Acoustic or optical cell-sorting integration into closed workflows for real-time selective expansion
SEP 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Acoustic-Optical Cell-Sorting Technology Background and Objectives
Cell sorting technologies have evolved significantly over the past decades, transitioning from basic mechanical separation methods to sophisticated acoustic and optical approaches. The integration of acoustic or optical cell-sorting technologies into closed workflows represents a critical advancement in bioprocessing and cellular therapy manufacturing. Historically, cell separation began with density gradient centrifugation and magnetic-based sorting in the 1970s, followed by fluorescence-activated cell sorting (FACS) in the 1980s. The past decade has witnessed remarkable progress in acoustic and optical sorting technologies, driven by demands for higher throughput, improved cell viability, and contamination-free processing environments.
The technological evolution has been propelled by breakthroughs in microfluidics, photonics, and ultrasonic manipulation of particles. Acoustic cell sorting utilizes ultrasonic standing waves to separate cells based on their physical properties, while optical sorting employs laser-induced forces or optical detection systems to identify and isolate specific cell populations. These technologies have demonstrated increasing precision and efficiency, gradually replacing traditional open-system approaches that risk contamination and process variability.
Current technological trends indicate a convergence toward fully automated, closed-system workflows that maintain sterility while enabling real-time monitoring and adjustment of cell populations. This trend aligns with the growing requirements in cell therapy manufacturing, where consistent cell quality and regulatory compliance are paramount. The integration of artificial intelligence and machine learning algorithms has further enhanced the capabilities of these systems, allowing for more sophisticated cell identification and sorting decisions.
The primary objective of acoustic-optical cell-sorting integration is to develop robust, scalable platforms that can perform selective cell expansion in real-time within closed systems. This involves creating seamless interfaces between cell identification, sorting mechanisms, and expansion environments while maintaining sterility and cell viability. Additional goals include reducing processing time, minimizing operator intervention, and ensuring reproducibility across manufacturing batches.
Technical objectives specifically focus on enhancing sorting accuracy to >99%, increasing throughput to billions of cells per hour, reducing cell stress during sorting, and developing feedback mechanisms that can dynamically adjust sorting parameters based on real-time cell analysis. The technology aims to support various therapeutic applications, including CAR-T cell therapy, stem cell processing, and exosome isolation, where selective expansion of specific cell populations is critical for therapeutic efficacy.
The ultimate vision is to establish a paradigm shift in cell manufacturing by enabling continuous processing rather than batch-based approaches, thereby improving efficiency, reducing costs, and enhancing the accessibility of advanced cellular therapies to broader patient populations.
The technological evolution has been propelled by breakthroughs in microfluidics, photonics, and ultrasonic manipulation of particles. Acoustic cell sorting utilizes ultrasonic standing waves to separate cells based on their physical properties, while optical sorting employs laser-induced forces or optical detection systems to identify and isolate specific cell populations. These technologies have demonstrated increasing precision and efficiency, gradually replacing traditional open-system approaches that risk contamination and process variability.
Current technological trends indicate a convergence toward fully automated, closed-system workflows that maintain sterility while enabling real-time monitoring and adjustment of cell populations. This trend aligns with the growing requirements in cell therapy manufacturing, where consistent cell quality and regulatory compliance are paramount. The integration of artificial intelligence and machine learning algorithms has further enhanced the capabilities of these systems, allowing for more sophisticated cell identification and sorting decisions.
The primary objective of acoustic-optical cell-sorting integration is to develop robust, scalable platforms that can perform selective cell expansion in real-time within closed systems. This involves creating seamless interfaces between cell identification, sorting mechanisms, and expansion environments while maintaining sterility and cell viability. Additional goals include reducing processing time, minimizing operator intervention, and ensuring reproducibility across manufacturing batches.
Technical objectives specifically focus on enhancing sorting accuracy to >99%, increasing throughput to billions of cells per hour, reducing cell stress during sorting, and developing feedback mechanisms that can dynamically adjust sorting parameters based on real-time cell analysis. The technology aims to support various therapeutic applications, including CAR-T cell therapy, stem cell processing, and exosome isolation, where selective expansion of specific cell populations is critical for therapeutic efficacy.
The ultimate vision is to establish a paradigm shift in cell manufacturing by enabling continuous processing rather than batch-based approaches, thereby improving efficiency, reducing costs, and enhancing the accessibility of advanced cellular therapies to broader patient populations.
Market Analysis for Closed-System Cell Sorting Solutions
The global market for closed-system cell sorting solutions is experiencing robust growth, driven primarily by increasing applications in cell therapy, regenerative medicine, and personalized healthcare. Current market valuations indicate that the cell sorting technology sector reached approximately $4.5 billion in 2022, with closed-system solutions representing a rapidly expanding segment projected to grow at a CAGR of 12-14% through 2028.
Acoustic and optical cell sorting technologies integrated into closed workflows address critical market needs for contamination-free processing environments, particularly in GMP-compliant manufacturing settings. The demand is especially pronounced in the cell and gene therapy sector, which has seen over 20 FDA approvals since 2017 and requires stringent processing standards to ensure product safety and efficacy.
Regional market analysis reveals North America currently dominates with approximately 45% market share, followed by Europe (30%) and Asia-Pacific (20%). The Asia-Pacific region, particularly China and Japan, demonstrates the fastest growth trajectory due to increasing investments in advanced healthcare infrastructure and rising adoption of cell-based therapies.
Key market segments include clinical applications (oncology, immunology, and regenerative medicine) representing 65% of the market, and research applications accounting for the remaining 35%. Within clinical applications, oncology-related cell sorting for CAR-T and other immunotherapies constitutes the largest subsegment at 40% of clinical applications.
End-user analysis indicates pharmaceutical and biotechnology companies comprise 45% of the market, academic and research institutions 30%, and healthcare providers 25%. The pharmaceutical segment shows the highest willingness to invest in premium closed-system solutions due to stringent regulatory requirements and scale-up needs.
Market drivers include increasing prevalence of chronic diseases requiring advanced therapeutic approaches, growing investments in cell therapy research, and evolving regulatory frameworks that favor closed processing systems. The COVID-19 pandemic has accelerated market growth by highlighting the importance of rapid cell isolation and processing capabilities in vaccine and therapeutic development.
Barriers to market adoption include high equipment costs (average system prices ranging from $250,000 to $500,000), technical complexity requiring specialized operator training, and integration challenges with existing workflows. Additionally, reimbursement uncertainties for downstream cell therapy products impact investment decisions in upstream processing technologies.
Acoustic and optical cell sorting technologies integrated into closed workflows address critical market needs for contamination-free processing environments, particularly in GMP-compliant manufacturing settings. The demand is especially pronounced in the cell and gene therapy sector, which has seen over 20 FDA approvals since 2017 and requires stringent processing standards to ensure product safety and efficacy.
Regional market analysis reveals North America currently dominates with approximately 45% market share, followed by Europe (30%) and Asia-Pacific (20%). The Asia-Pacific region, particularly China and Japan, demonstrates the fastest growth trajectory due to increasing investments in advanced healthcare infrastructure and rising adoption of cell-based therapies.
Key market segments include clinical applications (oncology, immunology, and regenerative medicine) representing 65% of the market, and research applications accounting for the remaining 35%. Within clinical applications, oncology-related cell sorting for CAR-T and other immunotherapies constitutes the largest subsegment at 40% of clinical applications.
End-user analysis indicates pharmaceutical and biotechnology companies comprise 45% of the market, academic and research institutions 30%, and healthcare providers 25%. The pharmaceutical segment shows the highest willingness to invest in premium closed-system solutions due to stringent regulatory requirements and scale-up needs.
Market drivers include increasing prevalence of chronic diseases requiring advanced therapeutic approaches, growing investments in cell therapy research, and evolving regulatory frameworks that favor closed processing systems. The COVID-19 pandemic has accelerated market growth by highlighting the importance of rapid cell isolation and processing capabilities in vaccine and therapeutic development.
Barriers to market adoption include high equipment costs (average system prices ranging from $250,000 to $500,000), technical complexity requiring specialized operator training, and integration challenges with existing workflows. Additionally, reimbursement uncertainties for downstream cell therapy products impact investment decisions in upstream processing technologies.
Current Challenges in Real-Time Selective Cell Expansion
Despite significant advancements in cell sorting technologies, real-time selective cell expansion within closed systems presents numerous technical challenges that impede widespread clinical and research applications. The integration of acoustic or optical cell-sorting mechanisms into closed workflows remains particularly problematic due to several interconnected factors.
Maintaining sterility throughout the entire process represents a primary concern. Traditional cell sorting methods often require open systems that expose cells to environmental contaminants, whereas closed systems must incorporate sophisticated interfaces between sorting mechanisms and expansion chambers without compromising sterility or cellular viability.
Throughput limitations constitute another significant barrier. Current acoustic and optical sorting technologies struggle to process large cell populations efficiently while maintaining high purity and recovery rates. This bottleneck becomes particularly pronounced when attempting to isolate rare cell subpopulations for subsequent expansion, where even minor inefficiencies can result in substantial cell loss.
Real-time monitoring capabilities present additional complications. Effective selective expansion requires continuous assessment of cell phenotype, proliferation rates, and functional characteristics. However, integrating non-invasive monitoring systems that can provide actionable data without disrupting the closed environment or damaging cells remains technically challenging.
Scalability issues further complicate implementation. Laboratory-scale acoustic and optical sorting systems often employ principles that do not translate efficiently to larger production volumes required for clinical applications. The physics of acoustic wave propagation or optical detection can change significantly with increased system dimensions.
Biocompatibility of materials used in sorting mechanisms represents another hurdle. Extended contact between cells and device components can trigger unwanted biological responses, including altered gene expression, reduced viability, or phenotypic drift during the expansion phase.
Energy dissipation, particularly in acoustic systems, can generate localized heating effects that compromise cellular integrity. Similarly, optical sorting methods may induce phototoxicity through prolonged exposure to high-intensity light sources, especially problematic during extended processing times required for larger samples.
Regulatory compliance adds another layer of complexity. Closed systems for clinical applications must meet stringent standards for materials, process validation, and quality control. The integration of novel sorting technologies into these workflows necessitates extensive validation studies and regulatory approvals.
Cost considerations also limit widespread adoption. Current high-precision acoustic and optical sorting technologies require expensive specialized equipment and expertise, making implementation economically unfeasible for many potential applications outside specialized research centers.
Maintaining sterility throughout the entire process represents a primary concern. Traditional cell sorting methods often require open systems that expose cells to environmental contaminants, whereas closed systems must incorporate sophisticated interfaces between sorting mechanisms and expansion chambers without compromising sterility or cellular viability.
Throughput limitations constitute another significant barrier. Current acoustic and optical sorting technologies struggle to process large cell populations efficiently while maintaining high purity and recovery rates. This bottleneck becomes particularly pronounced when attempting to isolate rare cell subpopulations for subsequent expansion, where even minor inefficiencies can result in substantial cell loss.
Real-time monitoring capabilities present additional complications. Effective selective expansion requires continuous assessment of cell phenotype, proliferation rates, and functional characteristics. However, integrating non-invasive monitoring systems that can provide actionable data without disrupting the closed environment or damaging cells remains technically challenging.
Scalability issues further complicate implementation. Laboratory-scale acoustic and optical sorting systems often employ principles that do not translate efficiently to larger production volumes required for clinical applications. The physics of acoustic wave propagation or optical detection can change significantly with increased system dimensions.
Biocompatibility of materials used in sorting mechanisms represents another hurdle. Extended contact between cells and device components can trigger unwanted biological responses, including altered gene expression, reduced viability, or phenotypic drift during the expansion phase.
Energy dissipation, particularly in acoustic systems, can generate localized heating effects that compromise cellular integrity. Similarly, optical sorting methods may induce phototoxicity through prolonged exposure to high-intensity light sources, especially problematic during extended processing times required for larger samples.
Regulatory compliance adds another layer of complexity. Closed systems for clinical applications must meet stringent standards for materials, process validation, and quality control. The integration of novel sorting technologies into these workflows necessitates extensive validation studies and regulatory approvals.
Cost considerations also limit widespread adoption. Current high-precision acoustic and optical sorting technologies require expensive specialized equipment and expertise, making implementation economically unfeasible for many potential applications outside specialized research centers.
Existing Integration Solutions for Closed Workflow Systems
01 Acoustic cell sorting technologies
Acoustic-based cell sorting technologies utilize sound waves to separate cells based on their physical properties such as size, density, and compressibility. These systems create acoustic standing waves that exert forces on cells, allowing for label-free separation in microfluidic channels. The technology enables real-time, gentle sorting of cells with high throughput and minimal damage, making it suitable for sensitive applications requiring viable cells for subsequent expansion.- Acoustic cell sorting technologies: Acoustic-based cell sorting technologies utilize sound waves to separate and sort cells based on their physical properties such as size, density, and compressibility. These systems create acoustic standing waves that exert forces on cells, allowing for label-free separation in microfluidic channels. The technology enables real-time, gentle cell manipulation without damaging cellular structures, making it ideal for sensitive applications requiring viable cells for subsequent expansion.
- Optical cell sorting systems: Optical cell sorting technologies employ light-based detection and separation methods such as laser-induced fluorescence, optical tweezers, or imaging cytometry to identify and isolate specific cell populations. These systems can detect multiple cellular parameters simultaneously and sort cells with high precision based on their optical properties or fluorescent markers. The technology allows for real-time monitoring and selective isolation of target cells for downstream expansion applications.
- Real-time cell expansion monitoring systems: Systems designed for real-time monitoring of cell expansion incorporate sensors and imaging technologies to track cell growth, proliferation, and metabolic activity continuously. These platforms integrate data acquisition and analysis capabilities to provide feedback on culture conditions, allowing for dynamic adjustments to optimize cell expansion. The technology enables selective expansion by identifying and promoting the growth of desired cell populations while monitoring their viability and functionality.
- Integrated sorting and expansion platforms: Integrated platforms combine cell sorting technologies with expansion capabilities in a single system, allowing for seamless transition from isolation to culture. These systems incorporate microfluidic components, controlled culture environments, and automated handling to maintain sterility and optimize cell viability. The integration enables selective expansion of sorted cell populations without the need for transfer between separate devices, reducing contamination risks and cell loss while improving efficiency.
- Selective cell expansion techniques: Techniques for selective cell expansion focus on creating optimal conditions that favor the growth of specific cell populations while inhibiting others. These methods incorporate specialized media formulations, growth factors, and physical stimuli to direct cell fate and proliferation. The approaches can be combined with sorting technologies to first isolate target cells and then provide tailored expansion conditions, resulting in higher purity and yield of desired cell types for research or therapeutic applications.
02 Optical cell sorting systems
Optical cell sorting technologies employ light-based detection and separation methods such as fluorescence-activated cell sorting (FACS) and laser capture microdissection. These systems use optical signals to identify cells of interest based on specific markers or properties, followed by physical separation mechanisms. The technology allows for high-precision selection of target cells in real-time, enabling subsequent selective expansion of specific cell populations for research or clinical applications.Expand Specific Solutions03 Microfluidic platforms for cell sorting
Microfluidic platforms integrate various cell sorting mechanisms within miniaturized devices, offering advantages such as reduced sample volume requirements, increased automation, and improved cell viability. These systems incorporate channels, chambers, and sorting junctions that enable precise manipulation of cells in suspension. The technology facilitates real-time monitoring and selective isolation of target cells that can be directly channeled into expansion chambers, creating seamless workflows for cell processing applications.Expand Specific Solutions04 Real-time cell analysis and expansion systems
Integrated systems that combine real-time cell analysis with selective expansion capabilities allow for continuous monitoring of cell populations during the sorting and expansion processes. These technologies incorporate sensors and imaging systems that provide feedback on cell characteristics, enabling dynamic adjustments to sorting parameters and expansion conditions. The approach maximizes the yield and purity of target cell populations while maintaining their functional properties for downstream applications.Expand Specific Solutions05 Automated cell selection and culture technologies
Automated platforms that integrate cell sorting with selective expansion capabilities minimize human intervention and reduce contamination risks. These systems employ robotics, computer vision, and machine learning algorithms to identify, isolate, and culture specific cell populations. The technology enables high-throughput processing of multiple samples simultaneously while maintaining sterility and consistency, making it valuable for clinical applications requiring standardized cell preparation protocols.Expand Specific Solutions
Leading Companies in Cell Sorting and Expansion Systems
The acoustic/optical cell-sorting integration market is currently in an early growth phase, characterized by increasing adoption in biopharmaceutical manufacturing and research applications. The global market size is estimated at approximately $1.5-2 billion, with projected annual growth of 15-20% driven by cell therapy advancements. Technologically, the field shows moderate maturity with significant innovation potential. Leading players include Terumo BCT and Cellares, who have developed closed-system automation platforms; Regenovo and Aokai Biotechnology, focusing on integration with 3D bioprinting; and research institutions like Oregon Health & Science University and Fraunhofer-Gesellschaft advancing novel optical sorting methods. Technology giants including Sony, Google, and Microsoft are entering the space with AI and imaging capabilities, indicating the field's cross-disciplinary nature and commercial potential.
Terumo BCT, Inc.
Technical Solution: Terumo BCT has developed the Quantum Cell Expansion System, which integrates acoustic cell sorting technology into a closed, automated workflow for real-time selective cell expansion. Their platform utilizes ultrasonic standing wave fields to separate cells based on physical properties like size, density, and compressibility without requiring cell labeling. The system incorporates microfluidic channels where acoustic forces align cells at pressure nodes, enabling continuous separation while maintaining sterility. This technology is particularly valuable for CAR-T cell manufacturing, where it can selectively isolate and expand specific T cell subpopulations with therapeutic potential. The closed system architecture minimizes contamination risks and reduces manual handling steps, which is critical for clinical applications. Recent advancements include integration with real-time monitoring systems that adjust separation parameters based on cell population characteristics during the expansion process.
Strengths: Maintains complete closed system integrity, reducing contamination risk and meeting GMP requirements for clinical manufacturing. The label-free approach preserves cell functionality and avoids regulatory complications associated with magnetic beads or fluorescent labels. Weaknesses: Acoustic-based separation may have lower resolution than FACS for complex cell populations, and throughput limitations may exist for large-scale manufacturing applications.
Aokai (Suzhou) Biotechnology Co., Ltd.
Technical Solution: Aokai Biotechnology has pioneered a microfluidic platform that integrates optical cell-sorting technology with closed-system bioreactors for continuous cell culture applications. Their system employs high-speed imaging and machine learning algorithms to identify target cells based on morphological characteristics and fluorescent markers, followed by precise optical manipulation using focused laser beams for cell displacement into collection channels. The technology enables real-time monitoring and selective expansion of specific cell populations without breaking system sterility. A key innovation is their "optical tweezers" approach that can manipulate individual cells with minimal stress, preserving cell viability and functionality. The platform incorporates automated feedback loops that continuously adjust culture conditions based on cell growth patterns and population dynamics, optimizing expansion of desired cell phenotypes. This system has shown particular promise in isolating rare stem cell populations and expanding them for regenerative medicine applications.
Strengths: Offers exceptional precision in single-cell manipulation and sorting, allowing for highly pure target cell populations. The integrated AI-driven imaging system enables label-free sorting based on complex morphological criteria beyond what conventional systems can achieve. Weaknesses: The optical manipulation approach has throughput limitations compared to bulk sorting methods, potentially limiting scalability for industrial applications. The sophisticated optical components also increase system complexity and maintenance requirements.
Key Patents in Acoustic and Optical Cell-Sorting Methods
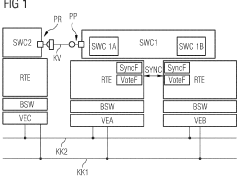
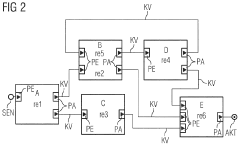
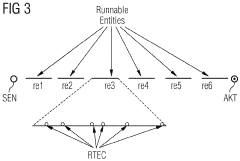
Method for the transparent replication of a software component of a software system
PatentWO2009013055A2
Innovation
- The method involves extending the AUTOSAR runtime environment with synchronization and selection functionality to create a virtual communication channel between replicated processing units, enabling location-transparent communication and ensuring redundancy through synchronized signal processing, using a virtual communication channel and dual-port RAM for synchronization.
Regulatory Considerations for Clinical Cell Processing
The integration of acoustic or optical cell-sorting technologies into closed workflows for clinical cell processing necessitates careful navigation of complex regulatory frameworks. In the United States, the FDA regulates cell-based products primarily through the Center for Biologics Evaluation and Research (CBER), with requirements varying based on whether the cells undergo substantial manipulation or are intended for homologous use.
For closed-system cell sorting technologies, compliance with Good Manufacturing Practice (GMP) standards is paramount. These systems must demonstrate consistent sterility maintenance, minimal cross-contamination risk, and reliable process validation. The FDA's risk-based approach requires manufacturers to implement robust quality control measures and validation protocols specific to real-time selective expansion processes.
European regulatory bodies, particularly under the European Medicines Agency (EMA), classify most cell-based therapies utilizing sorting technologies as Advanced Therapy Medicinal Products (ATMPs). This classification imposes stringent requirements for manufacturing facilities, process validation, and quality control. The closed nature of these workflows provides advantages in meeting these requirements by reducing contamination risks and enhancing process reproducibility.
Documentation requirements represent a significant regulatory consideration. Manufacturers must maintain comprehensive records of equipment qualification, process validation, operator training, and batch production. For real-time cell sorting systems, validation of the selection algorithms and demonstration of consistent cell population identification become critical components of the regulatory submission package.
Regulatory pathways may be expedited for certain applications through programs like the FDA's Regenerative Medicine Advanced Therapy (RMAT) designation or the EMA's Priority Medicines (PRIME) scheme. These pathways can accelerate development for technologies addressing unmet medical needs, potentially benefiting innovative closed-system cell sorting platforms.
International harmonization efforts, including those by the International Council for Harmonisation (ICH) and the International Standards Organization (ISO), are increasingly important as these technologies seek global implementation. Standards such as ISO 13485 for medical device quality management systems provide frameworks applicable to cell sorting instrumentation.
Emerging regulatory considerations include the validation of artificial intelligence components in optical sorting systems and the long-term safety monitoring requirements for cells processed through these novel workflows. Regulatory agencies are increasingly focusing on the entire processing ecosystem rather than individual components, necessitating integrated validation approaches.
For closed-system cell sorting technologies, compliance with Good Manufacturing Practice (GMP) standards is paramount. These systems must demonstrate consistent sterility maintenance, minimal cross-contamination risk, and reliable process validation. The FDA's risk-based approach requires manufacturers to implement robust quality control measures and validation protocols specific to real-time selective expansion processes.
European regulatory bodies, particularly under the European Medicines Agency (EMA), classify most cell-based therapies utilizing sorting technologies as Advanced Therapy Medicinal Products (ATMPs). This classification imposes stringent requirements for manufacturing facilities, process validation, and quality control. The closed nature of these workflows provides advantages in meeting these requirements by reducing contamination risks and enhancing process reproducibility.
Documentation requirements represent a significant regulatory consideration. Manufacturers must maintain comprehensive records of equipment qualification, process validation, operator training, and batch production. For real-time cell sorting systems, validation of the selection algorithms and demonstration of consistent cell population identification become critical components of the regulatory submission package.
Regulatory pathways may be expedited for certain applications through programs like the FDA's Regenerative Medicine Advanced Therapy (RMAT) designation or the EMA's Priority Medicines (PRIME) scheme. These pathways can accelerate development for technologies addressing unmet medical needs, potentially benefiting innovative closed-system cell sorting platforms.
International harmonization efforts, including those by the International Council for Harmonisation (ICH) and the International Standards Organization (ISO), are increasingly important as these technologies seek global implementation. Standards such as ISO 13485 for medical device quality management systems provide frameworks applicable to cell sorting instrumentation.
Emerging regulatory considerations include the validation of artificial intelligence components in optical sorting systems and the long-term safety monitoring requirements for cells processed through these novel workflows. Regulatory agencies are increasingly focusing on the entire processing ecosystem rather than individual components, necessitating integrated validation approaches.
Scalability and Cost Analysis of Integrated Cell-Sorting Systems
The integration of acoustic or optical cell-sorting technologies into closed workflows presents significant scalability and cost considerations that must be carefully evaluated. Current laboratory-scale systems typically process 10,000-50,000 cells per second, which becomes a critical bottleneck when scaling to industrial or clinical applications requiring millions or billions of cells. The capital expenditure for integrated cell-sorting systems ranges from $250,000 to $1.5 million, depending on sorting technology, automation level, and throughput capacity.
Operational costs present another dimension of economic analysis, with consumables accounting for approximately 30-45% of per-sample processing costs. Microfluidic chips for acoustic sorting systems typically cost $50-200 per unit, while optical sorting systems require specialized reagents and fluorescent markers costing $15-40 per sample. Labor costs decrease significantly with automation, potentially reducing from $25-35 per hour for manual operations to $5-10 per sample in fully automated systems.
Scaling considerations reveal important economic inflection points. Small-scale operations processing fewer than 100 samples weekly may find acoustic sorting more economical due to lower consumable costs, despite higher initial investment. Conversely, high-throughput facilities handling 500+ samples weekly might achieve better economies of scale with optical systems due to faster processing speeds, despite higher per-sample costs.
Energy consumption represents another scaling factor, with acoustic systems typically consuming 1.5-2.5 kW during operation compared to optical systems' 3-5 kW. This difference becomes significant in continuous operation scenarios, potentially adding $5,000-15,000 annually to operational costs for large-scale implementations.
Return on investment calculations indicate that integrated cell-sorting systems typically require 2-4 years to achieve financial breakeven, depending on utilization rates. Facilities achieving 70%+ utilization can reduce this to 18-24 months. Importantly, systems designed with modular architectures demonstrate 30-40% lower lifetime costs due to component upgradeability rather than complete system replacement.
Future cost reduction pathways include standardization of microfluidic components, development of reusable sorting chambers, and implementation of machine learning algorithms to optimize sorting parameters automatically. These innovations could potentially reduce per-sample costs by 40-60% within the next five years, significantly improving the economic viability of integrated cell-sorting for widespread clinical applications.
Operational costs present another dimension of economic analysis, with consumables accounting for approximately 30-45% of per-sample processing costs. Microfluidic chips for acoustic sorting systems typically cost $50-200 per unit, while optical sorting systems require specialized reagents and fluorescent markers costing $15-40 per sample. Labor costs decrease significantly with automation, potentially reducing from $25-35 per hour for manual operations to $5-10 per sample in fully automated systems.
Scaling considerations reveal important economic inflection points. Small-scale operations processing fewer than 100 samples weekly may find acoustic sorting more economical due to lower consumable costs, despite higher initial investment. Conversely, high-throughput facilities handling 500+ samples weekly might achieve better economies of scale with optical systems due to faster processing speeds, despite higher per-sample costs.
Energy consumption represents another scaling factor, with acoustic systems typically consuming 1.5-2.5 kW during operation compared to optical systems' 3-5 kW. This difference becomes significant in continuous operation scenarios, potentially adding $5,000-15,000 annually to operational costs for large-scale implementations.
Return on investment calculations indicate that integrated cell-sorting systems typically require 2-4 years to achieve financial breakeven, depending on utilization rates. Facilities achieving 70%+ utilization can reduce this to 18-24 months. Importantly, systems designed with modular architectures demonstrate 30-40% lower lifetime costs due to component upgradeability rather than complete system replacement.
Future cost reduction pathways include standardization of microfluidic components, development of reusable sorting chambers, and implementation of machine learning algorithms to optimize sorting parameters automatically. These innovations could potentially reduce per-sample costs by 40-60% within the next five years, significantly improving the economic viability of integrated cell-sorting for widespread clinical applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!