Optimization of stirred-tank single-use bioreactors for robust T-cell expansion with consistent phenotype
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Single-Use Bioreactor Technology Evolution and Objectives
Single-use bioreactors (SUBs) have revolutionized biopharmaceutical manufacturing since their introduction in the late 1990s. Initially developed for simple cell culture applications, these systems have evolved significantly over the past two decades to address increasingly complex bioprocessing needs, including the emerging field of cell therapy manufacturing.
The evolution of stirred-tank single-use bioreactors has progressed through several distinct phases. First-generation systems focused primarily on replacing traditional stainless steel vessels with flexible polymer bags, offering basic mixing capabilities but limited process control. Second-generation systems introduced improved sensor technologies and more sophisticated agitation mechanisms, enhancing process monitoring capabilities. Current third-generation systems feature advanced control systems, improved scalability, and specialized designs tailored to specific cell culture applications.
For T-cell expansion specifically, the technological trajectory has shifted from static culture systems (such as G-Rex vessels and culture flasks) toward more scalable and controllable stirred-tank configurations. This transition addresses critical manufacturing bottlenecks in cell therapy production, particularly for autologous therapies requiring consistent product quality across multiple small-scale batches.
The primary objectives driving single-use bioreactor development for T-cell expansion include achieving robust cell proliferation while maintaining consistent phenotypic characteristics. This requires precise control of critical process parameters such as dissolved oxygen, pH, temperature, and shear stress - all of which significantly impact T-cell functionality and therapeutic efficacy. Additionally, these systems aim to maximize volumetric productivity while minimizing process variability and contamination risks.
Recent technological advancements have focused on developing specialized impeller designs that provide efficient mixing while minimizing shear stress, implementing novel sensor technologies for real-time cell monitoring, and creating automated control algorithms that maintain optimal growth conditions throughout the expansion process. These innovations address the unique challenges of T-cell culture, which differs substantially from traditional protein production in terms of cell fragility, metabolic requirements, and quality attributes.
Looking forward, the field is trending toward fully integrated, closed-system bioreactors capable of supporting the entire T-cell manufacturing workflow from isolation through expansion and harvest. The ultimate goal is to develop standardized, scalable platforms that can reliably produce therapeutic T-cell products with consistent potency, purity, and functionality across multiple manufacturing runs and sites, thereby improving patient access to these transformative therapies.
The evolution of stirred-tank single-use bioreactors has progressed through several distinct phases. First-generation systems focused primarily on replacing traditional stainless steel vessels with flexible polymer bags, offering basic mixing capabilities but limited process control. Second-generation systems introduced improved sensor technologies and more sophisticated agitation mechanisms, enhancing process monitoring capabilities. Current third-generation systems feature advanced control systems, improved scalability, and specialized designs tailored to specific cell culture applications.
For T-cell expansion specifically, the technological trajectory has shifted from static culture systems (such as G-Rex vessels and culture flasks) toward more scalable and controllable stirred-tank configurations. This transition addresses critical manufacturing bottlenecks in cell therapy production, particularly for autologous therapies requiring consistent product quality across multiple small-scale batches.
The primary objectives driving single-use bioreactor development for T-cell expansion include achieving robust cell proliferation while maintaining consistent phenotypic characteristics. This requires precise control of critical process parameters such as dissolved oxygen, pH, temperature, and shear stress - all of which significantly impact T-cell functionality and therapeutic efficacy. Additionally, these systems aim to maximize volumetric productivity while minimizing process variability and contamination risks.
Recent technological advancements have focused on developing specialized impeller designs that provide efficient mixing while minimizing shear stress, implementing novel sensor technologies for real-time cell monitoring, and creating automated control algorithms that maintain optimal growth conditions throughout the expansion process. These innovations address the unique challenges of T-cell culture, which differs substantially from traditional protein production in terms of cell fragility, metabolic requirements, and quality attributes.
Looking forward, the field is trending toward fully integrated, closed-system bioreactors capable of supporting the entire T-cell manufacturing workflow from isolation through expansion and harvest. The ultimate goal is to develop standardized, scalable platforms that can reliably produce therapeutic T-cell products with consistent potency, purity, and functionality across multiple manufacturing runs and sites, thereby improving patient access to these transformative therapies.
T-Cell Therapy Market Demand Analysis
The T-cell therapy market has experienced exponential growth over the past decade, driven primarily by breakthrough approvals of CAR-T cell therapies for various hematological malignancies. The global market value reached approximately $5.8 billion in 2022 and is projected to grow at a CAGR of 23.9% through 2030, potentially reaching $27.4 billion by the end of the forecast period.
This remarkable growth trajectory is fueled by several key factors. First, the increasing prevalence of cancer worldwide has created an urgent need for more effective treatment options. According to the World Health Organization, cancer is responsible for nearly 10 million deaths annually, with this number expected to rise significantly in the coming decades due to aging populations and lifestyle factors.
The demonstrated clinical efficacy of approved T-cell therapies has been particularly compelling. Complete response rates of 60-90% in certain relapsed/refractory blood cancers have established these therapies as potentially curative options for patients who previously had limited treatment alternatives. This clinical success has accelerated investment in the field and expanded research into solid tumor applications.
Market analysis reveals significant regional variations in demand. North America currently dominates the market with approximately 60% share, followed by Europe at 25% and Asia-Pacific at 12%. However, the Asia-Pacific region is expected to witness the fastest growth rate due to increasing healthcare expenditure, improving regulatory frameworks, and growing awareness about advanced therapies.
A critical market driver is the expanding range of indications for T-cell therapies. While initial approvals focused on specific leukemias and lymphomas, ongoing clinical trials are investigating applications in multiple myeloma, solid tumors, and even certain autoimmune disorders. This indication expansion could potentially increase the eligible patient population by 300-400% over the next decade.
Manufacturing scalability remains a significant market constraint. Current production methods are labor-intensive, time-consuming, and expensive, resulting in therapy costs exceeding $350,000 per patient. This has created strong market demand for optimized bioreactor systems that can enable more efficient, consistent, and cost-effective T-cell expansion while maintaining critical quality attributes.
Healthcare systems worldwide are increasingly implementing value-based reimbursement models for cell therapies. This trend is driving demand for production technologies that can reduce manufacturing costs while ensuring consistent product quality, as payers seek evidence of long-term efficacy to justify the high upfront costs of these treatments.
This remarkable growth trajectory is fueled by several key factors. First, the increasing prevalence of cancer worldwide has created an urgent need for more effective treatment options. According to the World Health Organization, cancer is responsible for nearly 10 million deaths annually, with this number expected to rise significantly in the coming decades due to aging populations and lifestyle factors.
The demonstrated clinical efficacy of approved T-cell therapies has been particularly compelling. Complete response rates of 60-90% in certain relapsed/refractory blood cancers have established these therapies as potentially curative options for patients who previously had limited treatment alternatives. This clinical success has accelerated investment in the field and expanded research into solid tumor applications.
Market analysis reveals significant regional variations in demand. North America currently dominates the market with approximately 60% share, followed by Europe at 25% and Asia-Pacific at 12%. However, the Asia-Pacific region is expected to witness the fastest growth rate due to increasing healthcare expenditure, improving regulatory frameworks, and growing awareness about advanced therapies.
A critical market driver is the expanding range of indications for T-cell therapies. While initial approvals focused on specific leukemias and lymphomas, ongoing clinical trials are investigating applications in multiple myeloma, solid tumors, and even certain autoimmune disorders. This indication expansion could potentially increase the eligible patient population by 300-400% over the next decade.
Manufacturing scalability remains a significant market constraint. Current production methods are labor-intensive, time-consuming, and expensive, resulting in therapy costs exceeding $350,000 per patient. This has created strong market demand for optimized bioreactor systems that can enable more efficient, consistent, and cost-effective T-cell expansion while maintaining critical quality attributes.
Healthcare systems worldwide are increasingly implementing value-based reimbursement models for cell therapies. This trend is driving demand for production technologies that can reduce manufacturing costs while ensuring consistent product quality, as payers seek evidence of long-term efficacy to justify the high upfront costs of these treatments.
Current Challenges in T-Cell Expansion Technologies
Despite significant advancements in T-cell expansion technologies, several critical challenges persist in achieving robust and consistent cell manufacturing for immunotherapies. Current static culture systems and first-generation bioreactors demonstrate limited scalability, making them inadequate for meeting the increasing clinical demand for cellular therapies. These systems often suffer from heterogeneous microenvironments that create inconsistent cell expansion conditions, resulting in batch-to-batch variability in both cell yield and phenotypic characteristics.
The maintenance of consistent T-cell phenotype during expansion represents a particularly formidable challenge. Conventional stirred-tank bioreactors frequently induce undesirable phenotypic drift, with expanded T-cells showing altered cytokine production profiles, exhaustion markers, and diminished therapeutic efficacy compared to their starting populations. This phenotypic inconsistency directly impacts clinical outcomes and complicates regulatory approval processes.
Hydrodynamic stress in stirred-tank bioreactors presents another significant obstacle. T-cells are notably sensitive to shear forces generated by impeller agitation, which can trigger apoptosis, reduce viability, and alter cellular function. Current bioreactor designs struggle to balance the need for efficient mixing and mass transfer with the requirement to minimize detrimental mechanical forces on these delicate cells.
Nutrient and metabolite gradients within larger-scale bioreactors further complicate T-cell expansion. As culture volumes increase, maintaining homogeneous distribution of oxygen, nutrients, and growth factors becomes increasingly difficult. These gradients create microenvironments where cells experience varying conditions, contributing to inconsistent expansion rates and phenotypic heterogeneity across the culture.
Process monitoring and control systems for T-cell expansion remain inadequate. Unlike traditional biopharmaceutical processes, real-time monitoring of critical quality attributes for T-cells is challenging. Current technologies lack robust, non-invasive methods to continuously assess cell density, viability, phenotype, and functional characteristics during the expansion process, limiting the ability to implement feedback control strategies.
The high cost and complexity of current expansion technologies also present significant barriers to widespread adoption. Single-use bioreactor systems specifically designed for T-cell culture often require substantial capital investment and specialized expertise to operate effectively. This economic burden restricts access to advanced manufacturing capabilities, particularly for smaller clinical centers and academic institutions developing novel cellular therapies.
The maintenance of consistent T-cell phenotype during expansion represents a particularly formidable challenge. Conventional stirred-tank bioreactors frequently induce undesirable phenotypic drift, with expanded T-cells showing altered cytokine production profiles, exhaustion markers, and diminished therapeutic efficacy compared to their starting populations. This phenotypic inconsistency directly impacts clinical outcomes and complicates regulatory approval processes.
Hydrodynamic stress in stirred-tank bioreactors presents another significant obstacle. T-cells are notably sensitive to shear forces generated by impeller agitation, which can trigger apoptosis, reduce viability, and alter cellular function. Current bioreactor designs struggle to balance the need for efficient mixing and mass transfer with the requirement to minimize detrimental mechanical forces on these delicate cells.
Nutrient and metabolite gradients within larger-scale bioreactors further complicate T-cell expansion. As culture volumes increase, maintaining homogeneous distribution of oxygen, nutrients, and growth factors becomes increasingly difficult. These gradients create microenvironments where cells experience varying conditions, contributing to inconsistent expansion rates and phenotypic heterogeneity across the culture.
Process monitoring and control systems for T-cell expansion remain inadequate. Unlike traditional biopharmaceutical processes, real-time monitoring of critical quality attributes for T-cells is challenging. Current technologies lack robust, non-invasive methods to continuously assess cell density, viability, phenotype, and functional characteristics during the expansion process, limiting the ability to implement feedback control strategies.
The high cost and complexity of current expansion technologies also present significant barriers to widespread adoption. Single-use bioreactor systems specifically designed for T-cell culture often require substantial capital investment and specialized expertise to operate effectively. This economic burden restricts access to advanced manufacturing capabilities, particularly for smaller clinical centers and academic institutions developing novel cellular therapies.
Current Stirred-Tank Bioreactor Design Solutions
01 Single-use bioreactor design for T-cell expansion
Specialized single-use bioreactor systems designed specifically for T-cell cultivation provide advantages in terms of contamination control and process flexibility. These systems incorporate features such as optimized impeller designs, controlled agitation rates, and customized vessel geometries that promote gentle mixing while maintaining cell viability. The single-use nature eliminates cross-contamination risks and reduces cleaning validation requirements, making them ideal for clinical and commercial T-cell manufacturing.- Single-use bioreactor design for T-cell expansion: Specialized single-use bioreactor systems designed specifically for T-cell cultivation provide advantages in terms of contamination control and process flexibility. These systems incorporate features such as optimized impeller designs, controlled agitation rates, and customized gas exchange mechanisms that maintain ideal conditions for T-cell growth while minimizing shear stress. The bioreactor configuration ensures homogeneous distribution of nutrients and gases while preserving cell viability and functionality.
- Media formulation and supplementation strategies: Optimized media formulations containing specific growth factors, cytokines, and nutrients support robust T-cell expansion while maintaining consistent phenotypic characteristics. These formulations typically include components like IL-2, IL-7, IL-15, human serum or serum alternatives, and specialized supplements that promote T-cell activation and proliferation. The precise balance of these components is critical for achieving high cell densities while preserving the desired T-cell phenotype throughout the expansion process.
- Process parameters monitoring and control: Advanced monitoring and control systems for critical process parameters such as temperature, pH, dissolved oxygen, glucose concentration, and metabolite levels ensure optimal T-cell growth conditions. These systems employ sensors and automated feedback mechanisms to maintain parameters within tight ranges, allowing for consistent cell expansion outcomes. Real-time monitoring capabilities enable timely interventions to prevent conditions that might alter T-cell phenotype or reduce expansion efficiency.
- T-cell activation and expansion protocols: Standardized protocols for T-cell activation, using methods such as anti-CD3/CD28 antibodies, magnetic beads, or artificial antigen-presenting cells, followed by controlled expansion phases in stirred-tank bioreactors. These protocols define specific cell seeding densities, activation duration, expansion timelines, and harvesting procedures that collectively ensure reproducible outcomes. The carefully timed introduction of stimulatory signals and growth factors maintains the balance between proliferation and preservation of functional characteristics.
- Phenotype preservation and quality control: Methods for maintaining consistent T-cell phenotype throughout the expansion process, including specialized culture conditions that preserve key surface markers and functional properties. These approaches incorporate regular phenotypic analysis using flow cytometry, functional assays, and genomic profiling to ensure quality control. Specific strategies may include controlled cytokine exposure, optimized cell density maintenance, and carefully timed medium exchanges that collectively preserve the desired immunotherapeutic properties of the expanded T-cells.
02 Optimized culture parameters for consistent T-cell phenotype
Maintaining consistent T-cell phenotype during expansion requires precise control of culture parameters. This includes optimization of dissolved oxygen levels, pH control, temperature regulation, and nutrient feeding strategies. Specific parameter ranges have been identified that preserve key T-cell functional characteristics while allowing for robust expansion. These parameters can be programmatically controlled in stirred-tank bioreactors to ensure batch-to-batch consistency and preservation of therapeutic potential.Expand Specific Solutions03 Media formulation and supplementation strategies
Specialized media formulations support T-cell expansion while maintaining desired phenotypic characteristics. These formulations typically include base media supplemented with specific growth factors, cytokines (particularly IL-2), and other bioactive compounds that promote proliferation while preserving functionality. Controlled release or timed addition of these supplements in stirred-tank bioreactors helps maintain optimal growth conditions throughout the expansion process, resulting in higher cell yields with consistent phenotypic profiles.Expand Specific Solutions04 Monitoring and control systems for process consistency
Advanced monitoring and control systems are essential for maintaining consistent T-cell expansion conditions. These systems incorporate real-time sensors for critical parameters, automated feedback control loops, and process analytical technology (PAT) tools that enable continuous assessment of culture conditions. Some systems also include predictive modeling capabilities that can anticipate parameter shifts and make proactive adjustments, ensuring that cells maintain their desired phenotypic characteristics throughout the expansion process.Expand Specific Solutions05 Scale-up strategies for clinical and commercial manufacturing
Effective scale-up strategies are crucial for translating laboratory-scale T-cell expansion processes to clinical and commercial manufacturing. These strategies focus on maintaining consistent hydrodynamic conditions, oxygen transfer rates, and nutrient availability across different scales. Computational fluid dynamics modeling helps predict how changes in bioreactor geometry and operating parameters will affect cell growth and phenotype. Staged scale-up approaches with intermediate validation steps ensure that T-cell products maintain consistent quality attributes regardless of production scale.Expand Specific Solutions
Key Industry Players in Cell Therapy Manufacturing
The T-cell expansion bioreactor market is in a growth phase, with increasing demand driven by cell therapy advancements, particularly in immunotherapy applications. The global market is expanding rapidly, estimated to reach several billion dollars by 2025 as cell therapies gain regulatory approvals. Technologically, single-use bioreactors for T-cell expansion are advancing toward maturity, with key players like Cytiva, Lonza, and Life Technologies leading innovation. These companies have developed sophisticated systems addressing critical challenges in maintaining cell phenotype consistency during scale-up. Other significant contributors include Baxter International, Amgen, and Takeda Pharmaceutical, who are advancing proprietary technologies for optimized cell expansion while newer entrants like ProBioGen and Gamida Cell are introducing specialized solutions for specific therapeutic applications.
Global Life Sciences Solutions USA LLC
Technical Solution: Global Life Sciences Solutions (formerly part of GE Healthcare Life Sciences and now operating under Cytiva) has developed the Wave Bioreactor™ system, which offers an alternative approach to traditional stirred-tank bioreactors for T-cell expansion. Their technology utilizes a rocking motion platform that creates gentle waves within a single-use cultivation bag, providing efficient mixing and gas transfer while minimizing shear stress on sensitive T-cells. The system incorporates integrated optical sensors for non-invasive monitoring of key parameters including pH, dissolved oxygen, and temperature. Their approach includes specialized cell retention devices that enable perfusion culture modes, allowing for continuous media exchange while maintaining high cell densities[5]. The platform features automated control systems that maintain precise culture conditions through feedback mechanisms, adjusting rocking speed, angle, and gas flow rates in response to real-time measurements. Global Life Sciences Solutions has developed companion media formulations specifically optimized for wave-type bioreactors to enhance T-cell expansion while preserving critical phenotypic characteristics. The technology includes specialized harvest protocols that efficiently recover expanded T-cells while maintaining viability and functionality.
Strengths: Wave-induced mixing provides excellent gas transfer with minimal shear stress; single-use technology eliminates cleaning validation requirements; simple operation with fewer moving parts compared to traditional stirred-tank systems. Weaknesses: Potential limitations in scalability for very large production volumes; less precise control over mixing patterns compared to impeller-based systems; challenges with implementing certain process analytical technologies due to the rocking motion.
Amgen, Inc.
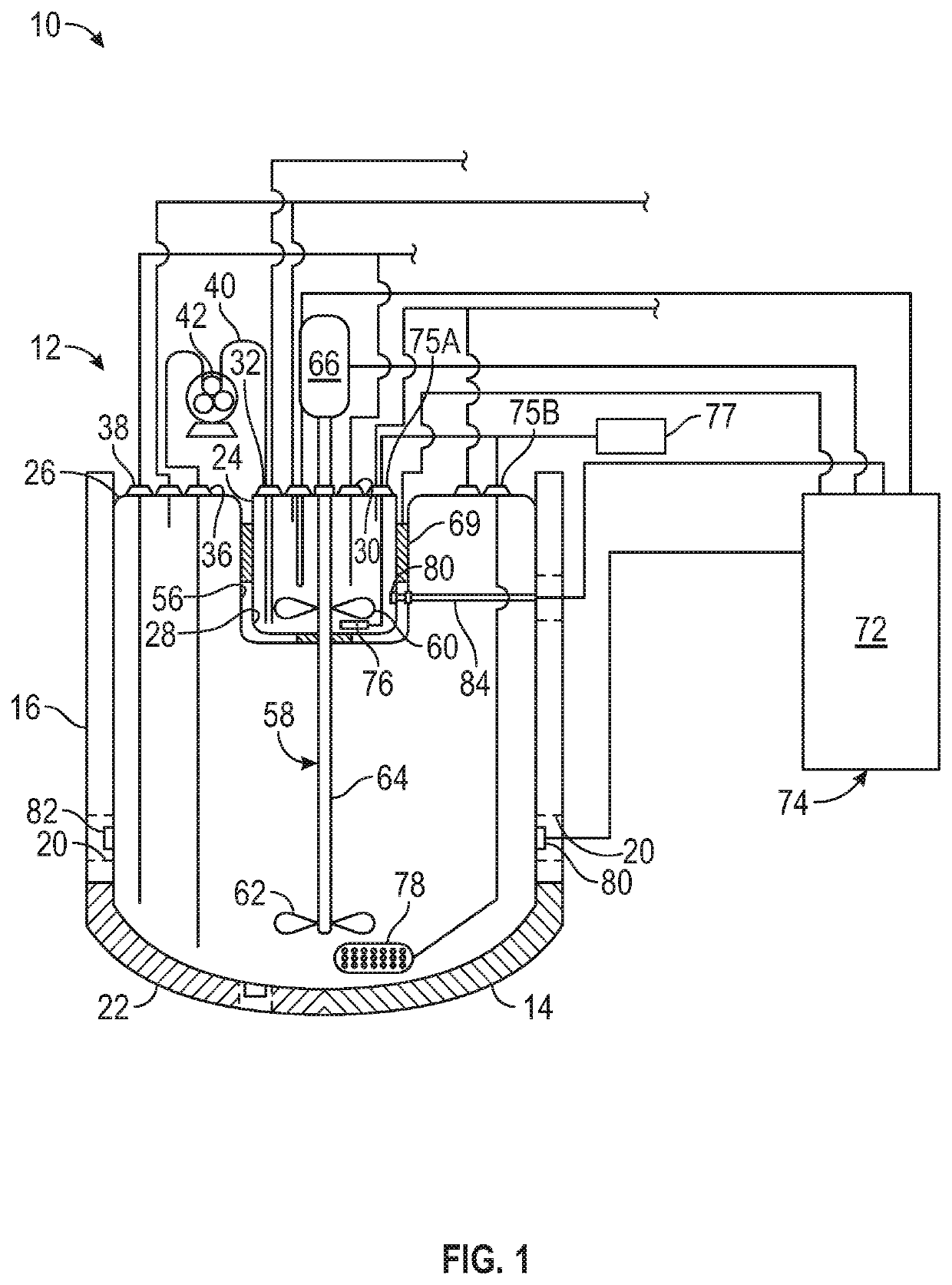
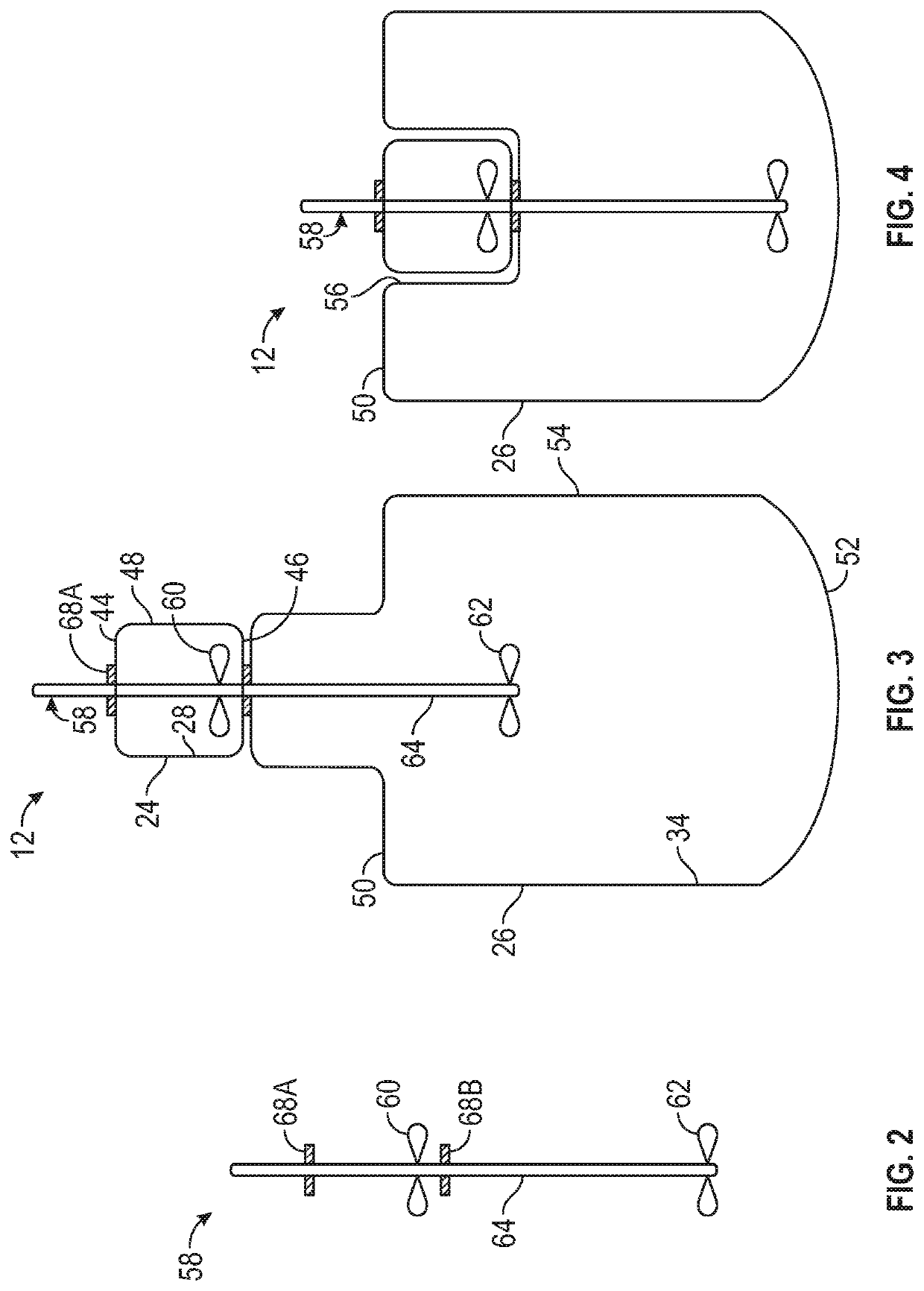
Technical Solution: Amgen has developed an advanced stirred-tank single-use bioreactor system specifically engineered for T-cell therapy manufacturing. Their technology incorporates a proprietary impeller design that creates optimal fluid dynamics for T-cell suspension culture while minimizing damaging shear forces. The system features precision control of critical process parameters through advanced sensor technology and automated feedback mechanisms that maintain tight control over temperature, pH, dissolved oxygen, and agitation speed. Amgen's approach includes specialized microcarrier technology with optimized surface chemistry that supports consistent T-cell attachment and growth while facilitating efficient harvest without compromising cell functionality[4]. Their platform incorporates automated sampling capabilities that enable real-time monitoring of key metabolites and growth parameters without compromising the closed system integrity. Amgen has developed proprietary media formulations specifically designed to support high-density T-cell cultures while preserving key phenotypic markers and functional characteristics. The system employs sophisticated process analytical technology (PAT) tools that provide continuous assessment of culture conditions and cell quality attributes throughout the manufacturing process, enabling early detection of deviations and implementation of corrective actions.
Strengths: Highly automated system with sophisticated process controls; integrated PAT tools for real-time quality monitoring; scalable platform supporting seamless technology transfer between clinical and commercial manufacturing. Weaknesses: Complex system requiring specialized expertise for operation; higher capital investment compared to conventional technologies; potential challenges with oxygen transfer limitations at extremely high cell densities.
Critical Parameters for Consistent T-Cell Phenotype Maintenance
Expansion and passaging of pluripotent stem cells using stirred-tank bioreactors
PatentActiveJP2019509047A
Innovation
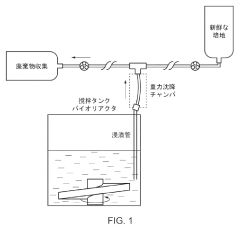
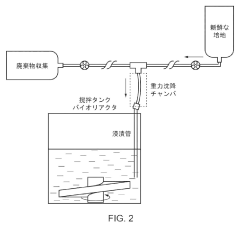
- A method utilizing gravitational sedimentation combined with automated perfusion in a closed system, employing a slicer for enzyme-free separation of cell aggregates, and avoiding the use of ROCK inhibitors to maintain cell viability and sterility.
Multi-chamber bioreactor apparatus
PatentActiveUS20200032185A1
Innovation
- A multi-chamber single-use bioreactor apparatus with interconnected bags of varying volumes, supported by a single rigid structure and controlled by a single unit, allowing for fluidic transfer between chambers without opening the system, reducing the need for multiple bioreactors and minimizing contamination risks.
Scale-Up Considerations for Clinical to Commercial Manufacturing
The transition from clinical-scale to commercial manufacturing of T-cell therapies presents significant challenges that must be addressed systematically. When scaling up stirred-tank single-use bioreactors for T-cell expansion, maintaining process consistency becomes increasingly complex. The critical parameters established during clinical trials must be preserved while adapting to larger volumes and different equipment configurations.
Process transfer requires careful evaluation of key performance indicators such as oxygen transfer rates, mixing efficiency, and shear stress profiles. These parameters directly impact cell growth kinetics, metabolic activity, and ultimately phenotypic consistency. Commercial manufacturing demands more robust control systems capable of maintaining these parameters within narrower tolerances across larger volumes.
Computational fluid dynamics modeling becomes essential during scale-up to predict flow patterns and identify potential dead zones or high-shear regions that could compromise cell quality. These models must be validated through experimental approaches to ensure accurate representation of the actual bioreactor environment at different scales.
Equipment selection presents another critical consideration, as commercial-scale bioreactors may utilize different impeller designs, sparging systems, or sensor technologies compared to their clinical counterparts. Each component must be evaluated for its impact on the cellular microenvironment and subsequent T-cell functionality.
Automation requirements increase substantially at commercial scale, necessitating more sophisticated control algorithms and feedback systems. Process analytical technology (PAT) implementation becomes crucial for real-time monitoring of critical quality attributes, enabling adaptive control strategies that maintain consistent culture conditions despite increased volume.
Supply chain considerations also gain prominence during scale-up, as larger batch sizes require greater quantities of raw materials with consistent quality. Supplier qualification and material testing protocols must be established to ensure batch-to-batch consistency in critical components such as media, cytokines, and activation reagents.
Regulatory expectations evolve with scale, requiring more comprehensive validation studies and process characterization. Establishing acceptable ranges for process parameters becomes more challenging as the impact of minor variations may be amplified in larger systems. Design space approaches can help define the operational boundaries within which consistent T-cell phenotype can be maintained.
Risk assessment methodologies must be adapted to address the unique challenges of commercial-scale operations, including contingency planning for equipment failures, contamination events, or unexpected process deviations that could impact larger, more valuable batches of therapeutic cells.
Process transfer requires careful evaluation of key performance indicators such as oxygen transfer rates, mixing efficiency, and shear stress profiles. These parameters directly impact cell growth kinetics, metabolic activity, and ultimately phenotypic consistency. Commercial manufacturing demands more robust control systems capable of maintaining these parameters within narrower tolerances across larger volumes.
Computational fluid dynamics modeling becomes essential during scale-up to predict flow patterns and identify potential dead zones or high-shear regions that could compromise cell quality. These models must be validated through experimental approaches to ensure accurate representation of the actual bioreactor environment at different scales.
Equipment selection presents another critical consideration, as commercial-scale bioreactors may utilize different impeller designs, sparging systems, or sensor technologies compared to their clinical counterparts. Each component must be evaluated for its impact on the cellular microenvironment and subsequent T-cell functionality.
Automation requirements increase substantially at commercial scale, necessitating more sophisticated control algorithms and feedback systems. Process analytical technology (PAT) implementation becomes crucial for real-time monitoring of critical quality attributes, enabling adaptive control strategies that maintain consistent culture conditions despite increased volume.
Supply chain considerations also gain prominence during scale-up, as larger batch sizes require greater quantities of raw materials with consistent quality. Supplier qualification and material testing protocols must be established to ensure batch-to-batch consistency in critical components such as media, cytokines, and activation reagents.
Regulatory expectations evolve with scale, requiring more comprehensive validation studies and process characterization. Establishing acceptable ranges for process parameters becomes more challenging as the impact of minor variations may be amplified in larger systems. Design space approaches can help define the operational boundaries within which consistent T-cell phenotype can be maintained.
Risk assessment methodologies must be adapted to address the unique challenges of commercial-scale operations, including contingency planning for equipment failures, contamination events, or unexpected process deviations that could impact larger, more valuable batches of therapeutic cells.
Quality Control and Regulatory Compliance Frameworks
Quality control and regulatory compliance are critical components in the optimization of stirred-tank single-use bioreactors for T-cell expansion. The manufacturing of cell therapies must adhere to stringent regulatory frameworks established by agencies such as the FDA, EMA, and PMDA, which govern the production of biological products intended for human use.
Current regulatory guidelines for cell therapy manufacturing emphasize the implementation of robust quality management systems (QMS) that ensure consistent product quality throughout the production process. For T-cell expansion in single-use bioreactors, this translates to comprehensive documentation of critical process parameters (CPPs) and critical quality attributes (CQAs) that influence cell phenotype consistency and expansion efficiency.
The FDA's guidance on Chemistry, Manufacturing, and Controls (CMC) for cell therapy products specifically addresses the need for validated analytical methods to monitor cell quality attributes during expansion. These methods must demonstrate sufficient sensitivity, specificity, and reproducibility to detect variations in T-cell phenotype, viability, and functional characteristics that may impact clinical efficacy.
Process validation represents another crucial aspect of regulatory compliance for single-use bioreactor systems. Manufacturers must establish acceptance criteria for key process parameters such as dissolved oxygen levels, pH, temperature, and agitation rates, all of which can significantly affect T-cell expansion kinetics and phenotypic stability.
Risk management frameworks, including Failure Mode and Effects Analysis (FMEA), are increasingly being applied to identify potential failure points in the bioreactor operation that could compromise product quality. This systematic approach helps manufacturers develop appropriate control strategies and establish meaningful in-process testing protocols.
Regulatory agencies are also focusing on supply chain integrity for single-use components, requiring manufacturers to implement supplier qualification programs and establish change control procedures for any modifications to bioreactor components or raw materials. This ensures that changes in supplier manufacturing processes do not adversely affect T-cell expansion outcomes.
The International Council for Harmonisation (ICH) guidelines, particularly ICH Q8-Q11, provide a framework for implementing Quality by Design (QbD) principles in biopharmaceutical manufacturing. Applied to T-cell expansion, these principles encourage the development of a design space that defines the acceptable ranges for process parameters that consistently yield cells with the desired phenotypic characteristics.
Emerging trends in regulatory compliance include the development of real-time release testing (RTRT) strategies, which leverage in-line or at-line monitoring technologies to assess product quality attributes during the manufacturing process rather than relying solely on end-product testing.
Current regulatory guidelines for cell therapy manufacturing emphasize the implementation of robust quality management systems (QMS) that ensure consistent product quality throughout the production process. For T-cell expansion in single-use bioreactors, this translates to comprehensive documentation of critical process parameters (CPPs) and critical quality attributes (CQAs) that influence cell phenotype consistency and expansion efficiency.
The FDA's guidance on Chemistry, Manufacturing, and Controls (CMC) for cell therapy products specifically addresses the need for validated analytical methods to monitor cell quality attributes during expansion. These methods must demonstrate sufficient sensitivity, specificity, and reproducibility to detect variations in T-cell phenotype, viability, and functional characteristics that may impact clinical efficacy.
Process validation represents another crucial aspect of regulatory compliance for single-use bioreactor systems. Manufacturers must establish acceptance criteria for key process parameters such as dissolved oxygen levels, pH, temperature, and agitation rates, all of which can significantly affect T-cell expansion kinetics and phenotypic stability.
Risk management frameworks, including Failure Mode and Effects Analysis (FMEA), are increasingly being applied to identify potential failure points in the bioreactor operation that could compromise product quality. This systematic approach helps manufacturers develop appropriate control strategies and establish meaningful in-process testing protocols.
Regulatory agencies are also focusing on supply chain integrity for single-use components, requiring manufacturers to implement supplier qualification programs and establish change control procedures for any modifications to bioreactor components or raw materials. This ensures that changes in supplier manufacturing processes do not adversely affect T-cell expansion outcomes.
The International Council for Harmonisation (ICH) guidelines, particularly ICH Q8-Q11, provide a framework for implementing Quality by Design (QbD) principles in biopharmaceutical manufacturing. Applied to T-cell expansion, these principles encourage the development of a design space that defines the acceptable ranges for process parameters that consistently yield cells with the desired phenotypic characteristics.
Emerging trends in regulatory compliance include the development of real-time release testing (RTRT) strategies, which leverage in-line or at-line monitoring technologies to assess product quality attributes during the manufacturing process rather than relying solely on end-product testing.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!