Integration of final product potency release assays into accelerated release strategies for urgent use products
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Potency Assay Integration Background and Objectives
The integration of potency assays into accelerated release strategies represents a critical advancement in biopharmaceutical manufacturing, particularly for products requiring urgent deployment. Historically, potency testing has been a rate-limiting step in product release, often requiring days or weeks to complete due to complex biological assays that measure functional activity rather than merely physical or chemical properties. This technical domain has evolved significantly over the past decade, transitioning from purely cell-based assays with high variability to more sophisticated hybrid approaches incorporating molecular techniques and automation.
The pharmaceutical industry has witnessed increasing pressure to expedite product release timelines, especially for vaccines during pandemics, emergency therapeutics, and personalized medicine applications where patient needs cannot accommodate traditional testing schedules. Regulatory frameworks have simultaneously evolved to recognize the need for accelerated pathways while maintaining stringent quality standards, as evidenced by FDA and EMA guidance documents published between 2018-2023.
Current technological trajectories point toward real-time release testing (RTRT) capabilities, where potency assessment occurs continuously throughout manufacturing rather than as an end-stage gate. This paradigm shift requires fundamental reconsideration of assay design principles, validation approaches, and integration with process analytical technology (PAT) frameworks.
The primary objective of this technical investigation is to evaluate methodologies for incorporating final product potency assays into accelerated release strategies without compromising product quality or regulatory compliance. Specifically, we aim to identify approaches that can reduce potency testing timelines by at least 50% while maintaining or improving assay precision and accuracy.
Secondary objectives include assessing the feasibility of surrogate potency markers that correlate with traditional assays but deliver results more rapidly, exploring the regulatory landscape for accelerated potency testing acceptance, and evaluating the cost-benefit relationship of implementing advanced technologies such as automated microfluidics platforms and AI-assisted data analysis for potency determination.
This research will focus particularly on protein-based therapeutics, including monoclonal antibodies, recombinant proteins, and advanced therapy medicinal products (ATMPs), where potency testing presents unique challenges due to complex mechanisms of action and biological variability. The findings will inform strategic technology investment decisions and regulatory strategy development for next-generation manufacturing platforms.
The pharmaceutical industry has witnessed increasing pressure to expedite product release timelines, especially for vaccines during pandemics, emergency therapeutics, and personalized medicine applications where patient needs cannot accommodate traditional testing schedules. Regulatory frameworks have simultaneously evolved to recognize the need for accelerated pathways while maintaining stringent quality standards, as evidenced by FDA and EMA guidance documents published between 2018-2023.
Current technological trajectories point toward real-time release testing (RTRT) capabilities, where potency assessment occurs continuously throughout manufacturing rather than as an end-stage gate. This paradigm shift requires fundamental reconsideration of assay design principles, validation approaches, and integration with process analytical technology (PAT) frameworks.
The primary objective of this technical investigation is to evaluate methodologies for incorporating final product potency assays into accelerated release strategies without compromising product quality or regulatory compliance. Specifically, we aim to identify approaches that can reduce potency testing timelines by at least 50% while maintaining or improving assay precision and accuracy.
Secondary objectives include assessing the feasibility of surrogate potency markers that correlate with traditional assays but deliver results more rapidly, exploring the regulatory landscape for accelerated potency testing acceptance, and evaluating the cost-benefit relationship of implementing advanced technologies such as automated microfluidics platforms and AI-assisted data analysis for potency determination.
This research will focus particularly on protein-based therapeutics, including monoclonal antibodies, recombinant proteins, and advanced therapy medicinal products (ATMPs), where potency testing presents unique challenges due to complex mechanisms of action and biological variability. The findings will inform strategic technology investment decisions and regulatory strategy development for next-generation manufacturing platforms.
Market Demand for Accelerated Release Strategies
The pharmaceutical and biotechnology industries are experiencing unprecedented pressure to accelerate product release timelines, particularly for urgent use products such as vaccines, emergency therapeutics, and critical care medications. Market research indicates that the global emergency medicine market is projected to reach $69.7 billion by 2026, growing at a CAGR of 6.5% from 2021. This growth is primarily driven by increasing incidences of natural disasters, disease outbreaks, and the ongoing need for rapid medical interventions in crisis situations.
The COVID-19 pandemic has fundamentally transformed market expectations regarding development and release timelines for urgent use products. What previously took years has been compressed into months, creating substantial market demand for accelerated release strategies. Healthcare providers, government agencies, and patients now expect critical medications to be available within dramatically shortened timeframes without compromising safety or efficacy.
Regulatory bodies have responded to this market pressure by implementing emergency use authorization (EUA) frameworks and other expedited approval pathways. These mechanisms have created a new market segment specifically focused on technologies and methodologies that can support accelerated product release while maintaining compliance with regulatory standards. Companies that can effectively navigate these pathways gain significant competitive advantages in terms of market entry timing and stakeholder trust.
From an economic perspective, the cost of delayed product releases during public health emergencies has become increasingly quantifiable. Studies estimate that each month of delay in releasing critical vaccines or therapeutics during a pandemic can result in billions of dollars in economic losses and thousands of preventable deaths. This economic reality has created a robust market for technologies that can compress release testing timelines.
Healthcare systems globally are investing in infrastructure to support rapid deployment of urgent use products. This includes dedicated supply chains, specialized storage facilities, and training programs for healthcare workers. These investments represent a significant market opportunity for companies offering integrated solutions that address the entire product lifecycle from manufacturing to patient administration.
The market is particularly receptive to innovative analytical technologies that can provide real-time or near-real-time potency assessments. Traditional potency assays that require days or weeks to complete are increasingly viewed as bottlenecks in the release process. Market analysis shows that technologies offering comparable accuracy with significantly reduced timeframes command premium pricing and rapid adoption rates across the pharmaceutical industry.
The COVID-19 pandemic has fundamentally transformed market expectations regarding development and release timelines for urgent use products. What previously took years has been compressed into months, creating substantial market demand for accelerated release strategies. Healthcare providers, government agencies, and patients now expect critical medications to be available within dramatically shortened timeframes without compromising safety or efficacy.
Regulatory bodies have responded to this market pressure by implementing emergency use authorization (EUA) frameworks and other expedited approval pathways. These mechanisms have created a new market segment specifically focused on technologies and methodologies that can support accelerated product release while maintaining compliance with regulatory standards. Companies that can effectively navigate these pathways gain significant competitive advantages in terms of market entry timing and stakeholder trust.
From an economic perspective, the cost of delayed product releases during public health emergencies has become increasingly quantifiable. Studies estimate that each month of delay in releasing critical vaccines or therapeutics during a pandemic can result in billions of dollars in economic losses and thousands of preventable deaths. This economic reality has created a robust market for technologies that can compress release testing timelines.
Healthcare systems globally are investing in infrastructure to support rapid deployment of urgent use products. This includes dedicated supply chains, specialized storage facilities, and training programs for healthcare workers. These investments represent a significant market opportunity for companies offering integrated solutions that address the entire product lifecycle from manufacturing to patient administration.
The market is particularly receptive to innovative analytical technologies that can provide real-time or near-real-time potency assessments. Traditional potency assays that require days or weeks to complete are increasingly viewed as bottlenecks in the release process. Market analysis shows that technologies offering comparable accuracy with significantly reduced timeframes command premium pricing and rapid adoption rates across the pharmaceutical industry.
Current Challenges in Potency Testing for Urgent Use Products
Potency testing for urgent use products faces significant challenges in the current biopharmaceutical landscape. Traditional potency assays, which measure a product's biological activity, typically require extensive time to complete—often ranging from 7 to 28 days depending on the product type and assay methodology. This extended timeline creates a fundamental conflict with the accelerated release needs for urgent use products such as vaccines during pandemics, emergency therapeutics, or personalized cell therapies where patient needs cannot accommodate conventional testing schedules.
Cell-based assays, which remain the gold standard for potency testing, present particular difficulties due to their inherent variability and complexity. These assays frequently suffer from high coefficients of variation (often 15-30%), making reliable interpretation challenging when attempting to implement accelerated testing paradigms. The requirement for live cells, specialized equipment, and trained personnel further complicates their integration into streamlined release strategies.
Regulatory frameworks present another significant hurdle. While agencies recognize the need for expedited release in emergency situations, they maintain stringent requirements for product quality and safety assurance. Current guidelines from FDA, EMA, and other global authorities lack clear pathways for implementing alternative or abbreviated potency testing approaches that maintain compliance while enabling faster product release.
Technical limitations of existing analytical methods also impede progress. Many current potency assays lack the sensitivity to detect subtle changes in product quality with reduced sample sizes or shortened incubation times. Additionally, the correlation between abbreviated testing protocols and traditional full-length assays often remains unvalidated, creating uncertainty about the predictive value of accelerated methods.
Resource constraints further exacerbate these challenges. Implementing dual testing approaches—where both traditional and accelerated methods run in parallel during validation phases—requires significant investment in equipment, personnel, and method development. Many organizations, particularly smaller biotech companies, struggle to allocate sufficient resources to these parallel efforts while maintaining their core production activities.
Data management systems present additional complications, as they must be adapted to handle accelerated testing paradigms, including appropriate specification setting, trending analysis, and integration with quality management systems. Legacy systems often lack the flexibility to accommodate these novel approaches, requiring significant IT infrastructure updates.
Finally, the scientific understanding of surrogate markers that could reliably predict potency remains incomplete for many biological products. The complex mechanisms of action for advanced therapeutics often involve multiple pathways and cellular interactions that cannot be fully captured in simplified or accelerated testing formats, creating uncertainty about their predictive value for clinical efficacy.
Cell-based assays, which remain the gold standard for potency testing, present particular difficulties due to their inherent variability and complexity. These assays frequently suffer from high coefficients of variation (often 15-30%), making reliable interpretation challenging when attempting to implement accelerated testing paradigms. The requirement for live cells, specialized equipment, and trained personnel further complicates their integration into streamlined release strategies.
Regulatory frameworks present another significant hurdle. While agencies recognize the need for expedited release in emergency situations, they maintain stringent requirements for product quality and safety assurance. Current guidelines from FDA, EMA, and other global authorities lack clear pathways for implementing alternative or abbreviated potency testing approaches that maintain compliance while enabling faster product release.
Technical limitations of existing analytical methods also impede progress. Many current potency assays lack the sensitivity to detect subtle changes in product quality with reduced sample sizes or shortened incubation times. Additionally, the correlation between abbreviated testing protocols and traditional full-length assays often remains unvalidated, creating uncertainty about the predictive value of accelerated methods.
Resource constraints further exacerbate these challenges. Implementing dual testing approaches—where both traditional and accelerated methods run in parallel during validation phases—requires significant investment in equipment, personnel, and method development. Many organizations, particularly smaller biotech companies, struggle to allocate sufficient resources to these parallel efforts while maintaining their core production activities.
Data management systems present additional complications, as they must be adapted to handle accelerated testing paradigms, including appropriate specification setting, trending analysis, and integration with quality management systems. Legacy systems often lack the flexibility to accommodate these novel approaches, requiring significant IT infrastructure updates.
Finally, the scientific understanding of surrogate markers that could reliably predict potency remains incomplete for many biological products. The complex mechanisms of action for advanced therapeutics often involve multiple pathways and cellular interactions that cannot be fully captured in simplified or accelerated testing formats, creating uncertainty about their predictive value for clinical efficacy.
Current Potency Assay Integration Solutions
01 Accelerated stability testing methods for pharmaceutical potency
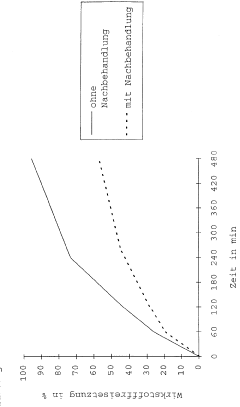
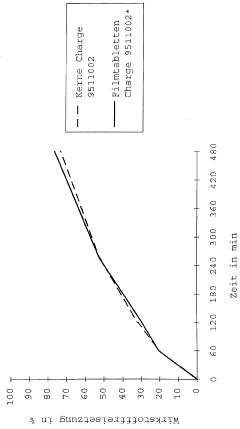
Accelerated stability testing methods are used to predict the long-term stability and potency of pharmaceutical products in a shorter timeframe. These methods involve subjecting the products to elevated temperatures, humidity, or other stress conditions to accelerate degradation processes. The data collected from these tests can be used to establish shelf life, determine appropriate storage conditions, and ensure that the product maintains its potency throughout its intended use period.- Accelerated stability testing methods for pharmaceutical potency: Accelerated stability testing methods are used to predict the long-term stability and potency of pharmaceutical products in a shorter timeframe. These methods involve subjecting the products to elevated temperatures, humidity, or other stress conditions to accelerate degradation processes. The data obtained from these tests can be used to establish shelf life, determine appropriate storage conditions, and ensure that the product maintains its potency throughout its intended use period.
- Analytical techniques for potency determination in release assays: Various analytical techniques are employed for determining the potency of final pharmaceutical products in release assays. These techniques include high-performance liquid chromatography (HPLC), enzyme-linked immunosorbent assay (ELISA), mass spectrometry, and bioassays. These methods provide quantitative measurements of active ingredients, ensuring that the final product meets specified potency requirements before release to the market.
- Controlled release formulations and potency assessment: Controlled release formulations require specialized potency release assays to evaluate the rate and extent of active ingredient release over time. These assays typically involve dissolution testing under various conditions to simulate in vivo performance. The potency of the released active ingredient is measured at predetermined time points to ensure consistent drug delivery and therapeutic efficacy throughout the intended release period.
- Real-time and accelerated release testing correlation: Establishing correlation between real-time and accelerated release testing is crucial for predicting long-term product performance. Mathematical models and statistical approaches are used to establish this correlation, allowing manufacturers to use accelerated testing data to make reliable predictions about product potency throughout its shelf life. This correlation helps in reducing the time required for product development and regulatory approval.
- Regulatory considerations for accelerated potency release testing: Regulatory frameworks provide guidelines for conducting accelerated potency release assays and interpreting their results. These guidelines specify the conditions for accelerated testing, acceptance criteria, and statistical methods for data analysis. Compliance with these regulatory requirements is essential for obtaining approval for pharmaceutical products and ensuring that accelerated testing accurately reflects the product's stability and potency characteristics.
02 In vitro release assays for biological product potency
In vitro release assays are used to evaluate the potency of biological products by measuring the release of active ingredients under controlled conditions. These assays can simulate physiological conditions and provide data on the rate and extent of drug release from formulations. For biological products such as vaccines, monoclonal antibodies, and gene therapies, specialized in vitro release assays are developed to accurately assess product potency and ensure batch-to-batch consistency.Expand Specific Solutions03 Analytical methods for accelerated release testing
Advanced analytical methods are employed for accelerated release testing to rapidly assess product potency. These methods include high-performance liquid chromatography (HPLC), mass spectrometry, enzyme-linked immunosorbent assays (ELISA), and cell-based potency assays. These techniques allow for precise quantification of active ingredients and their degradation products, enabling accurate assessment of product potency under accelerated conditions and prediction of long-term stability.Expand Specific Solutions04 Correlation between accelerated and real-time release testing
Establishing correlation between accelerated and real-time release testing is crucial for validating accelerated testing protocols. Mathematical models and statistical approaches are used to develop predictive relationships between data obtained from accelerated conditions and real-time stability. These correlations help in determining appropriate acceptance criteria for accelerated release tests and ensure that accelerated testing accurately predicts the long-term potency and stability of pharmaceutical products.Expand Specific Solutions05 Regulatory considerations for accelerated potency release assays
Regulatory frameworks provide guidance on the development, validation, and implementation of accelerated potency release assays. These considerations include establishing appropriate specifications, validation parameters, and acceptance criteria for accelerated testing protocols. Regulatory agencies require demonstration of the reliability and predictive capability of accelerated release assays before they can be used for product release decisions. Compliance with these regulatory requirements ensures that accelerated potency release assays are scientifically sound and appropriate for their intended use.Expand Specific Solutions
Key Industry Players in Accelerated Release Technologies
The integration of final product potency release assays into accelerated release strategies represents a critical challenge in the pharmaceutical industry, currently in a transitional phase as companies seek to balance regulatory compliance with urgent product availability. The market is experiencing significant growth, driven by increasing demand for rapid-release therapeutics, with an estimated value exceeding $2 billion. Leading pharmaceutical companies like Eli Lilly, GlaxoSmithKline, and Regeneron Pharmaceuticals have achieved moderate technological maturity in this field, while specialized firms such as PhaseBio, PolyPid, and Altus Formulation are developing innovative platforms that enhance release kinetics. Purdue Pharma and Boehringer Ingelheim are investing in advanced analytical methods to streamline potency testing, though regulatory harmonization remains a significant challenge across different markets.
Boehringer Ingelheim International GmbH
Technical Solution: Boehringer Ingelheim has developed an accelerated release strategy that focuses on integrating potency testing earlier in the manufacturing process. Their approach employs a combination of in-process potency monitoring and rapid release testing methodologies. The company has implemented automated cell-based potency assays that utilize luminescence-based readouts, reducing traditional assay times from days to hours. Their platform incorporates multivariate analysis of manufacturing process parameters to establish correlations with final product potency, enabling early prediction of potency outcomes. For biological products, Boehringer has developed abbreviated potency assays that focus on key functional epitopes that demonstrate strong correlation with full potency tests. Their system includes a risk-based release framework where products with consistent manufacturing history can follow an expedited pathway with reduced testing requirements. Boehringer has also implemented rapid analytical methods including capillary electrophoresis and mass spectrometry for structural characterization that serves as supporting evidence for potency consistency.
Strengths: Strong focus on process understanding and control as the foundation for accelerated release. Their risk-based approach allows customization based on product history and criticality. Weaknesses: Heavy reliance on manufacturing consistency may not account for unexpected variations. System requires extensive historical data to establish robust correlations between process parameters and potency.
GlaxoSmithKline Biologicals SA
Technical Solution: GSK Biologicals has developed an integrated accelerated release strategy for vaccines and biologics that incorporates surrogate potency assays into their release workflow. Their approach utilizes a combination of rapid physicochemical characterization methods coupled with abbreviated biological assays that target specific functional attributes. GSK's platform employs surface plasmon resonance (SPR) and biolayer interferometry to rapidly assess binding kinetics as surrogate markers for biological activity. For vaccines, they have implemented multiplexed immunoassays that can simultaneously evaluate multiple antigens in a single test, reducing testing time from weeks to days. GSK has also pioneered the use of flow cytometry-based potency assays that provide results within 24 hours compared to traditional methods requiring 7-14 days. Their system incorporates a staged release approach where products can be shipped to distribution centers based on preliminary potency data, with final authorization for use granted upon completion of confirmatory testing. This approach has been successfully implemented for seasonal influenza vaccines, reducing time-to-market by several weeks.
Strengths: Comprehensive validation package supporting correlation between rapid methods and traditional potency assays. Their staged release approach balances speed with safety considerations. Weaknesses: Some surrogate markers may not fully represent all aspects of biological activity. System requires significant regulatory interaction and approval for each product type.
Critical Technologies for Rapid Potency Assessment
Method for producing active agent preparations with controlled release from a matrix
PatentWO1999002134A2
Innovation
- A new process involving the granulation of active substances with binders in an intensive mixer, followed by cooling, sieving, and thermal post-treatment in a fluidized bed to achieve reproducible and controlled release rates, using binders with melting points between 35 and 90°C, such as macrogol, polymethacrylic acid derivatives, and lipophilic substances.
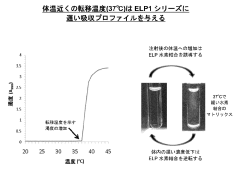
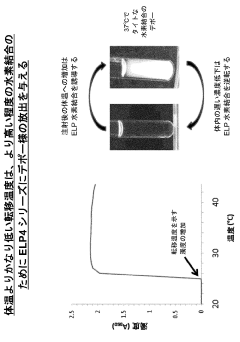
ELP fusion proteins for controlled and sustained release
PatentActiveJP2022176986A
Innovation
- Pharmaceutical formulations utilizing elastin-like protein (ELP) sequences that form reversible matrices at body temperature, providing sustained release through hydrogen bonds and hydrophobic interactions, allowing slow absorption and prolonged circulation, thereby enhancing pharmacokinetics with improved stability and reduced peak-to-trough ratios.
Regulatory Considerations for Expedited Release Pathways
Regulatory frameworks governing expedited release pathways vary significantly across global jurisdictions, with FDA, EMA, and NMPA each maintaining distinct approaches to accelerated approval mechanisms. These regulatory bodies have established specific guidelines for urgent use products, particularly evident in recent pandemic responses where emergency use authorizations became critical tools for rapid deployment of medical countermeasures.
The integration of final product potency release assays into these accelerated pathways requires careful navigation of regulatory expectations that balance speed with safety assurance. Regulatory agencies typically require robust validation data demonstrating that abbreviated testing protocols maintain adequate quality control standards while expediting availability. This often involves comparative studies showing correlation between accelerated and standard release testing methodologies.
Risk-based approaches have become increasingly accepted by regulators when considering expedited release strategies. Manufacturers must develop comprehensive risk assessment frameworks that clearly identify critical quality attributes and demonstrate how these will be monitored effectively within compressed timelines. Documentation of this risk-based decision-making process becomes a crucial component of regulatory submissions.
Post-approval commitments represent another important regulatory consideration, as agencies often grant accelerated pathways with the understanding that additional data collection will continue after initial distribution. These commitments may include enhanced pharmacovigilance, completion of validation studies, or implementation of more comprehensive testing once time constraints are alleviated.
Regulatory harmonization efforts have made progress in standardizing expectations for expedited release, though significant regional differences persist. The International Council for Harmonisation (ICH) has developed several guidelines that provide frameworks for quality considerations in accelerated development scenarios, though implementation varies across member countries.
Communication strategies with regulatory authorities constitute a critical success factor for expedited pathways. Early engagement through mechanisms like FDA's Breakthrough Therapy designation or EMA's PRIME (PRIority MEdicines) program can establish collaborative approaches to developing acceptable testing protocols. These interactions should focus on identifying minimum necessary data requirements and potential surrogate endpoints for potency determination.
Precedent cases demonstrate that regulatory flexibility increases proportionally with unmet medical need and public health urgency. Products addressing life-threatening conditions with limited treatment options typically receive greater consideration for novel release testing approaches, provided core safety parameters remain adequately controlled.
The integration of final product potency release assays into these accelerated pathways requires careful navigation of regulatory expectations that balance speed with safety assurance. Regulatory agencies typically require robust validation data demonstrating that abbreviated testing protocols maintain adequate quality control standards while expediting availability. This often involves comparative studies showing correlation between accelerated and standard release testing methodologies.
Risk-based approaches have become increasingly accepted by regulators when considering expedited release strategies. Manufacturers must develop comprehensive risk assessment frameworks that clearly identify critical quality attributes and demonstrate how these will be monitored effectively within compressed timelines. Documentation of this risk-based decision-making process becomes a crucial component of regulatory submissions.
Post-approval commitments represent another important regulatory consideration, as agencies often grant accelerated pathways with the understanding that additional data collection will continue after initial distribution. These commitments may include enhanced pharmacovigilance, completion of validation studies, or implementation of more comprehensive testing once time constraints are alleviated.
Regulatory harmonization efforts have made progress in standardizing expectations for expedited release, though significant regional differences persist. The International Council for Harmonisation (ICH) has developed several guidelines that provide frameworks for quality considerations in accelerated development scenarios, though implementation varies across member countries.
Communication strategies with regulatory authorities constitute a critical success factor for expedited pathways. Early engagement through mechanisms like FDA's Breakthrough Therapy designation or EMA's PRIME (PRIority MEdicines) program can establish collaborative approaches to developing acceptable testing protocols. These interactions should focus on identifying minimum necessary data requirements and potential surrogate endpoints for potency determination.
Precedent cases demonstrate that regulatory flexibility increases proportionally with unmet medical need and public health urgency. Products addressing life-threatening conditions with limited treatment options typically receive greater consideration for novel release testing approaches, provided core safety parameters remain adequately controlled.
Risk Management Strategies for Accelerated Product Release
Effective risk management is essential when implementing accelerated release strategies for urgent use products, particularly when integrating final product potency assays. These strategies must balance the need for rapid availability with quality and safety assurance through structured risk assessment frameworks.
A comprehensive risk management approach begins with risk identification, where potential failure points in the accelerated potency testing process are systematically cataloged. This includes analytical method variability, sample handling deviations, and equipment reliability concerns. Critical risk factors must be prioritized based on their potential impact on product efficacy and patient safety.
Risk mitigation strategies should incorporate redundant testing protocols where feasible, utilizing orthogonal methods to confirm potency results. Implementing real-time monitoring systems for critical process parameters during potency testing can provide early warning indicators of potential deviations, allowing for timely interventions before product release decisions are made.
Statistical process control methodologies offer valuable tools for establishing acceptable variability thresholds in accelerated potency assays. By defining appropriate control limits and decision rules, organizations can make data-driven release determinations while maintaining scientific rigor. Trend analysis of historical potency data further strengthens the risk assessment framework.
Contingency planning represents another crucial element, with predetermined response protocols for scenarios where potency results fall into gray zones or testing anomalies occur. These plans should include escalation pathways, additional verification steps, and decision matrices to guide release authorities through complex situations requiring scientific judgment.
Documentation strategies must be adapted for accelerated release contexts, ensuring that abbreviated testing approaches remain compliant with regulatory expectations. This includes justification of risk-based decisions, traceability of testing materials, and complete data integrity throughout the accelerated process.
Cross-functional collaboration between quality, manufacturing, regulatory affairs, and clinical teams is essential for holistic risk management. Regular simulation exercises testing the accelerated release pathway can identify operational vulnerabilities before implementation in actual emergency situations, strengthening organizational readiness and response capabilities.
A comprehensive risk management approach begins with risk identification, where potential failure points in the accelerated potency testing process are systematically cataloged. This includes analytical method variability, sample handling deviations, and equipment reliability concerns. Critical risk factors must be prioritized based on their potential impact on product efficacy and patient safety.
Risk mitigation strategies should incorporate redundant testing protocols where feasible, utilizing orthogonal methods to confirm potency results. Implementing real-time monitoring systems for critical process parameters during potency testing can provide early warning indicators of potential deviations, allowing for timely interventions before product release decisions are made.
Statistical process control methodologies offer valuable tools for establishing acceptable variability thresholds in accelerated potency assays. By defining appropriate control limits and decision rules, organizations can make data-driven release determinations while maintaining scientific rigor. Trend analysis of historical potency data further strengthens the risk assessment framework.
Contingency planning represents another crucial element, with predetermined response protocols for scenarios where potency results fall into gray zones or testing anomalies occur. These plans should include escalation pathways, additional verification steps, and decision matrices to guide release authorities through complex situations requiring scientific judgment.
Documentation strategies must be adapted for accelerated release contexts, ensuring that abbreviated testing approaches remain compliant with regulatory expectations. This includes justification of risk-based decisions, traceability of testing materials, and complete data integrity throughout the accelerated process.
Cross-functional collaboration between quality, manufacturing, regulatory affairs, and clinical teams is essential for holistic risk management. Regular simulation exercises testing the accelerated release pathway can identify operational vulnerabilities before implementation in actual emergency situations, strengthening organizational readiness and response capabilities.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!