Scalable electroporation methods for ex vivo nonviral gene insertion in primary T cells
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
T Cell Electroporation Background and Objectives
Electroporation has emerged as a pivotal technology for gene delivery in T cells, evolving significantly since its inception in the 1980s. Initially developed for bacterial transformation, this technique has been adapted for mammalian cells and has become increasingly sophisticated for therapeutic applications. The fundamental principle involves applying electrical pulses to temporarily disrupt cell membranes, allowing genetic material to enter the cytoplasm and eventually the nucleus.
The evolution of T cell electroporation technology has progressed from basic square wave systems to more advanced multi-pulse and flow-based platforms. Early methods suffered from low efficiency and high cell mortality, limiting clinical applications. However, recent advancements have dramatically improved viability and transfection rates, enabling the development of cellular immunotherapies including CAR-T cell products.
Current technological trends focus on scalability, automation, and standardization to meet the growing demand for cell-based therapies. The field is moving toward closed-system approaches that minimize contamination risks and operator variability while maximizing reproducibility across manufacturing batches. Additionally, there is increasing interest in combining electroporation with other technologies such as CRISPR-Cas9 for precise genetic modifications.
The primary objective of developing scalable electroporation methods for ex vivo nonviral gene insertion in primary T cells is to establish robust, GMP-compliant processes capable of supporting commercial-scale manufacturing of cellular immunotherapies. This includes achieving high transfection efficiencies (>50%) while maintaining excellent cell viability (>80%) and preserving T cell functionality and phenotype.
Secondary objectives include reducing the cost of goods through process optimization, minimizing the time from apheresis to final product, and developing universal platforms adaptable to various genetic payloads and T cell subtypes. These improvements aim to address the current limitations of viral vector-based approaches, including manufacturing complexity, safety concerns, and payload size restrictions.
Long-term goals encompass the development of point-of-care systems that could democratize access to cellular therapies by enabling local manufacturing at treatment centers rather than centralized facilities. This would significantly reduce logistical challenges and potentially decrease the time from patient sampling to treatment administration.
The achievement of these objectives would represent a paradigm shift in cellular immunotherapy, potentially expanding treatment options for various cancers, autoimmune disorders, and infectious diseases while improving accessibility through reduced costs and simplified manufacturing processes.
The evolution of T cell electroporation technology has progressed from basic square wave systems to more advanced multi-pulse and flow-based platforms. Early methods suffered from low efficiency and high cell mortality, limiting clinical applications. However, recent advancements have dramatically improved viability and transfection rates, enabling the development of cellular immunotherapies including CAR-T cell products.
Current technological trends focus on scalability, automation, and standardization to meet the growing demand for cell-based therapies. The field is moving toward closed-system approaches that minimize contamination risks and operator variability while maximizing reproducibility across manufacturing batches. Additionally, there is increasing interest in combining electroporation with other technologies such as CRISPR-Cas9 for precise genetic modifications.
The primary objective of developing scalable electroporation methods for ex vivo nonviral gene insertion in primary T cells is to establish robust, GMP-compliant processes capable of supporting commercial-scale manufacturing of cellular immunotherapies. This includes achieving high transfection efficiencies (>50%) while maintaining excellent cell viability (>80%) and preserving T cell functionality and phenotype.
Secondary objectives include reducing the cost of goods through process optimization, minimizing the time from apheresis to final product, and developing universal platforms adaptable to various genetic payloads and T cell subtypes. These improvements aim to address the current limitations of viral vector-based approaches, including manufacturing complexity, safety concerns, and payload size restrictions.
Long-term goals encompass the development of point-of-care systems that could democratize access to cellular therapies by enabling local manufacturing at treatment centers rather than centralized facilities. This would significantly reduce logistical challenges and potentially decrease the time from patient sampling to treatment administration.
The achievement of these objectives would represent a paradigm shift in cellular immunotherapy, potentially expanding treatment options for various cancers, autoimmune disorders, and infectious diseases while improving accessibility through reduced costs and simplified manufacturing processes.
Market Analysis for Ex Vivo Gene Therapy Technologies
The ex vivo gene therapy market has experienced remarkable growth, with the global market valued at approximately $1.2 billion in 2022 and projected to reach $5.3 billion by 2030, representing a compound annual growth rate (CAGR) of 20.4%. This substantial growth is primarily driven by increasing prevalence of genetic disorders, rising investment in gene therapy research, and advancements in delivery technologies.
T cell-based therapies, particularly CAR-T cell therapies, constitute a significant segment of this market. The FDA approval of Kymriah (Novartis) and Yescarta (Gilead/Kite) has validated the commercial viability of engineered T cell therapies, creating a robust demand for more efficient and cost-effective manufacturing processes.
Current market challenges center around manufacturing scalability and cost constraints. Traditional viral vector-based gene delivery methods face limitations including high production costs ($50,000-$200,000 per dose), limited payload capacity, and significant regulatory hurdles. These factors have created a substantial market opportunity for nonviral alternatives, particularly electroporation-based technologies.
The electroporation segment for gene delivery is growing at a CAGR of 23.7%, outpacing the overall market. This acceleration reflects the increasing recognition of electroporation's advantages in terms of safety profile, manufacturing simplicity, and cost-effectiveness. Industry analysts estimate that transitioning from viral to nonviral delivery methods could potentially reduce manufacturing costs by 40-60%.
Regional market distribution shows North America dominating with 45% market share, followed by Europe (30%) and Asia-Pacific (20%). However, the Asia-Pacific region is experiencing the fastest growth rate at 25.3% CAGR, driven by increasing research investments and expanding clinical trial infrastructure.
Key customer segments include academic research institutions, biotechnology companies, pharmaceutical corporations, and contract manufacturing organizations (CMOs). The pharmaceutical and biotechnology segment represents the largest market share at 65%, with CMOs showing the fastest growth as companies increasingly outsource manufacturing processes.
Market forecasts indicate that scalable electroporation technologies for T cell engineering will experience accelerated adoption, with projected market penetration increasing from 15% to 40% over the next five years. This growth trajectory is supported by the expanding pipeline of cell therapy candidates, with over 1,200 ongoing clinical trials globally involving engineered T cells, representing a 35% increase from 2020.
T cell-based therapies, particularly CAR-T cell therapies, constitute a significant segment of this market. The FDA approval of Kymriah (Novartis) and Yescarta (Gilead/Kite) has validated the commercial viability of engineered T cell therapies, creating a robust demand for more efficient and cost-effective manufacturing processes.
Current market challenges center around manufacturing scalability and cost constraints. Traditional viral vector-based gene delivery methods face limitations including high production costs ($50,000-$200,000 per dose), limited payload capacity, and significant regulatory hurdles. These factors have created a substantial market opportunity for nonviral alternatives, particularly electroporation-based technologies.
The electroporation segment for gene delivery is growing at a CAGR of 23.7%, outpacing the overall market. This acceleration reflects the increasing recognition of electroporation's advantages in terms of safety profile, manufacturing simplicity, and cost-effectiveness. Industry analysts estimate that transitioning from viral to nonviral delivery methods could potentially reduce manufacturing costs by 40-60%.
Regional market distribution shows North America dominating with 45% market share, followed by Europe (30%) and Asia-Pacific (20%). However, the Asia-Pacific region is experiencing the fastest growth rate at 25.3% CAGR, driven by increasing research investments and expanding clinical trial infrastructure.
Key customer segments include academic research institutions, biotechnology companies, pharmaceutical corporations, and contract manufacturing organizations (CMOs). The pharmaceutical and biotechnology segment represents the largest market share at 65%, with CMOs showing the fastest growth as companies increasingly outsource manufacturing processes.
Market forecasts indicate that scalable electroporation technologies for T cell engineering will experience accelerated adoption, with projected market penetration increasing from 15% to 40% over the next five years. This growth trajectory is supported by the expanding pipeline of cell therapy candidates, with over 1,200 ongoing clinical trials globally involving engineered T cells, representing a 35% increase from 2020.
Current Challenges in Nonviral T Cell Transfection
Despite significant advancements in nonviral gene delivery methods for T cell engineering, several critical challenges persist that limit widespread clinical application of these technologies. The current gold standard for nonviral transfection of primary T cells relies on electroporation, which involves applying electrical pulses to create temporary pores in cell membranes. However, this approach faces substantial scalability issues when transitioning from research to clinical manufacturing.
One primary challenge is the balance between transfection efficiency and cell viability. Higher electroporation voltages typically increase DNA uptake but simultaneously reduce cell survival rates. This trade-off becomes particularly problematic when working with limited patient-derived T cell samples, where maximizing both parameters is essential for therapeutic efficacy.
Reproducibility across different donor samples represents another significant hurdle. Primary T cells exhibit considerable donor-to-donor variability in their susceptibility to electroporation, necessitating optimization for each patient sample. This variability stems from differences in cell membrane composition, activation status, and metabolic state, making standardization difficult in clinical settings.
The physical limitations of current electroporation devices also impede scalability. Most systems are designed for small-scale research applications and lack the capacity to process the billions of cells required for therapeutic doses. Scaling up often leads to inconsistent field distribution across the cell suspension, resulting in heterogeneous transfection outcomes.
DNA toxicity presents an additional challenge, as primary T cells are particularly sensitive to exogenous DNA. The introduction of plasmid DNA can trigger innate immune sensors like cGAS-STING and TLR9, leading to interferon responses and potential cell death. This toxicity is exacerbated by the high DNA concentrations typically required to achieve sufficient transfection rates.
Integration efficiency remains suboptimal with nonviral approaches. Unlike viral vectors that have evolved mechanisms for efficient genomic integration, nonviral methods rely on the cell's DNA repair machinery for transgene insertion. This process is inherently inefficient in primary T cells, resulting in low stable integration rates and transient expression profiles.
Manufacturing complexity further complicates clinical translation. Current protocols involve multiple handling steps, specialized equipment, and precise timing requirements. These factors increase the risk of contamination, process variability, and manufacturing failures, while simultaneously driving up production costs and limiting accessibility.
Addressing these interconnected challenges requires innovative approaches that fundamentally rethink electroporation technology rather than incremental improvements to existing platforms. The ideal solution would combine high-throughput capabilities with gentle yet effective cell membrane permeabilization techniques that maintain critical T cell functions.
One primary challenge is the balance between transfection efficiency and cell viability. Higher electroporation voltages typically increase DNA uptake but simultaneously reduce cell survival rates. This trade-off becomes particularly problematic when working with limited patient-derived T cell samples, where maximizing both parameters is essential for therapeutic efficacy.
Reproducibility across different donor samples represents another significant hurdle. Primary T cells exhibit considerable donor-to-donor variability in their susceptibility to electroporation, necessitating optimization for each patient sample. This variability stems from differences in cell membrane composition, activation status, and metabolic state, making standardization difficult in clinical settings.
The physical limitations of current electroporation devices also impede scalability. Most systems are designed for small-scale research applications and lack the capacity to process the billions of cells required for therapeutic doses. Scaling up often leads to inconsistent field distribution across the cell suspension, resulting in heterogeneous transfection outcomes.
DNA toxicity presents an additional challenge, as primary T cells are particularly sensitive to exogenous DNA. The introduction of plasmid DNA can trigger innate immune sensors like cGAS-STING and TLR9, leading to interferon responses and potential cell death. This toxicity is exacerbated by the high DNA concentrations typically required to achieve sufficient transfection rates.
Integration efficiency remains suboptimal with nonviral approaches. Unlike viral vectors that have evolved mechanisms for efficient genomic integration, nonviral methods rely on the cell's DNA repair machinery for transgene insertion. This process is inherently inefficient in primary T cells, resulting in low stable integration rates and transient expression profiles.
Manufacturing complexity further complicates clinical translation. Current protocols involve multiple handling steps, specialized equipment, and precise timing requirements. These factors increase the risk of contamination, process variability, and manufacturing failures, while simultaneously driving up production costs and limiting accessibility.
Addressing these interconnected challenges requires innovative approaches that fundamentally rethink electroporation technology rather than incremental improvements to existing platforms. The ideal solution would combine high-throughput capabilities with gentle yet effective cell membrane permeabilization techniques that maintain critical T cell functions.
Current Scalable Electroporation Methodologies
01 Large-scale electroporation systems for industrial applications
Industrial-scale electroporation systems designed for high-throughput processing of biological materials. These systems incorporate advanced electrode configurations, flow-through chambers, and automated control mechanisms to enable continuous processing of large sample volumes. The scalable designs allow for consistent electroporation efficiency across increased throughput, making them suitable for commercial bioproduction, food processing, and pharmaceutical manufacturing applications.- Large-scale electroporation systems for industrial applications: Industrial-scale electroporation systems designed for high-throughput processing of biological materials. These systems incorporate advanced electrode configurations, flow-through chambers, and automated control mechanisms to enable continuous processing of large sample volumes. The scalable designs allow for consistent electroporation efficiency while maintaining cell viability across increased production volumes, making them suitable for biopharmaceutical manufacturing and food processing industries.
- Microfluidic electroporation platforms for scalable cell processing: Microfluidic-based electroporation technologies that enable precise control over electric field distribution and cell exposure conditions. These platforms utilize miniaturized channels and electrode arrays to process cells in a continuous flow manner, allowing for scalability through parallelization. The microfluidic approach reduces sample volume requirements while maintaining high transfection efficiency, making it suitable for applications ranging from research to clinical cell therapy manufacturing.
- Computational modeling for optimizing scalable electroporation processes: Advanced computational modeling techniques used to optimize electroporation parameters for scaling up processes. These models simulate electric field distribution, thermal effects, and cellular responses to predict performance at larger scales. By incorporating multiphysics simulations and machine learning algorithms, these approaches enable the design of more efficient electrode configurations and pulse protocols that maintain effectiveness when transitioning from laboratory to industrial scale applications.
- Pulse generation and delivery systems for high-throughput electroporation: Specialized pulse generation and delivery systems designed to support high-throughput electroporation applications. These systems incorporate advanced power electronics, precise timing controls, and multiple output channels to deliver consistent electric pulses across numerous samples simultaneously. The technology enables scalable processing by maintaining uniform field strength and pulse characteristics across larger treatment volumes or multiple parallel processing units.
- Electrode materials and configurations for scalable electroporation: Novel electrode materials and geometric configurations developed specifically to address scalability challenges in electroporation. These innovations include corrosion-resistant materials, 3D electrode arrays, and modular designs that can be expanded for larger processing volumes. The electrode systems are engineered to maintain uniform electric field distribution across increased treatment areas while minimizing unwanted effects such as heat generation and pH shifts that become more problematic at larger scales.
02 Microfluidic electroporation platforms for scalable cell processing
Microfluidic-based electroporation technologies that enable precise control over electric field parameters while allowing for scalable processing of cell populations. These platforms utilize miniaturized channels and electrode arrays to create uniform electroporation conditions across multiple parallel processing units. The integration of microfluidics with electroporation provides advantages in terms of reduced sample volumes, increased throughput, and improved cell viability compared to conventional bulk electroporation methods.Expand Specific Solutions03 Computational modeling for electroporation scale-up
Advanced computational modeling approaches that facilitate the scale-up of electroporation processes from laboratory to industrial scale. These models simulate electric field distribution, thermal effects, and cellular responses across different system geometries and operating conditions. By predicting performance at larger scales, these computational tools help optimize electrode design, pulse parameters, and flow dynamics to maintain electroporation efficiency during scale-up, reducing the need for extensive empirical testing.Expand Specific Solutions04 Electrode design innovations for scalable electroporation
Novel electrode configurations and materials specifically designed to address scalability challenges in electroporation. These innovations include multi-array electrode systems, flow-through electrode chambers, and specialized electrode geometries that maintain uniform electric field distribution across larger treatment volumes. Advanced electrode materials with improved durability and conductivity properties enable consistent performance during high-throughput operations while minimizing electrode degradation and sample contamination.Expand Specific Solutions05 Automated control systems for high-throughput electroporation
Sophisticated control and monitoring systems that enable reliable operation of electroporation processes at scale. These systems incorporate real-time feedback mechanisms, automated sample handling, and precise pulse delivery control to maintain consistent electroporation conditions across large sample volumes. Integration with data analytics platforms allows for continuous process optimization and quality control, ensuring reproducible results during scaled-up operations in research and manufacturing environments.Expand Specific Solutions
Key Industry Players in Cell Engineering and Gene Therapy
The field of scalable electroporation methods for ex vivo nonviral gene insertion in T cells is currently in a growth phase, with market size expanding rapidly due to increasing demand for cell therapies. The technology is approaching maturity with several established players demonstrating viable solutions. MaxCyte leads with its scalable transfection systems, while academic institutions like UC and University of Michigan provide foundational research. Commercial entities including Inovio Pharmaceuticals and CyteQuest are advancing microfluidic approaches, while Suzhou Yida Biotechnology has developed high-capacity flow electroporation systems processing up to 1×10^10 cells. The competitive landscape shows a mix of established biotech companies and emerging startups, with recent entrants like Cellectis and specialized players such as Triple Ring Technologies driving innovation in delivery efficiency and cell viability.
The Regents of the University of California
Technical Solution: The University of California has developed a microfluidic-based electroporation platform for high-efficiency gene insertion into primary T cells. This innovative approach combines precise microfluidic cell handling with localized electric field application, creating a more controlled and gentle transfection environment. The system features parallel microchannels with integrated electrodes that expose cells to optimized electric fields as they flow through the device. This design enables continuous processing of T cells while maintaining precise control over electroporation parameters. Research from UC Berkeley demonstrated transfection efficiencies exceeding 75% for mRNA and 50% for plasmid DNA in primary human T cells, with post-electroporation viabilities above 85%[7]. The platform has been successfully used for CRISPR-Cas9 delivery, achieving targeted gene knockout rates of 60-70% in primary T cells. A key innovation is the ability to process cells in their preferred media without specialized electroporation buffers, reducing cellular stress during the procedure. The system's scalability has been demonstrated through parallelization of microfluidic channels, enabling throughput of up to 10^7 cells per minute while maintaining consistent transfection performance[8].
Strengths: Gentle cell processing with minimal activation of stress pathways; continuous flow operation enables higher throughput than static systems; precise control over electric field parameters; compatible with standard cell culture media. Weaknesses: More complex fabrication and setup compared to conventional systems; requires specialized microfluidic expertise; current implementations have lower maximum throughput than commercial flow electroporation systems; technology still transitioning from academic to commercial applications.
MaxCyte, Inc.
Technical Solution: MaxCyte has developed the ExPERT™ platform, a scalable electroporation technology specifically designed for ex vivo gene modification of primary T cells. Their Flow Electroporation® Technology enables highly efficient transfection of a wide range of molecules, including mRNA, DNA, and proteins into primary T cells while maintaining high cell viability. The system utilizes disposable processing assemblies that can handle volumes from 0.5mL to 20L, making it suitable for both research and clinical manufacturing. MaxCyte's technology employs controlled electric fields to create temporary pores in cell membranes, allowing for the delivery of genetic material with transfection efficiencies exceeding 90% for primary human T cells[1]. Their GT® and VLX® instruments are specifically designed for large-scale clinical applications, capable of processing up to 2×10^11 cells in less than 30 minutes, addressing the manufacturing challenges in cell therapy production[2].
Strengths: Industry-leading scalability from small research batches to clinical-scale manufacturing; consistent performance across different scales; closed, GMP-compliant system suitable for clinical applications; high cell viability post-electroporation (>90%). Weaknesses: Higher capital investment compared to chemical transfection methods; requires specialized equipment and consumables; optimization may be needed for specific cell types or genetic cargoes.
Critical Patents and Innovations in T Cell Electroporation
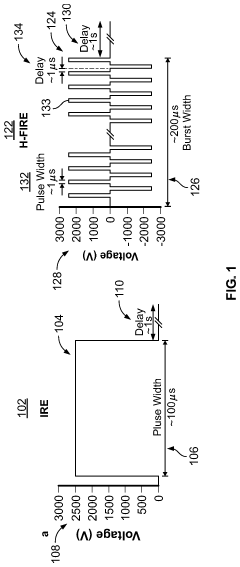
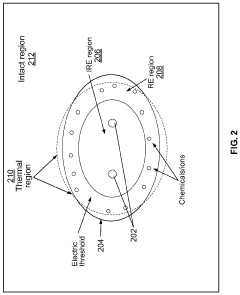
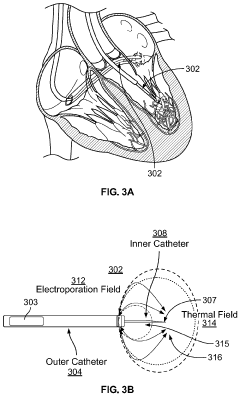
Methods and Systems for Thermal Enhancement of Electroporation
PatentPendingUS20240099769A1
Innovation
- A system and method combining thermal energy with nonthermal electroporation, using a catheter with a thermal chamber and electrodes to deliver controlled thermal energy and electrical fields, enhancing the irreversible electroporation region by converting reversible electroporation regions into irreversible ones, thereby improving ablation efficacy and safety.
System for dissociation and removal of proteinaceous tissue
PatentWO2007081474A2
Innovation
- A high-intensity short directionally changing electrical field is used to cause a transient change in tissue condition for traction-free removal of vitreous and intraocular tissues, employing a probe with multiple electrodes and fluidic techniques to create a localized ultrashort-pulsed electrical field for dissociation and aspiration.
Manufacturing and GMP Considerations for Clinical Applications
The transition from laboratory-scale T cell engineering to clinical-grade manufacturing presents significant challenges that must be addressed to ensure successful therapeutic applications. Good Manufacturing Practice (GMP) compliance is essential for clinical implementation of electroporation-based gene insertion technologies in T cells. Current manufacturing processes require specialized clean room facilities with controlled environments to prevent contamination and maintain product quality.
Closed-system electroporation devices have emerged as critical components for clinical manufacturing, reducing contamination risks while maintaining process consistency. Systems like MaxCyte's ExPERT platform and Lonza's Nucleofector technology have been adapted for GMP environments, featuring automated protocols and single-use components that minimize cross-contamination. These systems must demonstrate robust scalability while maintaining high cell viability and transfection efficiency across multiple production batches.
Quality control measures represent another crucial aspect of clinical manufacturing. Standardized testing protocols must be established to assess critical quality attributes including cell viability post-electroporation, transgene expression levels, vector copy number, and functional characteristics of the modified T cells. Release criteria must be clearly defined and validated to ensure batch-to-batch consistency and product safety.
Raw material qualification presents unique challenges for electroporation-based manufacturing. All components—including electroporation buffers, DNA/RNA constructs, and culture media—must be sourced from qualified suppliers and manufactured under GMP conditions. The transition from research-grade to GMP-grade materials often requires extensive comparability studies to ensure equivalent performance in the final product.
Process validation strategies must demonstrate that electroporation parameters can be consistently applied across multiple manufacturing runs. This includes validation of critical process parameters such as voltage settings, pulse duration, cell concentration, and DNA/RNA concentration. Statistical process control methods should be implemented to monitor these parameters and ensure they remain within established acceptance criteria.
Cost considerations significantly impact clinical implementation. Current GMP-compliant electroporation processes remain expensive, with costs driven by specialized equipment, facility requirements, and trained personnel. Economic analyses suggest that process optimization and automation could substantially reduce manufacturing costs, potentially expanding access to these therapies. Innovations in continuous processing and integration with downstream T cell expansion systems represent promising approaches to improve cost-effectiveness.
Closed-system electroporation devices have emerged as critical components for clinical manufacturing, reducing contamination risks while maintaining process consistency. Systems like MaxCyte's ExPERT platform and Lonza's Nucleofector technology have been adapted for GMP environments, featuring automated protocols and single-use components that minimize cross-contamination. These systems must demonstrate robust scalability while maintaining high cell viability and transfection efficiency across multiple production batches.
Quality control measures represent another crucial aspect of clinical manufacturing. Standardized testing protocols must be established to assess critical quality attributes including cell viability post-electroporation, transgene expression levels, vector copy number, and functional characteristics of the modified T cells. Release criteria must be clearly defined and validated to ensure batch-to-batch consistency and product safety.
Raw material qualification presents unique challenges for electroporation-based manufacturing. All components—including electroporation buffers, DNA/RNA constructs, and culture media—must be sourced from qualified suppliers and manufactured under GMP conditions. The transition from research-grade to GMP-grade materials often requires extensive comparability studies to ensure equivalent performance in the final product.
Process validation strategies must demonstrate that electroporation parameters can be consistently applied across multiple manufacturing runs. This includes validation of critical process parameters such as voltage settings, pulse duration, cell concentration, and DNA/RNA concentration. Statistical process control methods should be implemented to monitor these parameters and ensure they remain within established acceptance criteria.
Cost considerations significantly impact clinical implementation. Current GMP-compliant electroporation processes remain expensive, with costs driven by specialized equipment, facility requirements, and trained personnel. Economic analyses suggest that process optimization and automation could substantially reduce manufacturing costs, potentially expanding access to these therapies. Innovations in continuous processing and integration with downstream T cell expansion systems represent promising approaches to improve cost-effectiveness.
Regulatory Pathway for Engineered T Cell Therapies
The regulatory landscape for engineered T cell therapies represents a complex and evolving framework that developers must navigate to bring these innovative treatments to market. For ex vivo nonviral gene insertion technologies in primary T cells, regulatory considerations are particularly nuanced due to the combination of cellular therapy and genetic modification components.
The FDA classifies genetically modified cell therapies as biologics, requiring approval through the Biologics License Application (BLA) pathway. These products fall under the oversight of the Center for Biologics Evaluation and Research (CBER), specifically the Office of Tissues and Advanced Therapies (OTAT). In Europe, the European Medicines Agency (EMA) classifies these as Advanced Therapy Medicinal Products (ATMPs), specifically as gene therapy medicinal products (GTMPs) or somatic cell therapy medicinal products (sCTMPs).
Scalable electroporation methods for T cell engineering face specific regulatory scrutiny regarding manufacturing consistency, quality control, and safety profiles. Regulatory agencies require extensive characterization of the final cell product, including viability, identity, purity, potency, and genetic stability. For electroporation-based gene insertion, developers must demonstrate minimal off-target effects and consistent transgene expression levels across manufacturing batches.
Clinical development typically follows a phased approach, beginning with Phase 1 safety studies, progressing through efficacy evaluation, and culminating in pivotal trials. Accelerated approval pathways may be available for therapies addressing serious conditions with unmet medical needs, including Breakthrough Therapy Designation (FDA) or PRIME designation (EMA).
Manufacturing considerations present significant regulatory challenges, as scalable electroporation methods must demonstrate consistency in a GMP environment. Process analytical technology (PAT) implementation is increasingly expected to ensure real-time quality control. Developers must establish robust chain of custody procedures and demonstrate comparability when scaling production from clinical to commercial manufacturing.
Post-approval requirements include long-term safety monitoring, with particular attention to delayed adverse events and potential insertional mutagenesis risks. Risk Evaluation and Mitigation Strategies (REMS) may be required to manage specific safety concerns associated with engineered T cell products.
Recent regulatory trends indicate increasing flexibility toward innovative manufacturing approaches, with agencies developing framework documents specifically addressing cell and gene therapy products. The FDA's Regenerative Medicine Advanced Therapy (RMAT) designation and similar programs globally aim to expedite development of promising cellular therapies while maintaining appropriate safety standards.
The FDA classifies genetically modified cell therapies as biologics, requiring approval through the Biologics License Application (BLA) pathway. These products fall under the oversight of the Center for Biologics Evaluation and Research (CBER), specifically the Office of Tissues and Advanced Therapies (OTAT). In Europe, the European Medicines Agency (EMA) classifies these as Advanced Therapy Medicinal Products (ATMPs), specifically as gene therapy medicinal products (GTMPs) or somatic cell therapy medicinal products (sCTMPs).
Scalable electroporation methods for T cell engineering face specific regulatory scrutiny regarding manufacturing consistency, quality control, and safety profiles. Regulatory agencies require extensive characterization of the final cell product, including viability, identity, purity, potency, and genetic stability. For electroporation-based gene insertion, developers must demonstrate minimal off-target effects and consistent transgene expression levels across manufacturing batches.
Clinical development typically follows a phased approach, beginning with Phase 1 safety studies, progressing through efficacy evaluation, and culminating in pivotal trials. Accelerated approval pathways may be available for therapies addressing serious conditions with unmet medical needs, including Breakthrough Therapy Designation (FDA) or PRIME designation (EMA).
Manufacturing considerations present significant regulatory challenges, as scalable electroporation methods must demonstrate consistency in a GMP environment. Process analytical technology (PAT) implementation is increasingly expected to ensure real-time quality control. Developers must establish robust chain of custody procedures and demonstrate comparability when scaling production from clinical to commercial manufacturing.
Post-approval requirements include long-term safety monitoring, with particular attention to delayed adverse events and potential insertional mutagenesis risks. Risk Evaluation and Mitigation Strategies (REMS) may be required to manage specific safety concerns associated with engineered T cell products.
Recent regulatory trends indicate increasing flexibility toward innovative manufacturing approaches, with agencies developing framework documents specifically addressing cell and gene therapy products. The FDA's Regenerative Medicine Advanced Therapy (RMAT) designation and similar programs globally aim to expedite development of promising cellular therapies while maintaining appropriate safety standards.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!