Scalable microfluidic mixing systems for LNP-mRNA manufacturing: residence time, encapsulation and size control
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
LNP-mRNA Manufacturing Technology Evolution and Objectives
The evolution of Lipid Nanoparticle (LNP) delivery systems for mRNA therapeutics represents one of the most significant advancements in modern pharmaceutical technology. Initially developed in the early 2000s, LNP technology has undergone remarkable transformation from basic lipid formulations to sophisticated delivery vehicles capable of protecting and efficiently delivering mRNA to target cells.
The first generation of LNP systems faced significant challenges including poor encapsulation efficiency, inconsistent particle size distribution, and limited scalability. These early systems typically relied on batch processing methods that produced small quantities with considerable batch-to-batch variability, making them unsuitable for commercial-scale manufacturing.
A pivotal advancement occurred around 2010-2015 with the introduction of microfluidic mixing technologies, which dramatically improved control over critical parameters such as lipid-to-mRNA ratios, mixing conditions, and residence time. This technological leap enabled more consistent particle formation and significantly enhanced encapsulation efficiency, typically achieving over 90% compared to previous methods that struggled to exceed 60-70%.
The COVID-19 pandemic catalyzed unprecedented acceleration in LNP-mRNA manufacturing technology development. The urgent need for billions of vaccine doses pushed researchers and manufacturers to rapidly scale production while maintaining product quality. This pressure drove innovation in continuous flow microfluidic systems capable of producing LNPs at rates orders of magnitude higher than previous technologies.
Current technological objectives focus on developing truly scalable microfluidic mixing platforms that can maintain precise control over critical quality attributes while increasing throughput. Key parameters requiring optimization include residence time control (affecting lipid-mRNA interaction kinetics), encapsulation efficiency (impacting production costs and dosing requirements), and particle size distribution (influencing biodistribution and cellular uptake).
The industry aims to establish manufacturing platforms capable of consistent production from preclinical to commercial scale with minimal process modifications. This "scale-out" rather than "scale-up" approach seeks to maintain identical process parameters across production volumes, thereby reducing regulatory hurdles and accelerating time-to-market.
Looking forward, the field is moving toward fully automated, closed manufacturing systems with integrated in-line analytics for real-time process monitoring and control. These advanced platforms will incorporate artificial intelligence and machine learning algorithms to optimize process parameters dynamically, further enhancing consistency and efficiency in LNP-mRNA production while reducing manufacturing costs—a critical factor for expanding access to these revolutionary therapeutics beyond wealthy nations.
The first generation of LNP systems faced significant challenges including poor encapsulation efficiency, inconsistent particle size distribution, and limited scalability. These early systems typically relied on batch processing methods that produced small quantities with considerable batch-to-batch variability, making them unsuitable for commercial-scale manufacturing.
A pivotal advancement occurred around 2010-2015 with the introduction of microfluidic mixing technologies, which dramatically improved control over critical parameters such as lipid-to-mRNA ratios, mixing conditions, and residence time. This technological leap enabled more consistent particle formation and significantly enhanced encapsulation efficiency, typically achieving over 90% compared to previous methods that struggled to exceed 60-70%.
The COVID-19 pandemic catalyzed unprecedented acceleration in LNP-mRNA manufacturing technology development. The urgent need for billions of vaccine doses pushed researchers and manufacturers to rapidly scale production while maintaining product quality. This pressure drove innovation in continuous flow microfluidic systems capable of producing LNPs at rates orders of magnitude higher than previous technologies.
Current technological objectives focus on developing truly scalable microfluidic mixing platforms that can maintain precise control over critical quality attributes while increasing throughput. Key parameters requiring optimization include residence time control (affecting lipid-mRNA interaction kinetics), encapsulation efficiency (impacting production costs and dosing requirements), and particle size distribution (influencing biodistribution and cellular uptake).
The industry aims to establish manufacturing platforms capable of consistent production from preclinical to commercial scale with minimal process modifications. This "scale-out" rather than "scale-up" approach seeks to maintain identical process parameters across production volumes, thereby reducing regulatory hurdles and accelerating time-to-market.
Looking forward, the field is moving toward fully automated, closed manufacturing systems with integrated in-line analytics for real-time process monitoring and control. These advanced platforms will incorporate artificial intelligence and machine learning algorithms to optimize process parameters dynamically, further enhancing consistency and efficiency in LNP-mRNA production while reducing manufacturing costs—a critical factor for expanding access to these revolutionary therapeutics beyond wealthy nations.
Market Analysis for Scalable LNP-mRNA Production Systems
The global market for LNP-mRNA manufacturing systems is experiencing unprecedented growth, primarily driven by the success of mRNA vaccines during the COVID-19 pandemic. This market segment is projected to reach $15.49 billion by 2026, growing at a CAGR of 13.8% from 2021. The demand for scalable microfluidic mixing systems specifically has seen a sharp increase as pharmaceutical companies and CDMOs (Contract Development and Manufacturing Organizations) seek to optimize their production capabilities.
North America currently dominates the market with approximately 45% share, followed by Europe at 30% and Asia-Pacific at 20%. This regional distribution reflects the concentration of biopharmaceutical innovation and manufacturing infrastructure. Countries like the United States, Germany, Switzerland, and increasingly China and South Korea are making significant investments in mRNA technology platforms.
The market demand is segmented across three primary applications: vaccines (68%), therapeutics (22%), and research applications (10%). While COVID-19 vaccines catalyzed initial growth, the industry is rapidly diversifying toward other infectious diseases, cancer immunotherapies, and rare genetic disorders, creating sustained demand for advanced manufacturing solutions.
Key market drivers include the expanding pipeline of mRNA-based therapeutics, with over 180 candidates currently in clinical trials globally. Regulatory agencies have also established accelerated pathways for mRNA products, further stimulating market growth. Additionally, significant public and private funding—exceeding $5.7 billion in 2022 alone—continues to flow into this sector.
Customer requirements are evolving toward systems that offer greater scalability, consistency in nanoparticle size distribution, higher encapsulation efficiency, and reduced batch-to-batch variability. End-users increasingly demand manufacturing platforms that can seamlessly transition from clinical-scale to commercial-scale production without compromising product quality attributes.
Market challenges include high capital equipment costs, with advanced microfluidic systems ranging from $500,000 to several million dollars depending on throughput capacity. Technical barriers related to process standardization and the specialized expertise required for operation also limit market penetration in emerging economies.
The competitive landscape features established pharmaceutical equipment manufacturers expanding into this space, specialized microfluidics companies, and innovative startups developing disruptive technologies. Recent market consolidation through strategic acquisitions indicates the sector's maturity and recognition of its long-term value proposition in next-generation biopharmaceutical manufacturing.
North America currently dominates the market with approximately 45% share, followed by Europe at 30% and Asia-Pacific at 20%. This regional distribution reflects the concentration of biopharmaceutical innovation and manufacturing infrastructure. Countries like the United States, Germany, Switzerland, and increasingly China and South Korea are making significant investments in mRNA technology platforms.
The market demand is segmented across three primary applications: vaccines (68%), therapeutics (22%), and research applications (10%). While COVID-19 vaccines catalyzed initial growth, the industry is rapidly diversifying toward other infectious diseases, cancer immunotherapies, and rare genetic disorders, creating sustained demand for advanced manufacturing solutions.
Key market drivers include the expanding pipeline of mRNA-based therapeutics, with over 180 candidates currently in clinical trials globally. Regulatory agencies have also established accelerated pathways for mRNA products, further stimulating market growth. Additionally, significant public and private funding—exceeding $5.7 billion in 2022 alone—continues to flow into this sector.
Customer requirements are evolving toward systems that offer greater scalability, consistency in nanoparticle size distribution, higher encapsulation efficiency, and reduced batch-to-batch variability. End-users increasingly demand manufacturing platforms that can seamlessly transition from clinical-scale to commercial-scale production without compromising product quality attributes.
Market challenges include high capital equipment costs, with advanced microfluidic systems ranging from $500,000 to several million dollars depending on throughput capacity. Technical barriers related to process standardization and the specialized expertise required for operation also limit market penetration in emerging economies.
The competitive landscape features established pharmaceutical equipment manufacturers expanding into this space, specialized microfluidics companies, and innovative startups developing disruptive technologies. Recent market consolidation through strategic acquisitions indicates the sector's maturity and recognition of its long-term value proposition in next-generation biopharmaceutical manufacturing.
Microfluidic Mixing Challenges in LNP-mRNA Manufacturing
The current landscape of LNP-mRNA manufacturing faces significant challenges in microfluidic mixing systems that directly impact product quality, scalability, and manufacturing efficiency. One primary challenge is achieving consistent residence time during the mixing process. Residence time variations lead to heterogeneous lipid-mRNA interactions, resulting in batch-to-batch variability and compromised product quality. Even minor fluctuations in residence time can significantly alter LNP characteristics, making precise control essential for pharmaceutical-grade production.
Encapsulation efficiency represents another critical challenge, as incomplete mRNA encapsulation reduces therapeutic efficacy while wasting valuable genetic material. Current microfluidic systems struggle to maintain optimal encapsulation rates above 80-90% when scaled beyond laboratory settings. The delicate balance of lipid-to-mRNA ratios must be precisely maintained throughout the mixing process, requiring sophisticated flow control mechanisms that are difficult to implement at production scale.
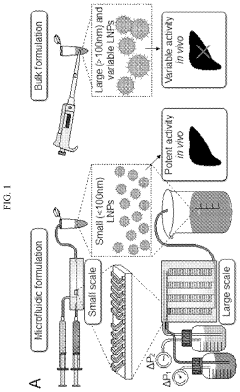
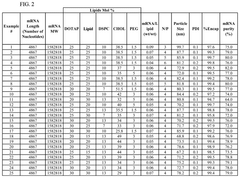
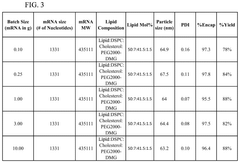
Size control and distribution uniformity present persistent technical hurdles. Therapeutic applications demand narrow particle size distributions (typically 80-100nm with PDI<0.2) for optimal cellular uptake and biodistribution. However, as production volumes increase, maintaining this tight size control becomes increasingly challenging due to flow instabilities, channel fouling, and mixing inconsistencies across parallel microfluidic units.
Manufacturing scalability itself introduces complex engineering challenges. Traditional approaches using single microfluidic chips cannot meet commercial production demands, necessitating parallelization strategies. However, parallelization introduces new complications including equal flow distribution, pressure balancing across multiple mixing units, and ensuring identical mixing conditions throughout the system. These challenges are compounded by the need to maintain sterile conditions and prevent cross-contamination.
Material compatibility issues further complicate microfluidic mixing system design. Many conventional microfluidic materials interact unfavorably with lipid components or solvents used in LNP formulation. Channel surface properties can induce lipid adsorption, leading to fouling, flow disruptions, and product contamination. Additionally, materials must withstand cleaning and sterilization procedures without degradation or leaching.
Process monitoring and quality control represent significant operational challenges. Real-time monitoring of critical parameters such as mixing efficiency, particle formation, and encapsulation rates remains limited with current technologies. The opacity of microfluidic channels and the nanoscale nature of LNP formation processes make direct observation difficult, complicating process validation and quality assurance efforts.
Encapsulation efficiency represents another critical challenge, as incomplete mRNA encapsulation reduces therapeutic efficacy while wasting valuable genetic material. Current microfluidic systems struggle to maintain optimal encapsulation rates above 80-90% when scaled beyond laboratory settings. The delicate balance of lipid-to-mRNA ratios must be precisely maintained throughout the mixing process, requiring sophisticated flow control mechanisms that are difficult to implement at production scale.
Size control and distribution uniformity present persistent technical hurdles. Therapeutic applications demand narrow particle size distributions (typically 80-100nm with PDI<0.2) for optimal cellular uptake and biodistribution. However, as production volumes increase, maintaining this tight size control becomes increasingly challenging due to flow instabilities, channel fouling, and mixing inconsistencies across parallel microfluidic units.
Manufacturing scalability itself introduces complex engineering challenges. Traditional approaches using single microfluidic chips cannot meet commercial production demands, necessitating parallelization strategies. However, parallelization introduces new complications including equal flow distribution, pressure balancing across multiple mixing units, and ensuring identical mixing conditions throughout the system. These challenges are compounded by the need to maintain sterile conditions and prevent cross-contamination.
Material compatibility issues further complicate microfluidic mixing system design. Many conventional microfluidic materials interact unfavorably with lipid components or solvents used in LNP formulation. Channel surface properties can induce lipid adsorption, leading to fouling, flow disruptions, and product contamination. Additionally, materials must withstand cleaning and sterilization procedures without degradation or leaching.
Process monitoring and quality control represent significant operational challenges. Real-time monitoring of critical parameters such as mixing efficiency, particle formation, and encapsulation rates remains limited with current technologies. The opacity of microfluidic channels and the nanoscale nature of LNP formation processes make direct observation difficult, complicating process validation and quality assurance efforts.
Current Microfluidic Approaches for LNP-mRNA Encapsulation
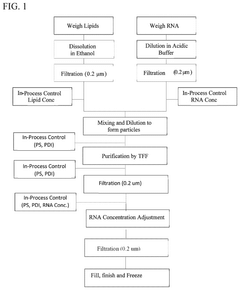
01 Microfluidic mixing device designs for LNP-mRNA formulation
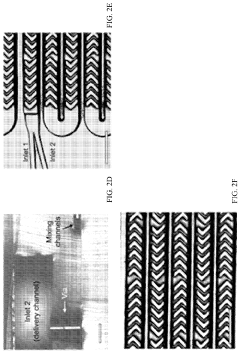
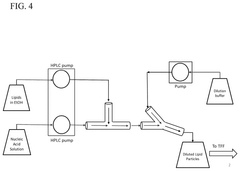
Various microfluidic mixing device designs can be employed for LNP-mRNA formulation to achieve optimal mixing conditions. These designs include T-junction mixers, herringbone mixers, and vortex mixers that create controlled turbulence for efficient lipid-mRNA interaction. The geometry of mixing channels and chambers significantly impacts the formation of uniform LNPs with consistent size distribution. Advanced designs incorporate multiple mixing stages or specialized flow patterns to ensure homogeneous mixing while maintaining gentle conditions for the sensitive mRNA cargo.- Microfluidic mixing device designs for LNP-mRNA formulation: Various microfluidic mixing device designs can be employed for LNP-mRNA formulation, including T-junction mixers, herringbone mixers, and vortex mixers. These designs create controlled mixing environments that enhance the interaction between lipid components and mRNA, leading to more uniform LNP formation. The geometry of the mixing channels and the flow patterns significantly impact the quality of the resulting LNPs, with optimized designs allowing for better control over particle size distribution and encapsulation efficiency.
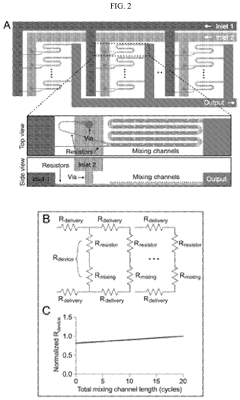
- Residence time optimization for LNP formation: The residence time in microfluidic mixing systems is a critical parameter that affects the quality of LNP-mRNA formulations. Controlling the flow rates and channel dimensions allows for precise adjustment of residence time, which directly impacts the lipid-mRNA interaction period. Optimal residence time ensures complete encapsulation of mRNA while preventing aggregation or degradation. Systems with tunable residence time capabilities enable process optimization for different LNP formulations, resulting in improved encapsulation efficiency and more consistent particle characteristics.
- Encapsulation efficiency enhancement techniques: Various techniques can be employed to enhance the encapsulation efficiency of mRNA in LNPs during microfluidic manufacturing. These include optimization of lipid composition ratios, pH adjustment of the aqueous phase, and implementation of specific mixing regimes. The charge interaction between cationic lipids and negatively charged mRNA plays a crucial role in encapsulation efficiency. Advanced microfluidic systems incorporate features that promote these interactions while maintaining the structural integrity of the mRNA, resulting in higher encapsulation rates and more effective delivery vehicles.
- Size control strategies for LNP production: Precise control over LNP size is essential for effective mRNA delivery and can be achieved through various microfluidic strategies. These include manipulation of flow rate ratios between aqueous and organic phases, adjustment of total flow rates, and implementation of specific mixer geometries. Post-formation processing techniques such as membrane filtration or tangential flow filtration can further refine size distribution. Advanced systems incorporate real-time size monitoring and feedback control mechanisms to maintain consistent particle dimensions throughout the manufacturing process, ensuring batch-to-batch reproducibility.
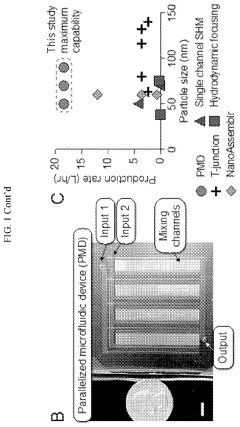
- Scalable manufacturing systems for clinical/commercial production: Scaling up microfluidic LNP-mRNA manufacturing from laboratory to clinical and commercial scales presents significant challenges. Parallel microfluidic systems with multiple mixing units can maintain the benefits of microfluidic mixing while increasing throughput. Continuous flow processes with integrated quality control monitoring enable consistent production of large batches. Advanced systems incorporate automation, process analytical technology, and closed-loop control to ensure consistent quality attributes across different production scales, facilitating the transition from development to commercial manufacturing of LNP-mRNA therapeutics.
02 Residence time optimization for LNP formation
Controlling residence time in microfluidic mixing systems is crucial for LNP-mRNA manufacturing. The duration that the lipid and mRNA components spend in the mixing zone directly affects encapsulation efficiency and particle characteristics. Optimal residence time allows sufficient interaction between components while preventing aggregation or degradation. Flow rate adjustments, channel length modifications, and incorporation of residence time chambers are strategies used to fine-tune this parameter. Systems with programmable residence time control enable process optimization for different LNP formulations.Expand Specific Solutions03 Encapsulation efficiency enhancement techniques
Maximizing encapsulation efficiency is essential for effective LNP-mRNA manufacturing. This can be achieved through precise control of mixing parameters such as flow rate ratios between lipid and mRNA streams, pH adjustment during mixing, and ionic strength optimization. Some systems incorporate specialized surface treatments to prevent component adsorption to channel walls. Advanced techniques include pulsatile flow patterns, temperature-controlled mixing zones, and the use of helper lipids that facilitate mRNA encapsulation. These approaches collectively improve the percentage of mRNA successfully encapsulated within the lipid nanoparticles.Expand Specific Solutions04 Size control and distribution management
Precise control over LNP size and size distribution is critical for therapeutic efficacy and stability. Microfluidic systems achieve this through controlled mixing intensities, solvent dilution rates, and specialized flow focusing techniques. The ratio of aqueous to organic phases and their respective flow rates significantly impact particle size. Some systems incorporate in-line size monitoring with feedback control mechanisms to maintain consistent particle dimensions. Post-formation size refinement can be achieved through integrated filtration or fractionation modules that ensure narrow size distributions suitable for therapeutic applications.Expand Specific Solutions05 Scalable and continuous manufacturing systems
Scalable microfluidic platforms enable transition from laboratory-scale to industrial production of LNP-mRNA formulations. These systems maintain critical quality attributes across different production scales through parallelization of mixing units, modular design approaches, and precise flow distribution. Continuous manufacturing configurations incorporate automated feedback control systems that monitor and adjust process parameters in real-time. Integration with downstream processing steps such as buffer exchange, sterilization, and fill-finish operations creates end-to-end manufacturing solutions. These platforms offer advantages in production efficiency, batch-to-batch consistency, and reduced contamination risk compared to traditional batch processes.Expand Specific Solutions
Leading Companies in LNP-mRNA Manufacturing Equipment
The scalable microfluidic mixing systems for LNP-mRNA manufacturing market is currently in a growth phase, with increasing demand driven by COVID-19 vaccine development and expanding therapeutic applications. The global market size for LNP-mRNA technologies is projected to reach billions of dollars by 2025, with microfluidic systems representing a critical manufacturing component. Leading players demonstrate varying levels of technical maturity: BioNTech and Translate Bio have established commercial-scale manufacturing capabilities, while Nutcracker Therapeutics and Arcturus Therapeutics are advancing innovative microfluidic platforms with enhanced control features. Academic institutions like Johns Hopkins University and University of British Columbia are contributing fundamental research, while pharmaceutical giants including AstraZeneca and Sanofi are investing heavily in scaling these technologies. Key technical challenges remain in achieving consistent residence time, optimal encapsulation efficiency, and precise size control at commercial scale.
Translate Bio, Inc.
Technical Solution: Translate Bio has developed a scalable microfluidic mixing platform called "MRT" (Messenger RNA Therapeutics) specifically optimized for LNP-mRNA manufacturing. Their system employs a specialized Y-junction microfluidic mixer with precisely controlled geometric features that create controlled turbulent mixing zones. The platform utilizes high-precision pumping systems to maintain flow rate ratios between lipid and mRNA solutions at 3:1, with total flow rates adjustable from 2-50 mL/min depending on scale. Residence time is carefully controlled between 1-5 milliseconds to minimize mRNA degradation while ensuring complete mixing. The system incorporates temperature control modules (20-40°C) to optimize lipid phase transitions during mixing. Translate Bio's technology achieves encapsulation efficiencies consistently above 90% with particle sizes tightly controlled between 80-100 nm and polydispersity indices below 0.15. Their platform includes automated dilution and buffer exchange modules to control the final ethanol concentration and remove unencapsulated components.
Strengths: Optimized specifically for mRNA therapeutics with demonstrated clinical manufacturing experience; high encapsulation efficiency; excellent batch-to-batch consistency. Weaknesses: Less flexible than some competing platforms; optimization may be required for different mRNA constructs; higher initial setup costs.
Nutcracker Therapeutics, Inc.
Technical Solution: Nutcracker Therapeutics has developed an integrated microfluidic platform called "ACORN" specifically designed for end-to-end mRNA-LNP manufacturing. Their system incorporates a novel serpentine microfluidic mixer with controlled geometric features that create precise Dean vortices for enhanced mixing efficiency. The platform utilizes programmable syringe pumps to achieve flow rate ratios between lipid and mRNA solutions ranging from 1:1 to 5:1, with total flow rates from 0.1-20 mL/min. Residence time is precisely controlled between 2-30 milliseconds through adjustable channel geometries. A key innovation in their system is the integration of in-line purification and buffer exchange modules that eliminate the need for separate downstream processing steps. The platform achieves encapsulation efficiencies of 85-95% with particle sizes tightly controlled between 70-100 nm. Nutcracker's technology enables rapid formulation optimization through automated parameter sweeps and can transition seamlessly from discovery to manufacturing scale.
Strengths: Fully integrated system combining LNP formation with purification steps; high throughput capability for rapid formulation screening; compact footprint suitable for distributed manufacturing. Weaknesses: Relatively new technology with limited long-term performance data; potential challenges in scaling to very high production volumes (>100L).
Critical Patents in Residence Time and Size Control Technologies
Microfluidic platforms for large scale nanoparticle formulations
PatentPendingUS20240050908A1
Innovation
- A scalable, parallelized microfluidic device (PMD) with an array of 128 microfluidic mixing channels is developed, incorporating a ladder geometry and flow resistors to ensure uniform fluid distribution and high production rates, achieving over 100-fold increase in production compared to single microfluidic channels while maintaining the physical properties and potency of LNPs.
Method of making lipid-encapsulated RNA nanoparticles
PatentPendingUS20250177305A1
Innovation
- A method involving the controlled mixing of an aqueous RNA solution with an ethanol solution containing cationic lipids, where the lipids are pumped through a smaller tube at one-third the flow rate of the RNA solution, creating a turbulent flow that produces lipid-encapsulated RNA nanoparticles with a bilayer structure.
Quality Control Standards for LNP-mRNA Formulations
Quality control standards for LNP-mRNA formulations represent a critical component in ensuring the safety, efficacy, and consistency of these advanced therapeutic products. As microfluidic mixing systems scale up for commercial manufacturing, robust quality control frameworks must evolve simultaneously to maintain product integrity throughout the production process.
The primary quality attributes requiring stringent monitoring include particle size distribution, polydispersity index (PDI), encapsulation efficiency, and lipid/mRNA ratio. These parameters directly influence the biological performance of the final product, with particle size typically targeted between 80-100 nm for optimal cellular uptake and biodistribution. Advanced analytical techniques such as dynamic light scattering (DLS), nanoparticle tracking analysis (NTA), and cryo-electron microscopy have become industry standards for size characterization.
Encapsulation efficiency represents another critical quality attribute, with regulatory expectations typically requiring >80% mRNA encapsulation. Ribogreen assays and HPLC-based methods have emerged as preferred analytical approaches for quantifying free versus encapsulated mRNA. The consistency of these measurements across manufacturing batches serves as a key indicator of process robustness.
Residence time control within microfluidic systems presents unique quality challenges, as variations can significantly impact LNP formation kinetics. Real-time monitoring systems utilizing spectroscopic methods have been developed to ensure consistent mixing conditions and residence time distributions, particularly important when scaling from laboratory to commercial production volumes.
Regulatory frameworks for LNP-mRNA products continue to evolve, with the FDA and EMA establishing increasingly specific guidance. Current standards emphasize process analytical technology (PAT) implementation to enable real-time quality assurance rather than relying solely on end-product testing. This approach aligns with Quality by Design (QbD) principles, where critical quality attributes are identified early and monitored throughout the manufacturing process.
Stability testing protocols for LNP-mRNA formulations have become increasingly standardized, with accelerated and real-time studies examining particle size growth, mRNA integrity, and lipid oxidation under various storage conditions. These protocols typically assess product quality at multiple timepoints over 6-24 months, providing crucial data for establishing shelf-life claims.
The primary quality attributes requiring stringent monitoring include particle size distribution, polydispersity index (PDI), encapsulation efficiency, and lipid/mRNA ratio. These parameters directly influence the biological performance of the final product, with particle size typically targeted between 80-100 nm for optimal cellular uptake and biodistribution. Advanced analytical techniques such as dynamic light scattering (DLS), nanoparticle tracking analysis (NTA), and cryo-electron microscopy have become industry standards for size characterization.
Encapsulation efficiency represents another critical quality attribute, with regulatory expectations typically requiring >80% mRNA encapsulation. Ribogreen assays and HPLC-based methods have emerged as preferred analytical approaches for quantifying free versus encapsulated mRNA. The consistency of these measurements across manufacturing batches serves as a key indicator of process robustness.
Residence time control within microfluidic systems presents unique quality challenges, as variations can significantly impact LNP formation kinetics. Real-time monitoring systems utilizing spectroscopic methods have been developed to ensure consistent mixing conditions and residence time distributions, particularly important when scaling from laboratory to commercial production volumes.
Regulatory frameworks for LNP-mRNA products continue to evolve, with the FDA and EMA establishing increasingly specific guidance. Current standards emphasize process analytical technology (PAT) implementation to enable real-time quality assurance rather than relying solely on end-product testing. This approach aligns with Quality by Design (QbD) principles, where critical quality attributes are identified early and monitored throughout the manufacturing process.
Stability testing protocols for LNP-mRNA formulations have become increasingly standardized, with accelerated and real-time studies examining particle size growth, mRNA integrity, and lipid oxidation under various storage conditions. These protocols typically assess product quality at multiple timepoints over 6-24 months, providing crucial data for establishing shelf-life claims.
Manufacturing Scale-up Strategies and Economic Considerations
Scaling up LNP-mRNA manufacturing from laboratory to commercial production presents significant challenges that require strategic planning and economic evaluation. The transition from small-scale microfluidic systems to industrial production necessitates careful consideration of both technical and financial factors to ensure consistent product quality while achieving cost-effectiveness.
Traditional batch processing approaches face limitations when scaling up LNP-mRNA production, particularly in maintaining uniform mixing conditions and consistent particle characteristics. Continuous flow manufacturing has emerged as a preferred strategy, offering advantages in process control, reduced batch-to-batch variability, and operational efficiency. Implementation of parallel microfluidic devices or scaled-up single units with optimized geometries can increase throughput while preserving critical quality attributes.
Capital expenditure considerations for scale-up include specialized equipment costs, facility modifications for GMP compliance, and automation systems. These initial investments must be balanced against long-term operational benefits. Manufacturers typically face a decision between modular expansion using multiple smaller units versus investing in larger custom systems, with the former offering flexibility and the latter potentially providing economies of scale.
Raw material costs represent a significant portion of production expenses, with lipid components and mRNA being particularly costly. Scale-up strategies must address supply chain reliability and potential volume discounts. Process intensification techniques that improve encapsulation efficiency can substantially reduce material waste, directly impacting cost of goods. Additionally, implementing in-line monitoring systems, while initially expensive, can reduce quality control costs and minimize batch failures.
Regulatory considerations significantly influence scale-up economics. Design of manufacturing systems must incorporate features that facilitate validation, including process analytical technology (PAT) for real-time monitoring of critical parameters such as residence time distribution and mixing efficiency. Documentation requirements increase substantially at commercial scale, necessitating robust data management systems.
Labor requirements shift during scale-up, with higher demand for specialized personnel during development phases and more operational staff during production. Training costs and knowledge transfer represent important economic factors that are often underestimated. Automation strategies can reduce labor costs but require significant upfront investment and specialized maintenance capabilities.
Risk mitigation strategies, including redundant systems and backup capacity, add to overall costs but are essential for commercial manufacturing. Economic models must account for these contingencies while balancing production flexibility against operational efficiency to optimize the total cost of ownership across the product lifecycle.
Traditional batch processing approaches face limitations when scaling up LNP-mRNA production, particularly in maintaining uniform mixing conditions and consistent particle characteristics. Continuous flow manufacturing has emerged as a preferred strategy, offering advantages in process control, reduced batch-to-batch variability, and operational efficiency. Implementation of parallel microfluidic devices or scaled-up single units with optimized geometries can increase throughput while preserving critical quality attributes.
Capital expenditure considerations for scale-up include specialized equipment costs, facility modifications for GMP compliance, and automation systems. These initial investments must be balanced against long-term operational benefits. Manufacturers typically face a decision between modular expansion using multiple smaller units versus investing in larger custom systems, with the former offering flexibility and the latter potentially providing economies of scale.
Raw material costs represent a significant portion of production expenses, with lipid components and mRNA being particularly costly. Scale-up strategies must address supply chain reliability and potential volume discounts. Process intensification techniques that improve encapsulation efficiency can substantially reduce material waste, directly impacting cost of goods. Additionally, implementing in-line monitoring systems, while initially expensive, can reduce quality control costs and minimize batch failures.
Regulatory considerations significantly influence scale-up economics. Design of manufacturing systems must incorporate features that facilitate validation, including process analytical technology (PAT) for real-time monitoring of critical parameters such as residence time distribution and mixing efficiency. Documentation requirements increase substantially at commercial scale, necessitating robust data management systems.
Labor requirements shift during scale-up, with higher demand for specialized personnel during development phases and more operational staff during production. Training costs and knowledge transfer represent important economic factors that are often underestimated. Automation strategies can reduce labor costs but require significant upfront investment and specialized maintenance capabilities.
Risk mitigation strategies, including redundant systems and backup capacity, add to overall costs but are essential for commercial manufacturing. Economic models must account for these contingencies while balancing production flexibility against operational efficiency to optimize the total cost of ownership across the product lifecycle.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!