Monitoring immunostimulatory impurities in mRNA batches: methods and acceptance criteria
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
mRNA Impurity Detection Background and Objectives
The evolution of messenger RNA (mRNA) therapeutics has witnessed remarkable progress over the past decade, culminating in the unprecedented rapid development and deployment of mRNA vaccines during the COVID-19 pandemic. This technological breakthrough has positioned mRNA as a promising platform for various therapeutic applications beyond vaccines, including protein replacement therapies and cancer immunotherapies.
Despite these advances, the manufacturing of pharmaceutical-grade mRNA presents significant quality control challenges. Immunostimulatory impurities in mRNA batches can trigger unintended immune responses, potentially leading to adverse effects and compromised therapeutic efficacy. These impurities may originate from various sources throughout the production process, including incomplete in vitro transcription, degradation products, double-stranded RNA contaminants, and residual DNA templates.
The regulatory landscape for mRNA therapeutics is still evolving, with agencies like the FDA and EMA working to establish comprehensive guidelines for quality control standards. Current regulatory frameworks emphasize the importance of thorough characterization and control of impurities, yet specific acceptance criteria for immunostimulatory contaminants remain under development as the field matures.
Detection and quantification of these impurities present technical challenges due to their diverse nature and the sensitivity required for meaningful analysis. Conventional analytical methods often lack the specificity or sensitivity needed to identify and quantify immunostimulatory components at clinically relevant concentrations, necessitating the development of novel analytical approaches.
The primary objective of this technical research is to comprehensively evaluate existing and emerging methodologies for monitoring immunostimulatory impurities in mRNA batches. This includes assessing analytical techniques such as high-performance liquid chromatography (HPLC), mass spectrometry, next-generation sequencing, and immunological assays for their applicability, sensitivity, specificity, and reproducibility in detecting relevant impurities.
Furthermore, this research aims to propose evidence-based acceptance criteria that balance safety considerations with manufacturing feasibility. These criteria must account for the relationship between impurity levels and immunological responses, considering both innate and adaptive immune activation pathways. The ultimate goal is to establish robust quality control parameters that ensure consistent safety and efficacy profiles across mRNA batches.
By addressing these challenges, we seek to contribute to the standardization of mRNA manufacturing processes and quality control protocols, thereby facilitating the broader adoption of mRNA therapeutics across various disease indications while maintaining the highest standards of patient safety.
Despite these advances, the manufacturing of pharmaceutical-grade mRNA presents significant quality control challenges. Immunostimulatory impurities in mRNA batches can trigger unintended immune responses, potentially leading to adverse effects and compromised therapeutic efficacy. These impurities may originate from various sources throughout the production process, including incomplete in vitro transcription, degradation products, double-stranded RNA contaminants, and residual DNA templates.
The regulatory landscape for mRNA therapeutics is still evolving, with agencies like the FDA and EMA working to establish comprehensive guidelines for quality control standards. Current regulatory frameworks emphasize the importance of thorough characterization and control of impurities, yet specific acceptance criteria for immunostimulatory contaminants remain under development as the field matures.
Detection and quantification of these impurities present technical challenges due to their diverse nature and the sensitivity required for meaningful analysis. Conventional analytical methods often lack the specificity or sensitivity needed to identify and quantify immunostimulatory components at clinically relevant concentrations, necessitating the development of novel analytical approaches.
The primary objective of this technical research is to comprehensively evaluate existing and emerging methodologies for monitoring immunostimulatory impurities in mRNA batches. This includes assessing analytical techniques such as high-performance liquid chromatography (HPLC), mass spectrometry, next-generation sequencing, and immunological assays for their applicability, sensitivity, specificity, and reproducibility in detecting relevant impurities.
Furthermore, this research aims to propose evidence-based acceptance criteria that balance safety considerations with manufacturing feasibility. These criteria must account for the relationship between impurity levels and immunological responses, considering both innate and adaptive immune activation pathways. The ultimate goal is to establish robust quality control parameters that ensure consistent safety and efficacy profiles across mRNA batches.
By addressing these challenges, we seek to contribute to the standardization of mRNA manufacturing processes and quality control protocols, thereby facilitating the broader adoption of mRNA therapeutics across various disease indications while maintaining the highest standards of patient safety.
Market Demand for mRNA Quality Control
The mRNA therapeutics and vaccine market has experienced unprecedented growth following the successful deployment of COVID-19 vaccines, creating an urgent demand for robust quality control mechanisms. The global mRNA therapeutics market, valued at $39.9 billion in 2022, is projected to reach $91.2 billion by 2028, representing a compound annual growth rate of 14.6%. This rapid expansion has intensified the need for sophisticated quality control processes specifically targeting immunostimulatory impurities in mRNA products.
Pharmaceutical companies and contract manufacturing organizations (CMOs) are increasingly seeking advanced analytical methods to detect and quantify immunostimulatory impurities, as these contaminants can trigger unwanted immune responses, compromising both safety and efficacy of mRNA-based products. Regulatory bodies, including the FDA and EMA, have heightened their scrutiny of mRNA product quality, creating market pressure for standardized testing protocols and acceptance criteria.
The demand for mRNA quality control is further driven by the diversification of therapeutic applications beyond vaccines. As mRNA technologies expand into cancer immunotherapy, protein replacement therapies, and genetic disorder treatments, manufacturers require more sensitive and specific analytical methods to ensure product consistency across different therapeutic modalities.
Investors and stakeholders in the biopharmaceutical sector have demonstrated willingness to fund research and development of novel quality control technologies, with venture capital investments in mRNA manufacturing and quality control reaching $4.7 billion in 2022 alone. This financial backing reflects market recognition of quality control as a critical factor in commercial success.
End-users, including healthcare providers and patients, are increasingly aware of product quality issues, creating downstream pressure for manufacturers to implement comprehensive quality control measures. Market research indicates that 78% of healthcare professionals consider manufacturing quality and purity profiles when selecting between competing mRNA products.
The competitive landscape is evolving rapidly, with specialized analytical service providers emerging to meet the growing demand for mRNA quality testing. These companies offer proprietary technologies for detecting immunostimulatory impurities, creating a secondary market estimated at $1.2 billion annually.
Geographically, North America dominates the market demand for mRNA quality control solutions (42%), followed by Europe (31%) and Asia-Pacific (21%), with the latter showing the fastest growth rate as regional manufacturing capabilities expand. This global distribution reflects the worldwide adoption of mRNA technologies and the universal need for standardized quality control methodologies.
Pharmaceutical companies and contract manufacturing organizations (CMOs) are increasingly seeking advanced analytical methods to detect and quantify immunostimulatory impurities, as these contaminants can trigger unwanted immune responses, compromising both safety and efficacy of mRNA-based products. Regulatory bodies, including the FDA and EMA, have heightened their scrutiny of mRNA product quality, creating market pressure for standardized testing protocols and acceptance criteria.
The demand for mRNA quality control is further driven by the diversification of therapeutic applications beyond vaccines. As mRNA technologies expand into cancer immunotherapy, protein replacement therapies, and genetic disorder treatments, manufacturers require more sensitive and specific analytical methods to ensure product consistency across different therapeutic modalities.
Investors and stakeholders in the biopharmaceutical sector have demonstrated willingness to fund research and development of novel quality control technologies, with venture capital investments in mRNA manufacturing and quality control reaching $4.7 billion in 2022 alone. This financial backing reflects market recognition of quality control as a critical factor in commercial success.
End-users, including healthcare providers and patients, are increasingly aware of product quality issues, creating downstream pressure for manufacturers to implement comprehensive quality control measures. Market research indicates that 78% of healthcare professionals consider manufacturing quality and purity profiles when selecting between competing mRNA products.
The competitive landscape is evolving rapidly, with specialized analytical service providers emerging to meet the growing demand for mRNA quality testing. These companies offer proprietary technologies for detecting immunostimulatory impurities, creating a secondary market estimated at $1.2 billion annually.
Geographically, North America dominates the market demand for mRNA quality control solutions (42%), followed by Europe (31%) and Asia-Pacific (21%), with the latter showing the fastest growth rate as regional manufacturing capabilities expand. This global distribution reflects the worldwide adoption of mRNA technologies and the universal need for standardized quality control methodologies.
Current Challenges in Immunostimulatory Impurity Detection
Despite significant advancements in mRNA vaccine and therapeutic production, the detection and monitoring of immunostimulatory impurities remain a critical challenge for manufacturers and regulatory bodies. Current analytical methods struggle with sensitivity limitations when identifying trace amounts of double-stranded RNA (dsRNA), residual DNA templates, and other immunogenic contaminants that can trigger undesired immune responses in patients.
The heterogeneous nature of these impurities presents a fundamental obstacle to standardized detection. Immunostimulatory contaminants vary widely in structure, size, and concentration, making comprehensive characterization through a single analytical technique virtually impossible. This complexity necessitates multiple complementary methods, increasing both cost and time requirements for quality control processes.
Regulatory frameworks compound these challenges by lacking harmonized acceptance criteria across different regions. The FDA, EMA, and other regulatory bodies maintain varying thresholds for impurity levels, creating compliance difficulties for companies operating in global markets. This regulatory inconsistency stems partly from the relatively recent emergence of mRNA as a therapeutic platform and insufficient long-term safety data to establish definitive standards.
Technical limitations of current analytical methods further exacerbate detection challenges. While techniques like HPLC-MS offer high sensitivity, they often require extensive sample preparation that can alter impurity profiles. Immunoassays provide specificity but may miss novel or unexpected contaminants. Next-generation sequencing approaches offer comprehensive analysis but remain prohibitively expensive and time-consuming for routine quality control.
Scale-up issues present additional complications as manufacturers transition from research to commercial production. Methods that perform adequately at laboratory scale frequently encounter sensitivity and reproducibility problems when applied to industrial-scale batches. This scalability gap creates significant risk during technology transfer and commercial manufacturing implementation.
The correlation between in vitro analytical results and in vivo immunogenicity remains poorly understood. Current methods may detect impurities without providing clear insights into their clinical relevance, leading to potentially unnecessary rejections of safe batches or, more concerning, approvals of problematic ones. This disconnect between analytical detection and biological significance represents perhaps the most fundamental challenge in the field.
Real-time monitoring capabilities are also severely limited, with most current methods requiring offline analysis that introduces delays in production workflows. This temporal gap between production and quality assessment increases manufacturing risks and costs, particularly for time-sensitive mRNA products with limited stability profiles.
AI and machine learning approaches show promise but remain in early development stages, hampered by limited training datasets and the inherent complexity of immunostimulatory mechanisms. These computational challenges reflect the broader scientific uncertainty surrounding the precise relationship between specific impurity profiles and adverse immune responses.
The heterogeneous nature of these impurities presents a fundamental obstacle to standardized detection. Immunostimulatory contaminants vary widely in structure, size, and concentration, making comprehensive characterization through a single analytical technique virtually impossible. This complexity necessitates multiple complementary methods, increasing both cost and time requirements for quality control processes.
Regulatory frameworks compound these challenges by lacking harmonized acceptance criteria across different regions. The FDA, EMA, and other regulatory bodies maintain varying thresholds for impurity levels, creating compliance difficulties for companies operating in global markets. This regulatory inconsistency stems partly from the relatively recent emergence of mRNA as a therapeutic platform and insufficient long-term safety data to establish definitive standards.
Technical limitations of current analytical methods further exacerbate detection challenges. While techniques like HPLC-MS offer high sensitivity, they often require extensive sample preparation that can alter impurity profiles. Immunoassays provide specificity but may miss novel or unexpected contaminants. Next-generation sequencing approaches offer comprehensive analysis but remain prohibitively expensive and time-consuming for routine quality control.
Scale-up issues present additional complications as manufacturers transition from research to commercial production. Methods that perform adequately at laboratory scale frequently encounter sensitivity and reproducibility problems when applied to industrial-scale batches. This scalability gap creates significant risk during technology transfer and commercial manufacturing implementation.
The correlation between in vitro analytical results and in vivo immunogenicity remains poorly understood. Current methods may detect impurities without providing clear insights into their clinical relevance, leading to potentially unnecessary rejections of safe batches or, more concerning, approvals of problematic ones. This disconnect between analytical detection and biological significance represents perhaps the most fundamental challenge in the field.
Real-time monitoring capabilities are also severely limited, with most current methods requiring offline analysis that introduces delays in production workflows. This temporal gap between production and quality assessment increases manufacturing risks and costs, particularly for time-sensitive mRNA products with limited stability profiles.
AI and machine learning approaches show promise but remain in early development stages, hampered by limited training datasets and the inherent complexity of immunostimulatory mechanisms. These computational challenges reflect the broader scientific uncertainty surrounding the precise relationship between specific impurity profiles and adverse immune responses.
Established Methods for Immunostimulatory Impurity Monitoring
01 Detection methods for immunostimulatory impurities in mRNA
Various analytical techniques can be employed to detect and quantify immunostimulatory impurities in mRNA batches. These methods include high-performance liquid chromatography (HPLC), mass spectrometry, and PCR-based assays that can identify double-stranded RNA contaminants and other immunogenic byproducts. These techniques allow for sensitive detection of impurities that could trigger unwanted immune responses when administered in mRNA therapeutics.- Detection methods for immunostimulatory impurities in mRNA: Various analytical techniques can be employed to detect and quantify immunostimulatory impurities in mRNA batches. These methods include high-performance liquid chromatography (HPLC), mass spectrometry, and PCR-based assays that can identify double-stranded RNA contaminants and other immunogenic molecules. These techniques allow for sensitive detection of impurities that could trigger unwanted immune responses when present in therapeutic mRNA formulations.
- Purification strategies to reduce immunostimulatory impurities: Various purification methods have been developed to minimize immunostimulatory impurities in mRNA batches. These include chromatographic techniques, enzymatic treatments to degrade double-stranded RNA contaminants, and modified synthesis protocols. Advanced purification strategies can significantly reduce the presence of immunogenic molecules that might trigger innate immune responses, thereby improving the safety profile of mRNA-based therapeutics.
- Acceptance criteria and quality control standards: Regulatory frameworks and industry standards have established acceptance criteria for immunostimulatory impurities in mRNA products. These criteria typically include maximum allowable limits for specific contaminants, such as double-stranded RNA and endotoxins. Quality control protocols involve multiple testing points throughout the manufacturing process to ensure that the final product meets predetermined specifications for purity and safety before release for clinical use.
- Impact of immunostimulatory impurities on efficacy and safety: Immunostimulatory impurities in mRNA batches can significantly affect both the efficacy and safety profiles of mRNA-based therapeutics. These impurities can trigger innate immune responses, leading to increased production of inflammatory cytokines and potentially causing adverse reactions in patients. Understanding the relationship between specific impurities and their immunological effects is crucial for developing safer mRNA products with predictable clinical outcomes.
- Novel technologies for real-time monitoring of impurities: Emerging technologies enable real-time monitoring of immunostimulatory impurities during mRNA production. These include biosensor-based systems, automated in-line analytical tools, and artificial intelligence algorithms that can predict the immunogenicity of mRNA batches based on their impurity profiles. Real-time monitoring allows for immediate corrective actions during manufacturing, reducing batch-to-batch variability and ensuring consistent quality of mRNA therapeutics.
02 Purification strategies to remove immunostimulatory impurities
Various purification methods have been developed to remove immunostimulatory impurities from mRNA batches. These include chromatographic techniques such as affinity chromatography, size exclusion chromatography, and ion exchange chromatography. Additional methods involve enzymatic treatment with specific nucleases to degrade double-stranded RNA contaminants, and precipitation techniques to separate mRNA from impurities based on their physicochemical properties.Expand Specific Solutions03 Acceptance criteria and quality control standards
Regulatory guidelines and industry standards have been established for acceptable levels of immunostimulatory impurities in mRNA products. These criteria typically include maximum allowable concentrations of double-stranded RNA, residual DNA templates, and other contaminants that could trigger immune responses. Quality control protocols involve batch testing against these standards to ensure consistency and safety of the final product before release for clinical use.Expand Specific Solutions04 Impact of immunostimulatory impurities on mRNA efficacy and safety
Immunostimulatory impurities in mRNA batches can significantly affect both the efficacy and safety profile of mRNA therapeutics. These impurities can trigger innate immune responses through pattern recognition receptors like Toll-like receptors, leading to increased production of inflammatory cytokines. This unwanted immune activation can result in reduced translation efficiency of the therapeutic mRNA, increased side effects, and potentially altered pharmacokinetic properties of the formulated product.Expand Specific Solutions05 Novel technologies for real-time monitoring of impurities
Emerging technologies enable real-time monitoring of immunostimulatory impurities during mRNA production processes. These include biosensor-based systems, automated in-line analytical platforms, and artificial intelligence algorithms that can predict the immunostimulatory potential of different impurity profiles. These technologies allow for continuous quality verification throughout the manufacturing process rather than relying solely on end-product testing, enabling faster batch release decisions and improved process control.Expand Specific Solutions
Key Players in mRNA Analytics and Quality Control
The mRNA immunostimulatory impurities monitoring landscape is currently in a growth phase, with the market expanding rapidly due to increased mRNA therapeutic development. The global market size is projected to reach significant value as companies invest heavily in quality control technologies. Technically, the field is advancing from early-stage development toward standardization, with varying levels of maturity across detection methods. Leading players like BioNTech SE and ModernaTX have established robust monitoring platforms following their COVID-19 vaccine success, while pharmaceutical giants such as Regeneron, Sanofi Pasteur, and Novo Nordisk are investing in proprietary technologies. Companies including TriLink BioTechnologies and Cellscript are developing specialized reagents and analytical tools, while FUJIFILM and Nitto Denko are contributing advanced materials for impurity reduction. Academic-industry partnerships with institutions like University of Strasbourg are accelerating method development and validation.
ModernaTX, Inc.
Technical Solution: Moderna has developed a comprehensive platform for monitoring immunostimulatory impurities in mRNA batches that combines multiple analytical techniques. Their approach utilizes high-performance liquid chromatography (HPLC) coupled with mass spectrometry to detect and quantify double-stranded RNA (dsRNA) contaminants with high sensitivity. Additionally, they employ enzyme-linked immunosorbent assays (ELISAs) specific for pattern recognition receptors to assess the immunostimulatory potential of residual impurities. Moderna has established a proprietary purification process that includes chromatographic steps and enzymatic treatments to remove dsRNA and other immunostimulatory contaminants. Their quality control system incorporates in vitro cell-based assays using human peripheral blood mononuclear cells (PBMCs) to measure cytokine induction as a functional readout of immunostimulatory activity, allowing for batch-to-batch consistency assessment.
Strengths: Industry-leading experience with mRNA therapeutics and vaccines provides extensive historical data for establishing acceptance criteria. Their integrated analytical platform offers high sensitivity and specificity for detecting various impurity types. Weaknesses: Their proprietary methods may require specialized equipment and expertise, potentially limiting broader application across the industry. The complexity of their analytical approach may increase production costs and time-to-release for batches.
BioNTech SE
Technical Solution: BioNTech has established a multi-tiered approach for monitoring immunostimulatory impurities in mRNA batches. Their methodology centers on the detection and quantification of double-stranded RNA (dsRNA) impurities using next-generation sequencing (NGS) combined with bioinformatics analysis to identify specific sequence motifs known to trigger immune responses. They have developed a proprietary purification process that includes HPLC-based methods and enzymatic treatments to remove dsRNA contaminants. BioNTech employs reporter cell lines expressing various pattern recognition receptors (PRRs) such as TLR3, TLR7, and RIG-I to assess the immunostimulatory potential of their mRNA products. These engineered cell lines produce measurable signals when exposed to immunostimulatory impurities, allowing for quantitative assessment. Their acceptance criteria are based on correlation studies between impurity levels and clinical outcomes, establishing thresholds that ensure product safety while maintaining efficacy. BioNTech also utilizes advanced analytical techniques including capillary electrophoresis and dynamic light scattering to characterize the physical attributes of mRNA products that may influence immunogenicity.
Strengths: Their integrated approach combining genomic analysis with functional cell-based assays provides comprehensive characterization of immunostimulatory potential. The company has extensive clinical experience that informs their acceptance criteria development. Weaknesses: The reliance on proprietary cell lines may introduce variability when comparing results across different manufacturing sites. Their methods may be resource-intensive and require specialized expertise in both molecular biology and immunology.
Critical Technologies for mRNA Batch Purity Assessment
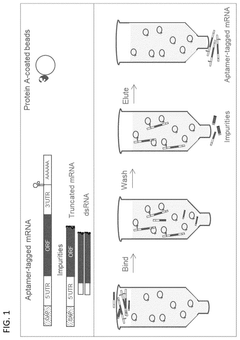
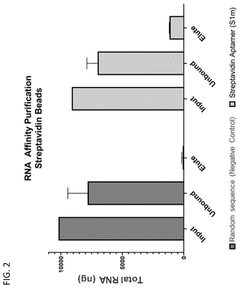
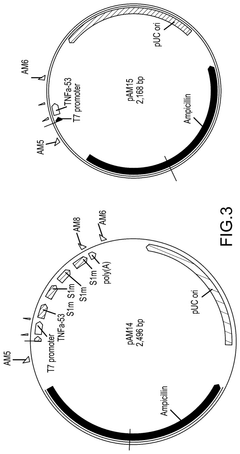
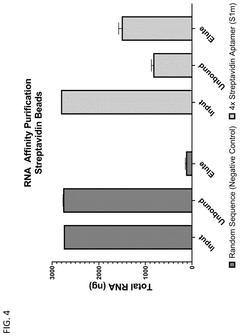
Compositions and methods for RNA affinity
PatentPendingUS20240327847A1
Innovation
- Incorporating RNA aptamers into the mRNA structure, specifically embedded in RNA scaffolds like tRNA or bioorthogonal scaffolds, which allows for affinity purification using immobilized affinity ligands on chromatography resins, effectively removing impurities while maintaining translation efficiency and minimizing immunogenicity.
Methods and therapeutics relating to mRNA biomarkers for clinical prognosis of cancer
PatentInactiveUS20170283882A1
Innovation
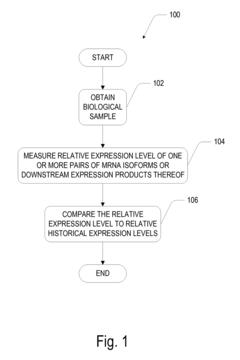
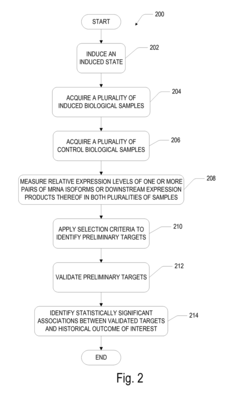
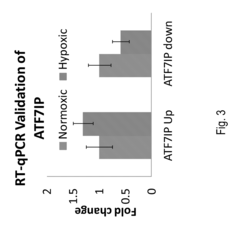
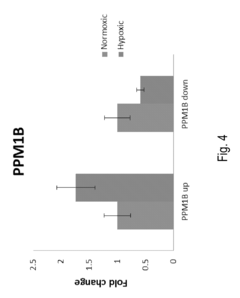
- A method involving the measurement and comparison of relative mRNA isoform expression levels in biological samples against historical or contemporary cohorts, combined with the identification of predictive targets through induced states like hypoxia, to provide accurate prognostic information and therapeutic insights.
Regulatory Framework for mRNA Therapeutics
The regulatory landscape for mRNA therapeutics has evolved significantly in recent years, particularly in response to the rapid development and deployment of mRNA vaccines during the COVID-19 pandemic. Regulatory bodies worldwide, including the FDA, EMA, and PMDA, have established frameworks that specifically address the unique characteristics and challenges of mRNA-based products.
These regulatory frameworks emphasize the critical importance of monitoring immunostimulatory impurities in mRNA batches, recognizing that such impurities can trigger unintended immune responses and potentially compromise both safety and efficacy. Double-stranded RNA (dsRNA) contaminants, residual DNA templates, and lipid nanoparticle components are particularly scrutinized due to their known immunostimulatory properties.
Current regulatory guidelines mandate comprehensive characterization of mRNA products, with specific requirements for purity assessment, impurity profiling, and batch-to-batch consistency. The FDA's guidance for industry on "Chemistry, Manufacturing, and Controls (CMC) Information for Human Gene Therapy Investigational New Drug Applications" provides a foundation, though specific mRNA-focused addendums continue to emerge.
Acceptance criteria for immunostimulatory impurities remain an evolving area, with regulatory agencies increasingly adopting risk-based approaches. These criteria typically include quantitative limits for dsRNA content (often <0.05% of total RNA), residual DNA (<10 ng per dose), and endotoxin levels (<5 EU/kg body weight). However, these thresholds may vary based on the specific application, route of administration, and target population.
Regulatory bodies are increasingly requiring manufacturers to implement validated analytical methods for detecting and quantifying immunostimulatory impurities. These methods must demonstrate sufficient sensitivity, specificity, and reproducibility to ensure reliable monitoring throughout the manufacturing process. The ICH Q2(R1) guideline on validation of analytical procedures serves as a reference point for method validation requirements.
Post-approval monitoring requirements are also becoming more stringent, with regulatory agencies mandating ongoing surveillance of immunostimulatory impurities as part of stability studies and continued process verification. This reflects the recognition that changes in manufacturing processes or storage conditions may impact impurity profiles over time.
International harmonization efforts are underway to standardize regulatory approaches to mRNA therapeutics, though significant regional variations persist. The International Coalition of Medicines Regulatory Authorities (ICMRA) has established working groups specifically focused on convergence of regulatory requirements for novel vaccine platforms, including mRNA technologies.
These regulatory frameworks emphasize the critical importance of monitoring immunostimulatory impurities in mRNA batches, recognizing that such impurities can trigger unintended immune responses and potentially compromise both safety and efficacy. Double-stranded RNA (dsRNA) contaminants, residual DNA templates, and lipid nanoparticle components are particularly scrutinized due to their known immunostimulatory properties.
Current regulatory guidelines mandate comprehensive characterization of mRNA products, with specific requirements for purity assessment, impurity profiling, and batch-to-batch consistency. The FDA's guidance for industry on "Chemistry, Manufacturing, and Controls (CMC) Information for Human Gene Therapy Investigational New Drug Applications" provides a foundation, though specific mRNA-focused addendums continue to emerge.
Acceptance criteria for immunostimulatory impurities remain an evolving area, with regulatory agencies increasingly adopting risk-based approaches. These criteria typically include quantitative limits for dsRNA content (often <0.05% of total RNA), residual DNA (<10 ng per dose), and endotoxin levels (<5 EU/kg body weight). However, these thresholds may vary based on the specific application, route of administration, and target population.
Regulatory bodies are increasingly requiring manufacturers to implement validated analytical methods for detecting and quantifying immunostimulatory impurities. These methods must demonstrate sufficient sensitivity, specificity, and reproducibility to ensure reliable monitoring throughout the manufacturing process. The ICH Q2(R1) guideline on validation of analytical procedures serves as a reference point for method validation requirements.
Post-approval monitoring requirements are also becoming more stringent, with regulatory agencies mandating ongoing surveillance of immunostimulatory impurities as part of stability studies and continued process verification. This reflects the recognition that changes in manufacturing processes or storage conditions may impact impurity profiles over time.
International harmonization efforts are underway to standardize regulatory approaches to mRNA therapeutics, though significant regional variations persist. The International Coalition of Medicines Regulatory Authorities (ICMRA) has established working groups specifically focused on convergence of regulatory requirements for novel vaccine platforms, including mRNA technologies.
Risk Assessment Strategies for mRNA Manufacturing
Risk assessment in mRNA manufacturing requires a comprehensive evaluation of potential immunostimulatory impurities that could compromise product safety and efficacy. These impurities, including double-stranded RNA (dsRNA), residual DNA templates, and endotoxins, can trigger innate immune responses that may lead to adverse events in patients or reduce therapeutic effectiveness.
The risk assessment process begins with identification of critical quality attributes (CQAs) related to immunostimulatory properties. This involves mapping the manufacturing process to identify potential sources of impurities at each step, from plasmid preparation through in vitro transcription to purification and formulation. Each identified impurity must be characterized for its immunostimulatory potential through in vitro assays measuring cytokine induction or activation of pattern recognition receptors.
A risk prioritization matrix should be established based on severity of potential immune response, likelihood of impurity presence, and detectability. High-risk impurities require more stringent control strategies and monitoring protocols. For instance, dsRNA contamination presents a high-risk profile due to its potent activation of TLR3 and RIG-I pathways, necessitating validated removal steps and sensitive detection methods.
Implementation of risk mitigation strategies involves establishing process controls at critical points where immunostimulatory impurities may be introduced or removed. This includes optimization of DNA template quality, enzymatic digestion of residual DNA, chromatographic purification steps, and filtration processes. Each control point requires validation to demonstrate consistent reduction of impurities to acceptable levels.
Continuous risk assessment throughout product development and commercial manufacturing is essential. This involves periodic reassessment as manufacturing scale increases, analytical methods improve, or regulatory expectations evolve. A risk-based approach to sampling and testing frequency should be implemented, with more intensive monitoring during process validation and initial commercial batches, potentially decreasing as process consistency is demonstrated.
Documentation of the risk assessment process, including scientific rationale for acceptance criteria and control strategies, forms a critical component of regulatory submissions. This should include correlation of impurity levels with clinical outcomes where possible, demonstrating that established acceptance criteria are protective of patient safety while ensuring product efficacy.
The risk assessment process begins with identification of critical quality attributes (CQAs) related to immunostimulatory properties. This involves mapping the manufacturing process to identify potential sources of impurities at each step, from plasmid preparation through in vitro transcription to purification and formulation. Each identified impurity must be characterized for its immunostimulatory potential through in vitro assays measuring cytokine induction or activation of pattern recognition receptors.
A risk prioritization matrix should be established based on severity of potential immune response, likelihood of impurity presence, and detectability. High-risk impurities require more stringent control strategies and monitoring protocols. For instance, dsRNA contamination presents a high-risk profile due to its potent activation of TLR3 and RIG-I pathways, necessitating validated removal steps and sensitive detection methods.
Implementation of risk mitigation strategies involves establishing process controls at critical points where immunostimulatory impurities may be introduced or removed. This includes optimization of DNA template quality, enzymatic digestion of residual DNA, chromatographic purification steps, and filtration processes. Each control point requires validation to demonstrate consistent reduction of impurities to acceptable levels.
Continuous risk assessment throughout product development and commercial manufacturing is essential. This involves periodic reassessment as manufacturing scale increases, analytical methods improve, or regulatory expectations evolve. A risk-based approach to sampling and testing frequency should be implemented, with more intensive monitoring during process validation and initial commercial batches, potentially decreasing as process consistency is demonstrated.
Documentation of the risk assessment process, including scientific rationale for acceptance criteria and control strategies, forms a critical component of regulatory submissions. This should include correlation of impurity levels with clinical outcomes where possible, demonstrating that established acceptance criteria are protective of patient safety while ensuring product efficacy.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!