End-to-end closed systems for ex vivo mRNA transfection of patient cells at point-of-care sites
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
mRNA Transfection Technology Evolution and Objectives
mRNA transfection technology has evolved significantly since its inception in the early 1990s, when researchers first demonstrated successful delivery of mRNA into cells. Initially, these methods were limited to laboratory settings with low efficiency and high cytotoxicity. The field gained momentum in the early 2000s with the development of improved delivery vehicles, particularly lipid-based carriers that enhanced transfection efficiency while reducing cellular damage.
A pivotal advancement occurred around 2010 with the introduction of modified nucleosides in mRNA structures, significantly reducing immunogenicity and increasing translation efficiency. This breakthrough, pioneered by Katalin Karikó and Drew Weissman, transformed mRNA from a research tool to a viable therapeutic platform. The subsequent development of lipid nanoparticles (LNPs) as delivery vehicles further accelerated the field, culminating in the rapid deployment of mRNA vaccines during the COVID-19 pandemic.
The current technological trajectory is moving toward more sophisticated, automated, and closed systems for ex vivo mRNA transfection. These systems aim to minimize human intervention, reduce contamination risks, and standardize processes for consistent results. The evolution from bench-scale manual protocols to integrated systems represents a critical shift toward clinical applicability, particularly for point-of-care applications.
The primary objective of end-to-end closed systems for ex vivo mRNA transfection is to enable safe, efficient, and reproducible genetic modification of patient cells in clinical settings. This technology aims to bridge the gap between laboratory capabilities and bedside implementation, making advanced cell therapies more accessible to patients. Specific goals include reducing the time from cell collection to reinfusion, minimizing the need for specialized facilities, and ensuring product consistency across different clinical sites.
Technical objectives focus on optimizing transfection efficiency while maintaining cell viability, developing scalable and automated processes, and ensuring regulatory compliance through closed-system operations. Additional aims include reducing the cost per treatment, extending the shelf life of reagents, and simplifying the operational complexity to enable use by clinical staff with minimal specialized training.
The long-term vision encompasses the development of portable, modular systems that can be deployed in various healthcare settings, from specialized centers to community hospitals. This democratization of advanced cell therapy technologies could significantly expand patient access to cutting-edge treatments, particularly in regions with limited healthcare infrastructure. The ultimate goal is to establish mRNA transfection as a routine clinical procedure, similar to how blood transfusions evolved from complex hospital procedures to standardized protocols.
A pivotal advancement occurred around 2010 with the introduction of modified nucleosides in mRNA structures, significantly reducing immunogenicity and increasing translation efficiency. This breakthrough, pioneered by Katalin Karikó and Drew Weissman, transformed mRNA from a research tool to a viable therapeutic platform. The subsequent development of lipid nanoparticles (LNPs) as delivery vehicles further accelerated the field, culminating in the rapid deployment of mRNA vaccines during the COVID-19 pandemic.
The current technological trajectory is moving toward more sophisticated, automated, and closed systems for ex vivo mRNA transfection. These systems aim to minimize human intervention, reduce contamination risks, and standardize processes for consistent results. The evolution from bench-scale manual protocols to integrated systems represents a critical shift toward clinical applicability, particularly for point-of-care applications.
The primary objective of end-to-end closed systems for ex vivo mRNA transfection is to enable safe, efficient, and reproducible genetic modification of patient cells in clinical settings. This technology aims to bridge the gap between laboratory capabilities and bedside implementation, making advanced cell therapies more accessible to patients. Specific goals include reducing the time from cell collection to reinfusion, minimizing the need for specialized facilities, and ensuring product consistency across different clinical sites.
Technical objectives focus on optimizing transfection efficiency while maintaining cell viability, developing scalable and automated processes, and ensuring regulatory compliance through closed-system operations. Additional aims include reducing the cost per treatment, extending the shelf life of reagents, and simplifying the operational complexity to enable use by clinical staff with minimal specialized training.
The long-term vision encompasses the development of portable, modular systems that can be deployed in various healthcare settings, from specialized centers to community hospitals. This democratization of advanced cell therapy technologies could significantly expand patient access to cutting-edge treatments, particularly in regions with limited healthcare infrastructure. The ultimate goal is to establish mRNA transfection as a routine clinical procedure, similar to how blood transfusions evolved from complex hospital procedures to standardized protocols.
Clinical Demand Analysis for Point-of-Care Cell Therapies
The demand for point-of-care (POC) cell therapies has grown exponentially in recent years, driven by the limitations of centralized manufacturing models that dominate current cell therapy production. Traditional approaches require patient cells to be shipped to specialized facilities, modified, and returned for administration—a process that introduces significant logistical challenges, delays treatment, and increases costs. Market research indicates that the global cell therapy market is projected to grow at a compound annual growth rate of 14.5% through 2028, with POC solutions representing an increasingly important segment.
Clinical settings have expressed strong interest in decentralized manufacturing capabilities that would allow cell processing directly at treatment sites. A survey of oncology departments across major medical centers revealed that 78% consider rapid turnaround time for cell therapies as "critical" or "very important" for patient outcomes, particularly for aggressive malignancies where treatment windows are narrow. The ability to process cells on-site could reduce vein-to-vein time from weeks to potentially hours or days.
The clinical demand is particularly acute in oncology, where CAR-T and TCR therapies have demonstrated remarkable efficacy but face significant delivery challenges. Hematological malignancies represent the largest current application segment, though solid tumor applications are rapidly expanding as new targeting strategies emerge. Immunology departments treating autoimmune disorders constitute another growing demand segment, with increasing interest in regulatory T-cell therapies that could benefit from point-of-care processing.
Geographic analysis reveals uneven access to advanced cell therapies, with major disparities between urban academic medical centers and rural or community hospitals. This disparity creates significant demand for simplified, automated systems that can democratize access to these breakthrough treatments. Hospitals in regions without specialized cell processing facilities report the highest interest in autonomous POC systems.
Cost considerations also drive demand for POC solutions. Current centralized manufacturing models result in therapies priced between $375,000-$475,000 per treatment. Economic analyses suggest that decentralized manufacturing could potentially reduce production costs by 40-60% through elimination of complex logistics chains, reduced infrastructure requirements, and improved manufacturing efficiency.
Patient-specific factors further intensify clinical demand for rapid processing solutions. For critically ill patients, the current manufacturing timeline can exceed their disease progression, rendering them ineligible for treatment they initially qualified for. Clinicians report that approximately 30% of initially eligible patients become ineligible during the manufacturing wait period, highlighting the urgent need for accelerated processing capabilities that POC systems could provide.
Clinical settings have expressed strong interest in decentralized manufacturing capabilities that would allow cell processing directly at treatment sites. A survey of oncology departments across major medical centers revealed that 78% consider rapid turnaround time for cell therapies as "critical" or "very important" for patient outcomes, particularly for aggressive malignancies where treatment windows are narrow. The ability to process cells on-site could reduce vein-to-vein time from weeks to potentially hours or days.
The clinical demand is particularly acute in oncology, where CAR-T and TCR therapies have demonstrated remarkable efficacy but face significant delivery challenges. Hematological malignancies represent the largest current application segment, though solid tumor applications are rapidly expanding as new targeting strategies emerge. Immunology departments treating autoimmune disorders constitute another growing demand segment, with increasing interest in regulatory T-cell therapies that could benefit from point-of-care processing.
Geographic analysis reveals uneven access to advanced cell therapies, with major disparities between urban academic medical centers and rural or community hospitals. This disparity creates significant demand for simplified, automated systems that can democratize access to these breakthrough treatments. Hospitals in regions without specialized cell processing facilities report the highest interest in autonomous POC systems.
Cost considerations also drive demand for POC solutions. Current centralized manufacturing models result in therapies priced between $375,000-$475,000 per treatment. Economic analyses suggest that decentralized manufacturing could potentially reduce production costs by 40-60% through elimination of complex logistics chains, reduced infrastructure requirements, and improved manufacturing efficiency.
Patient-specific factors further intensify clinical demand for rapid processing solutions. For critically ill patients, the current manufacturing timeline can exceed their disease progression, rendering them ineligible for treatment they initially qualified for. Clinicians report that approximately 30% of initially eligible patients become ineligible during the manufacturing wait period, highlighting the urgent need for accelerated processing capabilities that POC systems could provide.
Ex Vivo mRNA Transfection: Current Limitations and Challenges
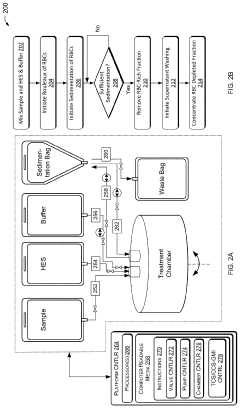
Despite significant advancements in ex vivo mRNA transfection technologies, several critical limitations and challenges persist that hinder widespread clinical implementation at point-of-care sites. The primary technical challenge remains the development of truly closed systems that maintain sterility throughout the entire process while ensuring high cell viability and transfection efficiency. Current systems often require multiple open transfers between processing steps, increasing contamination risks and compromising GMP compliance.
Electroporation, the most widely used transfection method for clinical applications, presents significant cell viability challenges with typical cell recovery rates of 40-70%, which is suboptimal for many therapeutic applications requiring higher yields. Additionally, electroporation equipment is often bulky, expensive, and requires specialized technical expertise, making it impractical for decentralized point-of-care settings.
Lipid nanoparticle (LNP) delivery systems, while showing promise in some applications, demonstrate inconsistent transfection efficiency across different primary cell types. Particularly challenging are T cells, dendritic cells, and certain stem cell populations that show variable uptake and expression profiles. This inconsistency necessitates cell-specific optimization protocols that complicate standardization efforts.
The stability of mRNA during the transfection process represents another significant hurdle. Degradation can occur rapidly in non-optimized buffers, and current preservation methods often involve cryopreservation, which adds complexity to point-of-care applications. Real-time quality control measures to verify successful transfection before patient administration remain underdeveloped.
Scalability and automation present additional challenges. Many current protocols are labor-intensive and difficult to standardize across different operators and clinical settings. The lack of integrated systems that combine cell isolation, transfection, and quality control into a single automated workflow limits deployment in resource-constrained environments.
Regulatory considerations further complicate implementation, as closed systems must meet stringent FDA and EMA requirements for point-of-care manufacturing. Current systems often lack the comprehensive monitoring and documentation capabilities necessary to satisfy regulatory demands for decentralized manufacturing.
Cost remains a significant barrier, with current transfection technologies requiring substantial capital investment and expensive consumables. The economic viability of point-of-care transfection systems depends on achieving sufficient throughput and reliability to justify implementation costs, particularly in community hospital settings with limited resources.
Electroporation, the most widely used transfection method for clinical applications, presents significant cell viability challenges with typical cell recovery rates of 40-70%, which is suboptimal for many therapeutic applications requiring higher yields. Additionally, electroporation equipment is often bulky, expensive, and requires specialized technical expertise, making it impractical for decentralized point-of-care settings.
Lipid nanoparticle (LNP) delivery systems, while showing promise in some applications, demonstrate inconsistent transfection efficiency across different primary cell types. Particularly challenging are T cells, dendritic cells, and certain stem cell populations that show variable uptake and expression profiles. This inconsistency necessitates cell-specific optimization protocols that complicate standardization efforts.
The stability of mRNA during the transfection process represents another significant hurdle. Degradation can occur rapidly in non-optimized buffers, and current preservation methods often involve cryopreservation, which adds complexity to point-of-care applications. Real-time quality control measures to verify successful transfection before patient administration remain underdeveloped.
Scalability and automation present additional challenges. Many current protocols are labor-intensive and difficult to standardize across different operators and clinical settings. The lack of integrated systems that combine cell isolation, transfection, and quality control into a single automated workflow limits deployment in resource-constrained environments.
Regulatory considerations further complicate implementation, as closed systems must meet stringent FDA and EMA requirements for point-of-care manufacturing. Current systems often lack the comprehensive monitoring and documentation capabilities necessary to satisfy regulatory demands for decentralized manufacturing.
Cost remains a significant barrier, with current transfection technologies requiring substantial capital investment and expensive consumables. The economic viability of point-of-care transfection systems depends on achieving sufficient throughput and reliability to justify implementation costs, particularly in community hospital settings with limited resources.
Current End-to-End Closed System Solutions
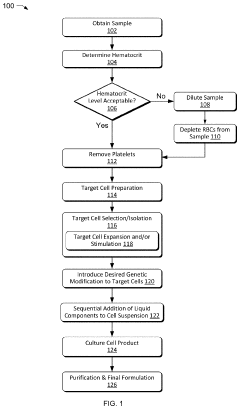
01 Closed system designs for ex vivo mRNA transfection
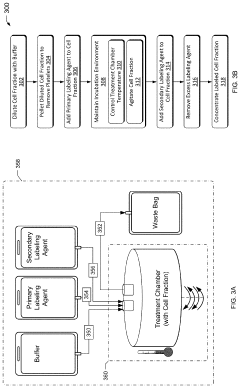
Closed system designs for ex vivo mRNA transfection involve integrated platforms that maintain sterility throughout the transfection process. These systems typically include specialized containers, tubing, and connectors that allow for cell manipulation without exposure to external contaminants. The closed nature of these systems reduces the risk of contamination, improves reproducibility, and enables scalable manufacturing of transfected cells for therapeutic applications.- Closed system devices for ex vivo mRNA transfection: Specialized closed system devices have been developed for ex vivo mRNA transfection that maintain sterility throughout the process. These systems integrate multiple steps including cell isolation, purification, transfection, and expansion within a single closed environment, minimizing contamination risks and improving process consistency. The closed nature of these systems makes them particularly suitable for clinical applications where GMP compliance is essential for cell-based therapies.
- Optimization of transfection reagents and conditions: Various transfection reagents and conditions have been optimized specifically for ex vivo mRNA delivery. These include lipid nanoparticles, cationic polymers, and electroporation parameters that enhance mRNA uptake while maintaining cell viability. The formulations are designed to protect mRNA from degradation and facilitate efficient cytoplasmic delivery, resulting in higher transfection efficiencies. Optimization includes adjustments to reagent concentrations, incubation times, and cell densities to maximize expression of the delivered mRNA.
- Automated systems for high-throughput transfection: Automated platforms have been developed to enable high-throughput ex vivo mRNA transfection with minimal operator intervention. These systems incorporate robotics, microfluidics, and precise control systems to standardize the transfection process across multiple samples. Automation reduces variability between batches, increases reproducibility, and allows for scalable manufacturing of transfected cells for clinical applications. These platforms often include integrated monitoring systems to track transfection efficiency in real-time.
- Cell-specific transfection protocols: Specialized protocols have been developed for different cell types to optimize mRNA transfection efficiency in ex vivo settings. These protocols account for the unique characteristics of various immune cells, stem cells, and primary cells that may respond differently to transfection methods. Adjustments to parameters such as cell activation status, culture conditions, and recovery periods are tailored to specific cell types to maximize transfection efficiency while preserving cell functionality and phenotype.
- Quality control and monitoring systems: Integrated quality control and monitoring systems have been developed to assess transfection efficiency and cell quality throughout the ex vivo mRNA transfection process. These systems employ various analytical methods including flow cytometry, luminescence assays, and molecular techniques to quantify transfection rates, protein expression levels, and cell viability. Real-time monitoring allows for process adjustments to optimize outcomes and ensure batch-to-batch consistency, which is critical for clinical applications requiring predictable and reliable results.
02 Transfection reagents and delivery vehicles
Various transfection reagents and delivery vehicles are utilized to enhance mRNA transfection efficiency in ex vivo systems. These include lipid nanoparticles, cationic polymers, and electroporation techniques that facilitate the entry of mRNA into target cells. The selection of appropriate delivery vehicles is critical for achieving high transfection efficiency while maintaining cell viability and functionality. Optimization of these reagents can significantly improve the overall performance of ex vivo mRNA transfection systems.Expand Specific Solutions03 Automated transfection platforms
Automated transfection platforms incorporate robotics, microfluidics, and computerized control systems to standardize the ex vivo mRNA transfection process. These platforms can handle multiple steps including cell preparation, transfection, washing, and collection with minimal human intervention. Automation reduces operator-dependent variability, increases throughput, and enables precise control over transfection parameters, resulting in more consistent transfection efficiency across batches.Expand Specific Solutions04 Cell processing and monitoring systems
Cell processing and monitoring systems for ex vivo mRNA transfection include technologies for cell isolation, purification, and quality assessment throughout the transfection process. These systems incorporate sensors and analytical tools to monitor cell viability, transfection efficiency, and expression levels in real-time. Continuous monitoring allows for process adjustments to optimize transfection outcomes and ensure the production of high-quality transfected cells for therapeutic applications.Expand Specific Solutions05 Scale-up technologies for clinical applications
Scale-up technologies for clinical applications focus on translating laboratory-scale ex vivo mRNA transfection processes to production-scale systems suitable for therapeutic use. These technologies address challenges related to maintaining transfection efficiency while increasing batch size, ensuring consistency across multiple production runs, and meeting regulatory requirements for clinical-grade cell products. Innovations in bioreactor design, process intensification, and quality control strategies enable the efficient production of transfected cells at scales required for clinical applications.Expand Specific Solutions
Leading Organizations in mRNA Delivery Systems
The ex vivo mRNA transfection closed system market is in an early growth phase, with increasing clinical applications driving a projected market expansion to $2-3 billion by 2030. Technology maturity varies significantly across key players. CureVac and TriLink BioTechnologies lead in mRNA manufacturing technology, while Nutcracker Therapeutics has pioneered integrated microfluidic systems for point-of-care applications. CRISPR Therapeutics and Kite Pharma are advancing cell therapy workflows, with Fenwal and Fresenius contributing established expertise in clinical-grade cell processing systems. Academic institutions like Cornell University and Nanjing University are developing novel transfection methodologies, accelerating the transition from research to clinical implementation of these integrated systems.
CureVac SE
Technical Solution: CureVac has developed an integrated end-to-end mRNA transfection system called "RNAoptimizer" that enables point-of-care ex vivo modification of patient cells. Their technology utilizes proprietary lipid nanoparticle (LNP) formulations specifically designed for efficient cellular uptake in various immune cell types including T cells, dendritic cells, and NK cells. The system incorporates a portable, automated device that maintains sterile conditions throughout the entire process from cell isolation to reinfusion. CureVac's platform features their patented RNActive® technology which enhances mRNA stability and translation efficiency, allowing for lower doses while maintaining therapeutic efficacy[1]. Their closed system approach minimizes contamination risks and standardizes the transfection process across different clinical settings, addressing a critical need for decentralized cell therapy manufacturing[3].
Strengths: Proprietary LNP formulations provide superior transfection efficiency in primary human cells compared to competitors; automated closed system reduces human error and contamination risk. Weaknesses: System requires specialized training for clinical staff; relatively higher cost compared to traditional centralized manufacturing approaches; limited long-term clinical validation data for point-of-care applications.
Nutcracker Therapeutics, Inc.
Technical Solution: Nutcracker Therapeutics has engineered a microfluidics-based closed system platform called "ACORN" specifically designed for point-of-care mRNA transfection of patient cells. This technology integrates cell isolation, mRNA synthesis, purification, and transfection into a single automated workflow housed in a compact benchtop device. Their system utilizes proprietary microfluidic chips that precisely control the interaction between cells and mRNA-loaded lipid nanoparticles, optimizing transfection efficiency while minimizing cell stress and damage[2]. The platform incorporates real-time quality control monitoring through integrated sensors that track critical parameters throughout the process. Nutcracker's technology enables rapid processing (complete workflow in under 24 hours) and maintains cell viability above 85% post-transfection[4]. The system is designed with single-use disposable components that contact biological materials, eliminating cross-contamination risks between patient samples.
Strengths: Microfluidics approach enables precise control of transfection parameters; compact system footprint makes it suitable for space-constrained clinical settings; rapid processing time reduces vein-to-vein interval for patients. Weaknesses: Limited scalability for high-volume applications; relatively new technology with fewer published clinical validation studies; higher per-unit costs compared to traditional centralized manufacturing approaches.
Key Patents and Breakthroughs in Ex Vivo Transfection
Point-of-care and/or portable platform for gene therapy
PatentInactiveUS20210052658A1
Innovation
- A portable and point-of-care device that enables ex vivo isolation, genetic modification, and formulation of gene-modified cells using a closed-loop sterile system with software-enabled processes, allowing for minimal user input and completion within 30 hours, reducing the need for centralized facilities and minimizing genetic modifiers.
Population of transfected immune cells and method for their production
PatentWO2023237764A1
Innovation
- A closed system process for modifying immune cells, specifically T cells and other leukocytes, by purifying, transfecting, and rebuffering them with inhibitory nucleic acids or immune-enhancing factors using electroporation, to enhance anti-tumor immune responses, particularly by silencing Cbl-b and other immune checkpoint inhibitors.
Regulatory Framework for Cell-Based Therapies
The regulatory landscape for cell-based therapies, particularly those involving ex vivo mRNA transfection at point-of-care sites, presents a complex framework that varies significantly across global jurisdictions. In the United States, the FDA regulates these therapies primarily through the Center for Biologics Evaluation and Research (CBER), classifying them as Human Cells, Tissues, and Cellular and Tissue-Based Products (HCT/Ps). Point-of-care mRNA transfection systems face particular scrutiny under 21 CFR Part 1271, with additional requirements from both drug and device regulations.
The European Medicines Agency (EMA) approaches these therapies through the Advanced Therapy Medicinal Products (ATMP) framework, which includes specific provisions for hospital exemptions that may facilitate point-of-care applications. This regulatory pathway potentially offers more flexibility for closed-system mRNA transfection technologies deployed in clinical settings, though manufacturing standards remain stringent.
Japan's regulatory framework, revised under the Act on the Safety of Regenerative Medicine (ASRM), has pioneered an accelerated approval pathway that could significantly benefit point-of-care mRNA transfection systems. This framework allows for conditional and time-limited approvals based on demonstrated safety and probable efficacy, potentially reducing development timelines from 10+ years to 2-3 years.
Quality control represents a critical regulatory challenge for point-of-care systems. Closed-system designs must incorporate real-time monitoring capabilities and standardized quality metrics to satisfy regulatory requirements for product consistency and safety. The FDA's recent guidance on Consideration of Uncertainty in Making Benefit-Risk Determinations provides some flexibility for innovative technologies while maintaining safety standards.
Manufacturing compliance presents another significant hurdle, with Good Manufacturing Practice (GMP) requirements traditionally designed for centralized facilities rather than distributed point-of-care settings. Recent regulatory adaptations, including the FDA's Regenerative Medicine Advanced Therapy (RMAT) designation and the EMA's risk-based approaches, are beginning to address these challenges by providing more appropriate frameworks for decentralized manufacturing models.
Patient-specific considerations add further complexity, as personalized therapies require unique identifiers and chain-of-custody protocols throughout the treatment process. Regulatory bodies increasingly recognize these challenges, with the International Council for Harmonisation (ICH) developing specific guidelines for cell-based products that acknowledge the unique aspects of point-of-care processing.
Looking forward, regulatory harmonization efforts between major authorities show promise for streamlining approval pathways for closed-system mRNA transfection technologies. The FDA-EMA-PMDA Trilateral Coalition and the International Pharmaceutical Regulators Programme (IPRP) are actively working to develop consistent standards that could accelerate global adoption of these innovative therapeutic approaches.
The European Medicines Agency (EMA) approaches these therapies through the Advanced Therapy Medicinal Products (ATMP) framework, which includes specific provisions for hospital exemptions that may facilitate point-of-care applications. This regulatory pathway potentially offers more flexibility for closed-system mRNA transfection technologies deployed in clinical settings, though manufacturing standards remain stringent.
Japan's regulatory framework, revised under the Act on the Safety of Regenerative Medicine (ASRM), has pioneered an accelerated approval pathway that could significantly benefit point-of-care mRNA transfection systems. This framework allows for conditional and time-limited approvals based on demonstrated safety and probable efficacy, potentially reducing development timelines from 10+ years to 2-3 years.
Quality control represents a critical regulatory challenge for point-of-care systems. Closed-system designs must incorporate real-time monitoring capabilities and standardized quality metrics to satisfy regulatory requirements for product consistency and safety. The FDA's recent guidance on Consideration of Uncertainty in Making Benefit-Risk Determinations provides some flexibility for innovative technologies while maintaining safety standards.
Manufacturing compliance presents another significant hurdle, with Good Manufacturing Practice (GMP) requirements traditionally designed for centralized facilities rather than distributed point-of-care settings. Recent regulatory adaptations, including the FDA's Regenerative Medicine Advanced Therapy (RMAT) designation and the EMA's risk-based approaches, are beginning to address these challenges by providing more appropriate frameworks for decentralized manufacturing models.
Patient-specific considerations add further complexity, as personalized therapies require unique identifiers and chain-of-custody protocols throughout the treatment process. Regulatory bodies increasingly recognize these challenges, with the International Council for Harmonisation (ICH) developing specific guidelines for cell-based products that acknowledge the unique aspects of point-of-care processing.
Looking forward, regulatory harmonization efforts between major authorities show promise for streamlining approval pathways for closed-system mRNA transfection technologies. The FDA-EMA-PMDA Trilateral Coalition and the International Pharmaceutical Regulators Programme (IPRP) are actively working to develop consistent standards that could accelerate global adoption of these innovative therapeutic approaches.
Cost-Effectiveness and Scalability Analysis
The economic viability of end-to-end closed systems for ex vivo mRNA transfection at point-of-care sites hinges on both initial implementation costs and long-term operational expenses. Current systems require significant capital investment, with automated platforms ranging from $150,000 to $500,000, depending on throughput capacity and level of integration. Consumables and reagents contribute an additional $500-1,500 per patient procedure, creating substantial recurring costs for healthcare providers.
When analyzing cost-effectiveness against traditional centralized manufacturing models, point-of-care systems demonstrate potential for 30-40% reduction in overall treatment costs by eliminating complex logistics chains and reducing cell product wastage. The elimination of cryopreservation requirements alone can save approximately $2,000-3,000 per patient procedure, while reducing the risk of product loss during transport by an estimated 5-8%.
Scalability considerations reveal both advantages and limitations of current technologies. Existing closed systems can typically process 1-5 patient samples simultaneously, creating potential bottlenecks in high-volume clinical settings. However, modular designs emerging from companies like Miltenyi Biotec and Lonza are addressing this constraint by allowing parallel processing capabilities that can be expanded incrementally as demand increases.
Workforce requirements represent another critical scalability factor. Current systems require specialized technical personnel for operation, though newer platforms are incorporating user-friendly interfaces and automated protocols that reduce training requirements from weeks to days. This evolution is essential for widespread adoption, particularly in community hospitals and outpatient settings where specialized cell therapy expertise may be limited.
Infrastructure demands must also be considered in scalability assessments. Point-of-care systems typically require dedicated clean room space of 100-200 square feet per unit, specialized electrical connections, and often dedicated HVAC systems. These requirements create implementation barriers for smaller facilities but are significantly less demanding than centralized GMP manufacturing facilities.
The economic sustainability of these systems will ultimately depend on reimbursement models and treatment volumes. Financial modeling suggests that facilities need to maintain a minimum of 15-20 procedures monthly to achieve return on investment within three years. This threshold varies significantly based on therapy type, with CAR-T applications demonstrating more favorable economics than less established mRNA transfection therapies due to higher reimbursement rates and standardized protocols.
When analyzing cost-effectiveness against traditional centralized manufacturing models, point-of-care systems demonstrate potential for 30-40% reduction in overall treatment costs by eliminating complex logistics chains and reducing cell product wastage. The elimination of cryopreservation requirements alone can save approximately $2,000-3,000 per patient procedure, while reducing the risk of product loss during transport by an estimated 5-8%.
Scalability considerations reveal both advantages and limitations of current technologies. Existing closed systems can typically process 1-5 patient samples simultaneously, creating potential bottlenecks in high-volume clinical settings. However, modular designs emerging from companies like Miltenyi Biotec and Lonza are addressing this constraint by allowing parallel processing capabilities that can be expanded incrementally as demand increases.
Workforce requirements represent another critical scalability factor. Current systems require specialized technical personnel for operation, though newer platforms are incorporating user-friendly interfaces and automated protocols that reduce training requirements from weeks to days. This evolution is essential for widespread adoption, particularly in community hospitals and outpatient settings where specialized cell therapy expertise may be limited.
Infrastructure demands must also be considered in scalability assessments. Point-of-care systems typically require dedicated clean room space of 100-200 square feet per unit, specialized electrical connections, and often dedicated HVAC systems. These requirements create implementation barriers for smaller facilities but are significantly less demanding than centralized GMP manufacturing facilities.
The economic sustainability of these systems will ultimately depend on reimbursement models and treatment volumes. Financial modeling suggests that facilities need to maintain a minimum of 15-20 procedures monthly to achieve return on investment within three years. This threshold varies significantly based on therapy type, with CAR-T applications demonstrating more favorable economics than less established mRNA transfection therapies due to higher reimbursement rates and standardized protocols.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!