rapidly growing market segment, with hydrogen fuel cell vehicles gaining traction in heavy-duty transport applications. Major automotive manufacturers including Toyota, Hyundai, and Daimler have made significant investments in hydrogen mobility solutions, signaling strong industry confidence in hydrogen's role in the future transportation mix.
Energy storage applications represent a third major market driver, with hydrogen increasingly viewed as a solution for long-duration energy storage to complement intermittent renewable energy sources. Grid operators and utilities in regions with high renewable penetration are exploring hydrogen as a seasonal storage medium, creating demand for efficient electrolysis technologies that can utilize excess renewable electricity.
Geographically, Europe leads in green hydrogen initiatives, with the European Union's Hydrogen Strategy targeting 40 GW of electrolyzer capacity by 2030. Asia-Pacific follows closely, with Japan, South Korea, and increasingly China making substantial investments in hydrogen infrastructure. North America is rapidly accelerating its hydrogen ambitions, particularly following the introduction of production tax credits for clean hydrogen in the Inflation Reduction Act.
The market demand for green hydrogen production technologies is further bolstered by declining renewable electricity costs, which constitute the primary operational expense for electrolysis operations. As renewable energy continues to become more affordable, the economic case for green hydrogen production strengthens, creating favorable conditions for advanced electrolysis technologies like SOECs to achieve commercial viability.
SOEC Development Status and Technical Barriers
Solid Oxide Electrolysis Cells (SOECs) have emerged as a promising technology for clean energy production, particularly in hydrogen generation and carbon utilization. Currently, SOECs have reached early commercialization stages with several demonstration projects operational worldwide. Leading regions include Europe, particularly Denmark and Germany, along with the United States, Japan, and increasingly China, where significant R&D investments are being made.
Despite progress, SOECs face substantial technical barriers that limit widespread adoption. The most critical challenge remains cell durability and degradation rates. Current state-of-the-art systems typically show degradation rates of 1-2% per 1000 hours of operation, significantly higher than the 0.1-0.2% target needed for commercial viability in most applications. This degradation primarily stems from microstructural changes in electrodes and electrolyte interfaces during high-temperature operation.
Material stability presents another major hurdle. The harsh operating environment (700-850°C) combined with varying steam content and electrical potentials leads to accelerated materials degradation. Chromium poisoning from metallic interconnects and silicon contamination from sealing materials continue to compromise long-term performance. Advanced materials such as scandium-doped zirconia electrolytes show promise but remain costly for large-scale implementation.
System-level integration challenges persist, particularly in thermal management and balance-of-plant components. The high operating temperatures necessitate specialized heat exchangers, insulation systems, and control mechanisms that significantly increase system complexity and cost. Current SOEC stacks achieve electrical efficiencies of 80-90% in laboratory settings, but integrated system efficiencies typically fall to 60-75% when accounting for thermal management and auxiliary components.
Scale-up and manufacturing barriers represent significant obstacles to commercialization. Current production methods remain largely semi-automated or batch processes, resulting in high unit costs and inconsistent quality. The industry lacks standardized manufacturing protocols and quality control metrics, hampering rapid scaling and cost reduction. Production costs currently range from $2,000-5,000/kW, substantially above the $500-800/kW target needed for market competitiveness.
Economic viability remains challenging with current levelized costs of hydrogen from SOEC systems at $4-7/kg, compared to conventional methods at $1-3/kg. However, when integrated with renewable energy sources and considering carbon pricing scenarios, the economic gap narrows significantly. Recent techno-economic analyses suggest cost parity could be achieved by 2030 with continued technological improvements and supportive policy frameworks.
Despite progress, SOECs face substantial technical barriers that limit widespread adoption. The most critical challenge remains cell durability and degradation rates. Current state-of-the-art systems typically show degradation rates of 1-2% per 1000 hours of operation, significantly higher than the 0.1-0.2% target needed for commercial viability in most applications. This degradation primarily stems from microstructural changes in electrodes and electrolyte interfaces during high-temperature operation.
Material stability presents another major hurdle. The harsh operating environment (700-850°C) combined with varying steam content and electrical potentials leads to accelerated materials degradation. Chromium poisoning from metallic interconnects and silicon contamination from sealing materials continue to compromise long-term performance. Advanced materials such as scandium-doped zirconia electrolytes show promise but remain costly for large-scale implementation.
System-level integration challenges persist, particularly in thermal management and balance-of-plant components. The high operating temperatures necessitate specialized heat exchangers, insulation systems, and control mechanisms that significantly increase system complexity and cost. Current SOEC stacks achieve electrical efficiencies of 80-90% in laboratory settings, but integrated system efficiencies typically fall to 60-75% when accounting for thermal management and auxiliary components.
Scale-up and manufacturing barriers represent significant obstacles to commercialization. Current production methods remain largely semi-automated or batch processes, resulting in high unit costs and inconsistent quality. The industry lacks standardized manufacturing protocols and quality control metrics, hampering rapid scaling and cost reduction. Production costs currently range from $2,000-5,000/kW, substantially above the $500-800/kW target needed for market competitiveness.
Economic viability remains challenging with current levelized costs of hydrogen from SOEC systems at $4-7/kg, compared to conventional methods at $1-3/kg. However, when integrated with renewable energy sources and considering carbon pricing scenarios, the economic gap narrows significantly. Recent techno-economic analyses suggest cost parity could be achieved by 2030 with continued technological improvements and supportive policy frameworks.
Current SOEC System Designs and Operating Principles
01 Electrode materials and structures for solid oxide electrolysis cells
Various electrode materials and structures are used in solid oxide electrolysis cells to improve performance and durability. These include specialized cathode and anode materials that enhance electrochemical reactions, reduce degradation, and improve conductivity. Advanced electrode structures such as porous designs facilitate gas diffusion and increase active reaction sites, while composite electrodes combining multiple materials can provide synergistic benefits for overall cell efficiency.- Electrode materials and structures for SOECs: The choice of electrode materials and their structural design significantly impacts the performance of solid oxide electrolysis cells. Advanced materials such as perovskites, cermets, and composite electrodes can enhance electrochemical activity and durability. Optimized electrode structures with controlled porosity and thickness facilitate efficient gas diffusion and electrochemical reactions at the triple-phase boundaries, leading to improved cell efficiency and longevity.
- Electrolyte development for high-temperature operation: Electrolyte materials for solid oxide electrolysis cells must exhibit high ionic conductivity while maintaining stability at elevated temperatures. Yttria-stabilized zirconia (YSZ), gadolinium-doped ceria (GDC), and scandia-stabilized zirconia (ScSZ) are commonly used electrolytes. Recent developments focus on reducing electrolyte thickness to minimize ohmic resistance and developing new compositions that can operate at intermediate temperatures (600-800°C) while maintaining performance and durability.
- System integration and stack design: Effective integration of solid oxide electrolysis cells into stacks and complete systems is crucial for commercial viability. Advanced stack designs incorporate improved sealing technologies, interconnect materials resistant to oxidation and chromium evaporation, and optimized flow field configurations. System-level considerations include thermal management, gas handling, and control strategies to maintain stable operation and extend stack lifetime while maximizing efficiency.
- Reversible operation and co-electrolysis capabilities: Reversible solid oxide cells capable of operating in both electrolysis and fuel cell modes offer flexibility for energy storage and conversion applications. Additionally, co-electrolysis of steam and carbon dioxide enables direct production of syngas (H₂ + CO), which can be further processed into synthetic fuels. These advanced operational modes require specialized materials and designs that can withstand redox cycling and maintain stability under varying gas compositions and operating conditions.
- Degradation mechanisms and durability enhancement: Understanding and mitigating degradation mechanisms is essential for improving the long-term durability of solid oxide electrolysis cells. Key degradation processes include electrode delamination, chromium poisoning, nickel agglomeration, and electrolyte cracking. Protective coatings, compositional modifications, and microstructural engineering approaches can enhance resistance to these degradation mechanisms, leading to extended cell lifetimes and more reliable operation under various conditions.
02 Electrolyte compositions for high-temperature operation
Specialized electrolyte compositions are developed for solid oxide electrolysis cells operating at high temperatures. These electrolytes typically feature enhanced ionic conductivity, thermal stability, and mechanical strength. Materials such as yttria-stabilized zirconia (YSZ), gadolinium-doped ceria (GDC), and other ceramic composites are engineered to maintain performance under extreme operating conditions while minimizing degradation and extending cell lifetime.Expand Specific Solutions03 System integration and stack design for solid oxide electrolysis
Advanced stack designs and system integration approaches are crucial for solid oxide electrolysis cell deployment. These include innovative cell stacking configurations, sealing technologies, and interconnect designs that minimize electrical resistance and thermal stress. Complete systems incorporate thermal management, gas handling, and control subsystems to optimize efficiency, ensure safe operation, and enable practical applications such as hydrogen production or syngas generation.Expand Specific Solutions04 Degradation mechanisms and durability enhancement
Research focuses on understanding and mitigating degradation mechanisms in solid oxide electrolysis cells to enhance durability. Key challenges include electrode poisoning, delamination at interfaces, chromium poisoning from interconnects, and microstructural changes during operation. Solutions involve protective coatings, dopants to stabilize materials, optimized operating protocols, and novel material combinations that resist degradation while maintaining electrochemical performance over extended operational periods.Expand Specific Solutions05 Co-electrolysis and multi-functional applications
Solid oxide electrolysis cells can be designed for co-electrolysis of multiple feedstocks (such as steam and carbon dioxide) to produce syngas or other valuable products. These multi-functional applications leverage the high operating temperatures and versatile electrode chemistries to enable efficient conversion processes. Advanced catalyst formulations and optimized operating conditions allow for selective product formation, opening pathways for carbon utilization, renewable fuel production, and chemical manufacturing.Expand Specific Solutions
Leading Organizations in SOEC Research and Commercialization
Solid oxide electrolysis cells (SOECs) are emerging as a critical technology for future energy solutions, with the market currently in its early growth phase. The global SOEC market is projected to expand significantly as green hydrogen and carbon-neutral fuel demands increase. Leading academic institutions like Xi'an Jiaotong University, Tsinghua University, and University of South Carolina are advancing fundamental research, while commercial players demonstrate varying levels of technological maturity. Companies like DynElectro ApS and Topsoe A/S in Europe show specialized expertise in extending SOEC lifespans and system integration, while Asian corporations including Hyundai, Kia, and Sinopec are investing in applications for transportation and industrial sectors. The competitive landscape reflects a blend of established energy companies and specialized startups working to overcome durability and cost challenges.
Dalian Institute of Chemical Physics of CAS
Technical Solution: The Dalian Institute has pioneered innovative SOEC designs featuring novel electrode materials that significantly enhance electrochemical performance and durability. Their research focuses on developing composite electrodes with mixed ionic-electronic conductivity, achieving current densities exceeding 1.5 A/cm² at 800°C while maintaining stability over 3,000+ operating hours. Their proprietary infiltration techniques for electrode fabrication have demonstrated 30% improvements in electrode kinetics compared to conventional methods. The institute has developed specialized protective coatings that mitigate chromium poisoning and sulfur contamination, extending cell lifetimes by up to 40%. Their integrated SOEC-methanation system demonstrates closed-loop carbon utilization, converting CO2 to synthetic natural gas with system efficiencies approaching 85%. Recent breakthroughs include proton-conducting SOECs operating at intermediate temperatures (500-650°C), reducing material degradation while maintaining high performance.
Strengths: Cutting-edge materials science expertise; demonstrated long-term stability improvements; integrated system designs for multiple energy conversion pathways. Weaknesses: Technology primarily at laboratory/pilot scale; higher manufacturing complexity for specialized materials; requires further scale-up validation.
Topsoe A/S
Technical Solution: Topsoe has developed advanced solid oxide electrolysis cell (SOEC) technology that operates at high temperatures (700-850°C), enabling electricity-to-fuel conversion with exceptional efficiency. Their proprietary SOEC stack design incorporates specialized ceramic materials with optimized microstructures that significantly enhance durability and performance. Topsoe's integrated systems can achieve electricity-to-hydrogen conversion efficiencies exceeding 90% when utilizing waste heat, compared to 60-70% for conventional electrolyzers. Their technology enables co-electrolysis of steam and CO2 to produce syngas directly, which can be further processed into various e-fuels through their established catalytic processes. Topsoe has demonstrated industrial-scale implementation with their 2.6 MW SOEC plant in Denmark, producing over 1,200 kg of hydrogen daily while achieving significant cost reductions through their vertically integrated manufacturing approach.
Strengths: Industry-leading electrical efficiency (>90% with heat integration); capability for direct co-electrolysis of H2O and CO2; established large-scale manufacturing infrastructure. Weaknesses: Higher capital costs compared to alkaline electrolyzers; requires high-quality heat sources for optimal efficiency; ceramic components present challenges for rapid thermal cycling.
Critical Materials and Cell Architecture Innovations
Solid oxide electrolysis cell and cell assembly including the same
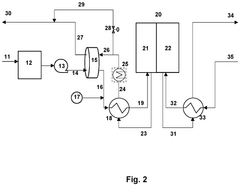
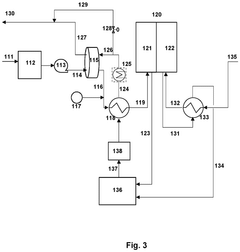
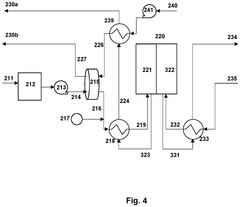
PatentPendingUS20250051932A1
Innovation
- The design includes a unit comprising two unit cells with a porous conductive layer in between, along with a separator outside the unit, allowing for parallel electrical connection of multiple units to prevent high voltage application and incorporating circuit breakers for individual unit control.
Solid oxide cell systems
PatentPendingEP4611079A1
Innovation
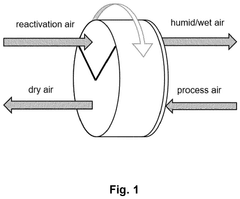
- Integration of a low-pressure desiccant dehumidifier and air recuperator in the SOC system, utilizing waste heat for desiccant regeneration and eliminating the need for separate heaters or blowers, with optimized thermal management to dehumidify process air and prevent chromium migration.
Economic Viability and Cost Reduction Strategies
The economic viability of solid oxide electrolysis cells (SOECs) remains a critical factor determining their widespread adoption in future energy systems. Currently, high capital costs present a significant barrier, with SOEC systems typically costing between $800-1,500 per kilowatt, substantially higher than competing hydrogen production technologies. These costs are primarily driven by expensive ceramic materials, complex manufacturing processes, and the need for high-temperature operation systems.
Material innovation represents a promising cost reduction pathway. Research into alternative electrode materials that maintain performance while reducing reliance on expensive rare earth elements and precious metals could significantly lower production costs. Advanced manufacturing techniques, including 3D printing and roll-to-roll processing, offer potential for streamlined production and reduced labor costs, potentially decreasing manufacturing expenses by 30-40% over the next decade.
Scale economies will play a crucial role in SOEC commercialization. As production volumes increase from current pilot scales to industrial manufacturing, fixed costs can be distributed across larger output volumes. Industry projections suggest that scaling production from megawatt to gigawatt capacity could reduce unit costs by up to 60%, making SOECs increasingly competitive with conventional hydrogen production methods.
System integration improvements offer additional economic benefits. Optimizing thermal management to recover waste heat can increase overall system efficiency by 10-15%, improving the economics of operation. Extending cell lifetimes from current 20,000-30,000 hours to 50,000+ hours would substantially reduce lifetime costs and improve return on investment metrics for industrial adopters.
Policy support mechanisms remain essential for near-term SOEC deployment. Carbon pricing, renewable energy subsidies, and dedicated hydrogen production incentives can significantly improve the economic equation. Analysis indicates that a carbon price of approximately $50-100 per ton could make SOEC hydrogen production cost-competitive with steam methane reforming in many markets, even before achieving full manufacturing scale.
Long-term economic projections suggest SOECs could achieve hydrogen production costs of $2-3/kg by 2030, approaching parity with fossil-based production methods. This economic trajectory, combined with SOECs' unique advantages in integration with renewable energy systems, positions them as increasingly viable components of future decarbonized energy infrastructure, particularly in sectors requiring both electricity storage and clean fuel production capabilities.
Material innovation represents a promising cost reduction pathway. Research into alternative electrode materials that maintain performance while reducing reliance on expensive rare earth elements and precious metals could significantly lower production costs. Advanced manufacturing techniques, including 3D printing and roll-to-roll processing, offer potential for streamlined production and reduced labor costs, potentially decreasing manufacturing expenses by 30-40% over the next decade.
Scale economies will play a crucial role in SOEC commercialization. As production volumes increase from current pilot scales to industrial manufacturing, fixed costs can be distributed across larger output volumes. Industry projections suggest that scaling production from megawatt to gigawatt capacity could reduce unit costs by up to 60%, making SOECs increasingly competitive with conventional hydrogen production methods.
System integration improvements offer additional economic benefits. Optimizing thermal management to recover waste heat can increase overall system efficiency by 10-15%, improving the economics of operation. Extending cell lifetimes from current 20,000-30,000 hours to 50,000+ hours would substantially reduce lifetime costs and improve return on investment metrics for industrial adopters.
Policy support mechanisms remain essential for near-term SOEC deployment. Carbon pricing, renewable energy subsidies, and dedicated hydrogen production incentives can significantly improve the economic equation. Analysis indicates that a carbon price of approximately $50-100 per ton could make SOEC hydrogen production cost-competitive with steam methane reforming in many markets, even before achieving full manufacturing scale.
Long-term economic projections suggest SOECs could achieve hydrogen production costs of $2-3/kg by 2030, approaching parity with fossil-based production methods. This economic trajectory, combined with SOECs' unique advantages in integration with renewable energy systems, positions them as increasingly viable components of future decarbonized energy infrastructure, particularly in sectors requiring both electricity storage and clean fuel production capabilities.
Integration with Renewable Energy Systems
The integration of Solid Oxide Electrolysis Cells (SOECs) with renewable energy systems represents a critical synergy for advancing sustainable energy solutions. Renewable energy sources such as solar and wind power are inherently intermittent, creating significant challenges for grid stability and energy storage. SOECs offer a compelling solution by converting excess renewable electricity into storable hydrogen or syngas during peak production periods, effectively functioning as large-scale energy storage systems.
When connected to solar photovoltaic arrays or wind farms, SOECs can operate during periods of surplus generation, utilizing electricity that might otherwise be curtailed. This integration creates a flexible energy ecosystem where renewable electricity can be stored chemically rather than lost, significantly improving the overall efficiency of renewable installations. The high-temperature operation of SOECs (typically 600-900°C) further enhances this integration, as waste heat from other industrial processes can be utilized to improve electrolysis efficiency.
Several demonstration projects worldwide have validated this integration concept. For instance, the HELMETH project in Europe demonstrated the coupling of SOECs with methanation reactors to convert renewable electricity to synthetic natural gas with system efficiencies exceeding 75%. Similarly, the HyBalance project in Denmark showcased how wind power could be effectively balanced through hydrogen production via electrolysis.
The scalability of SOEC technology presents another advantage for renewable integration. Systems can be designed from kilowatt to megawatt scale, allowing deployment across diverse renewable energy installations from distributed microgrids to utility-scale solar farms. This flexibility enables renewable energy developers to incorporate energy storage solutions proportionate to their generation capacity and grid requirements.
From a grid management perspective, SOEC integration provides valuable grid services including demand response and frequency regulation. By dynamically adjusting their operation based on grid conditions, SOECs can help balance supply and demand, potentially reducing the need for conventional spinning reserves and peaker plants. This capability becomes increasingly valuable as renewable penetration increases in modern grids.
Looking forward, the integration of SOECs with renewable energy systems will likely evolve toward more sophisticated hybrid systems incorporating multiple technologies. These might include combined heat and power systems, carbon capture technologies, and direct air capture systems—all powered by renewable electricity and utilizing the versatility of SOECs to produce various energy carriers and valuable chemicals.
When connected to solar photovoltaic arrays or wind farms, SOECs can operate during periods of surplus generation, utilizing electricity that might otherwise be curtailed. This integration creates a flexible energy ecosystem where renewable electricity can be stored chemically rather than lost, significantly improving the overall efficiency of renewable installations. The high-temperature operation of SOECs (typically 600-900°C) further enhances this integration, as waste heat from other industrial processes can be utilized to improve electrolysis efficiency.
Several demonstration projects worldwide have validated this integration concept. For instance, the HELMETH project in Europe demonstrated the coupling of SOECs with methanation reactors to convert renewable electricity to synthetic natural gas with system efficiencies exceeding 75%. Similarly, the HyBalance project in Denmark showcased how wind power could be effectively balanced through hydrogen production via electrolysis.
The scalability of SOEC technology presents another advantage for renewable integration. Systems can be designed from kilowatt to megawatt scale, allowing deployment across diverse renewable energy installations from distributed microgrids to utility-scale solar farms. This flexibility enables renewable energy developers to incorporate energy storage solutions proportionate to their generation capacity and grid requirements.
From a grid management perspective, SOEC integration provides valuable grid services including demand response and frequency regulation. By dynamically adjusting their operation based on grid conditions, SOECs can help balance supply and demand, potentially reducing the need for conventional spinning reserves and peaker plants. This capability becomes increasingly valuable as renewable penetration increases in modern grids.
Looking forward, the integration of SOECs with renewable energy systems will likely evolve toward more sophisticated hybrid systems incorporating multiple technologies. These might include combined heat and power systems, carbon capture technologies, and direct air capture systems—all powered by renewable electricity and utilizing the versatility of SOECs to produce various energy carriers and valuable chemicals.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!