Automation of complex QC workflows to meet high-throughput clinical demand: robotics + analytics
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Clinical QC Automation Background and Objectives
Quality control (QC) in clinical laboratories has evolved significantly over the past decades, transitioning from manual processes to increasingly automated systems. The integration of robotics and analytics in QC workflows represents a critical advancement in meeting the growing demands of modern healthcare. Historically, clinical laboratories have faced challenges in maintaining consistent quality while handling increasing sample volumes, with technicians performing repetitive tasks prone to human error and variability.
The evolution of clinical QC automation began with simple mechanical devices in the 1970s, progressing through semi-automated systems in the 1990s, to today's sophisticated robotic platforms integrated with advanced analytics. This technological progression has been driven by the exponential growth in testing volumes, increasingly complex assays, and heightened regulatory requirements in clinical diagnostics.
Current clinical laboratories face unprecedented pressure to deliver accurate results at scale. With the global clinical laboratory services market projected to reach $426.8 billion by 2028, growing at a CAGR of 7.2%, there is an urgent need for solutions that can maintain quality while increasing throughput. The COVID-19 pandemic further highlighted this necessity, with laboratories worldwide struggling to scale testing capabilities while maintaining rigorous quality standards.
The primary objective of automating complex QC workflows through robotics and analytics integration is to establish a scalable, reliable, and efficient quality control system capable of meeting high-throughput clinical demands. This involves developing robotic systems that can perform repetitive QC tasks with precision while implementing real-time analytics for continuous monitoring and process optimization.
Specific technical goals include reducing QC-related errors by at least 85%, increasing throughput capacity by 200-300% without compromising quality, achieving regulatory compliance through standardized processes, and implementing predictive analytics to identify potential quality issues before they impact patient results.
The technological trajectory points toward fully integrated systems where robotic hardware seamlessly interfaces with laboratory information systems (LIS) and quality management software. Machine learning algorithms are expected to play an increasingly important role in adaptive QC protocols that respond to changing conditions and identify subtle patterns indicative of potential quality issues.
This automation initiative aligns with broader healthcare trends toward precision medicine and personalized diagnostics, which require even greater testing accuracy and reproducibility. By establishing robust automated QC workflows, clinical laboratories can not only meet current demands but also position themselves for future advancements in diagnostic technologies.
The evolution of clinical QC automation began with simple mechanical devices in the 1970s, progressing through semi-automated systems in the 1990s, to today's sophisticated robotic platforms integrated with advanced analytics. This technological progression has been driven by the exponential growth in testing volumes, increasingly complex assays, and heightened regulatory requirements in clinical diagnostics.
Current clinical laboratories face unprecedented pressure to deliver accurate results at scale. With the global clinical laboratory services market projected to reach $426.8 billion by 2028, growing at a CAGR of 7.2%, there is an urgent need for solutions that can maintain quality while increasing throughput. The COVID-19 pandemic further highlighted this necessity, with laboratories worldwide struggling to scale testing capabilities while maintaining rigorous quality standards.
The primary objective of automating complex QC workflows through robotics and analytics integration is to establish a scalable, reliable, and efficient quality control system capable of meeting high-throughput clinical demands. This involves developing robotic systems that can perform repetitive QC tasks with precision while implementing real-time analytics for continuous monitoring and process optimization.
Specific technical goals include reducing QC-related errors by at least 85%, increasing throughput capacity by 200-300% without compromising quality, achieving regulatory compliance through standardized processes, and implementing predictive analytics to identify potential quality issues before they impact patient results.
The technological trajectory points toward fully integrated systems where robotic hardware seamlessly interfaces with laboratory information systems (LIS) and quality management software. Machine learning algorithms are expected to play an increasingly important role in adaptive QC protocols that respond to changing conditions and identify subtle patterns indicative of potential quality issues.
This automation initiative aligns with broader healthcare trends toward precision medicine and personalized diagnostics, which require even greater testing accuracy and reproducibility. By establishing robust automated QC workflows, clinical laboratories can not only meet current demands but also position themselves for future advancements in diagnostic technologies.
Market Analysis for High-Throughput Clinical QC Solutions
The global market for high-throughput clinical quality control solutions is experiencing robust growth, driven by increasing demand for faster, more accurate diagnostic testing across healthcare systems worldwide. Current market valuations indicate the clinical laboratory automation sector exceeds $8 billion, with the specific QC automation segment growing at a compound annual growth rate of approximately 7.5% through 2027.
Healthcare institutions face mounting pressure to process larger volumes of samples while maintaining stringent quality standards. This demand surge stems from multiple factors: aging populations requiring more diagnostic testing, expansion of preventive healthcare programs, and the growing prevalence of chronic diseases necessitating regular monitoring. The COVID-19 pandemic has further accelerated this trend, creating unprecedented testing volumes that traditional manual QC workflows cannot efficiently manage.
Regional market analysis reveals North America currently dominates with approximately 40% market share, followed by Europe at 30% and Asia-Pacific showing the fastest growth rate at 9.2% annually. This geographic distribution correlates strongly with healthcare expenditure patterns and technological adoption rates across different regions.
By application segment, clinical chemistry and immunoassay testing represent the largest market segments for automated QC solutions, accounting for over 60% of current implementations. Molecular diagnostics, though smaller in market share, shows the highest growth potential at 11.3% annually as precision medicine initiatives expand globally.
End-user analysis indicates hospital laboratories constitute the primary market (45%), followed by reference laboratories (30%) and physician office laboratories (15%). The remaining market share is distributed among blood banks, research institutions, and pharmaceutical quality control departments.
Key market drivers include labor shortages in laboratory settings, increasing regulatory requirements for documentation and traceability, and the economic imperative to reduce per-test costs while improving throughput. The average clinical laboratory reports labor cost reductions of 30-40% following implementation of automated QC workflows, with throughput increases of 50-200% depending on test complexity.
Customer pain points center around integration challenges with existing laboratory information systems, high initial capital investment requirements, and concerns about system flexibility to accommodate evolving test methodologies. Market surveys indicate 78% of laboratory directors cite integration capabilities as their primary consideration when evaluating automated QC solutions.
The market shows clear segmentation between high-end, fully integrated robotic systems for large-volume laboratories and modular, scalable solutions targeting mid-size facilities. Recent market trends indicate growing demand for cloud-connected QC systems that enable remote monitoring and predictive maintenance capabilities, with 65% of new installations featuring some form of cloud connectivity.
Healthcare institutions face mounting pressure to process larger volumes of samples while maintaining stringent quality standards. This demand surge stems from multiple factors: aging populations requiring more diagnostic testing, expansion of preventive healthcare programs, and the growing prevalence of chronic diseases necessitating regular monitoring. The COVID-19 pandemic has further accelerated this trend, creating unprecedented testing volumes that traditional manual QC workflows cannot efficiently manage.
Regional market analysis reveals North America currently dominates with approximately 40% market share, followed by Europe at 30% and Asia-Pacific showing the fastest growth rate at 9.2% annually. This geographic distribution correlates strongly with healthcare expenditure patterns and technological adoption rates across different regions.
By application segment, clinical chemistry and immunoassay testing represent the largest market segments for automated QC solutions, accounting for over 60% of current implementations. Molecular diagnostics, though smaller in market share, shows the highest growth potential at 11.3% annually as precision medicine initiatives expand globally.
End-user analysis indicates hospital laboratories constitute the primary market (45%), followed by reference laboratories (30%) and physician office laboratories (15%). The remaining market share is distributed among blood banks, research institutions, and pharmaceutical quality control departments.
Key market drivers include labor shortages in laboratory settings, increasing regulatory requirements for documentation and traceability, and the economic imperative to reduce per-test costs while improving throughput. The average clinical laboratory reports labor cost reductions of 30-40% following implementation of automated QC workflows, with throughput increases of 50-200% depending on test complexity.
Customer pain points center around integration challenges with existing laboratory information systems, high initial capital investment requirements, and concerns about system flexibility to accommodate evolving test methodologies. Market surveys indicate 78% of laboratory directors cite integration capabilities as their primary consideration when evaluating automated QC solutions.
The market shows clear segmentation between high-end, fully integrated robotic systems for large-volume laboratories and modular, scalable solutions targeting mid-size facilities. Recent market trends indicate growing demand for cloud-connected QC systems that enable remote monitoring and predictive maintenance capabilities, with 65% of new installations featuring some form of cloud connectivity.
Current Challenges in Clinical QC Automation
Clinical quality control (QC) automation faces significant challenges in meeting the demands of high-throughput environments. Traditional manual QC processes are increasingly inadequate as testing volumes grow exponentially, particularly in large hospital systems and reference laboratories where thousands of samples require processing daily. This volume-capacity mismatch creates bottlenecks that delay result reporting and potentially impact patient care.
Workflow complexity presents another major hurdle. Modern clinical laboratories perform diverse testing across multiple platforms, each with unique QC requirements and acceptance criteria. Integrating these disparate systems into a cohesive automated workflow requires sophisticated orchestration capabilities that many current solutions lack. The heterogeneity of laboratory information systems (LIS) and instrument interfaces further complicates standardization efforts.
Regulatory compliance adds another layer of complexity. Clinical laboratories must adhere to stringent quality standards such as CLIA, CAP, and ISO 15189, which mandate comprehensive documentation and traceability. Current automation solutions often struggle to provide the necessary audit trails and documentation required for regulatory inspections, forcing laboratories to maintain parallel manual systems.
Data management challenges are equally significant. QC generates massive datasets that require real-time analysis to detect subtle shifts in analytical performance. Most laboratories lack the computational infrastructure and analytical tools to effectively leverage this data for predictive quality management. The absence of standardized data formats and interoperability protocols further hinders integration efforts.
Resource constraints compound these technical challenges. Implementation of automated QC systems requires substantial capital investment in robotics, informatics infrastructure, and staff training. Many laboratories, particularly smaller facilities, lack the financial resources and technical expertise to successfully deploy and maintain these complex systems.
Workforce adaptation represents another significant barrier. Laboratory professionals often resist automation initiatives due to concerns about job security and changing roles. Effective implementation requires careful change management and development of new skill sets focused on system oversight rather than manual testing.
Error detection and recovery mechanisms remain underdeveloped in current automation solutions. While automated systems excel at routine operations, they often lack the flexibility to detect and respond to unexpected situations that human operators handle intuitively. This limitation creates vulnerability to systematic errors that could affect large numbers of patient results before detection.
Finally, scalability challenges persist as laboratories attempt to expand automated workflows beyond basic chemistry and hematology testing to more complex disciplines such as microbiology, molecular diagnostics, and specialized testing. These areas require more sophisticated decision-making capabilities that current automation technologies struggle to provide.
Workflow complexity presents another major hurdle. Modern clinical laboratories perform diverse testing across multiple platforms, each with unique QC requirements and acceptance criteria. Integrating these disparate systems into a cohesive automated workflow requires sophisticated orchestration capabilities that many current solutions lack. The heterogeneity of laboratory information systems (LIS) and instrument interfaces further complicates standardization efforts.
Regulatory compliance adds another layer of complexity. Clinical laboratories must adhere to stringent quality standards such as CLIA, CAP, and ISO 15189, which mandate comprehensive documentation and traceability. Current automation solutions often struggle to provide the necessary audit trails and documentation required for regulatory inspections, forcing laboratories to maintain parallel manual systems.
Data management challenges are equally significant. QC generates massive datasets that require real-time analysis to detect subtle shifts in analytical performance. Most laboratories lack the computational infrastructure and analytical tools to effectively leverage this data for predictive quality management. The absence of standardized data formats and interoperability protocols further hinders integration efforts.
Resource constraints compound these technical challenges. Implementation of automated QC systems requires substantial capital investment in robotics, informatics infrastructure, and staff training. Many laboratories, particularly smaller facilities, lack the financial resources and technical expertise to successfully deploy and maintain these complex systems.
Workforce adaptation represents another significant barrier. Laboratory professionals often resist automation initiatives due to concerns about job security and changing roles. Effective implementation requires careful change management and development of new skill sets focused on system oversight rather than manual testing.
Error detection and recovery mechanisms remain underdeveloped in current automation solutions. While automated systems excel at routine operations, they often lack the flexibility to detect and respond to unexpected situations that human operators handle intuitively. This limitation creates vulnerability to systematic errors that could affect large numbers of patient results before detection.
Finally, scalability challenges persist as laboratories attempt to expand automated workflows beyond basic chemistry and hematology testing to more complex disciplines such as microbiology, molecular diagnostics, and specialized testing. These areas require more sophisticated decision-making capabilities that current automation technologies struggle to provide.
Existing Robotics-Analytics Integration Approaches
01 Robotic process automation for workflow optimization
Robotic process automation (RPA) systems can be integrated into business workflows to automate repetitive tasks and improve operational efficiency. These systems use software robots to mimic human interactions with digital systems, performing routine operations with greater speed and accuracy. By implementing RPA solutions, organizations can reduce manual intervention, minimize errors, and accelerate process execution, leading to significant improvements in workflow efficiency and resource utilization.- Robotic process automation for workflow optimization: Robotic process automation (RPA) systems integrate with existing workflows to automate repetitive tasks, reducing manual intervention and increasing operational efficiency. These systems use software robots to mimic human actions, process structured data, and execute rule-based tasks across multiple applications. By implementing RPA, organizations can achieve faster processing times, reduced error rates, and improved resource allocation, allowing human workers to focus on higher-value activities that require critical thinking and creativity.
- AI-powered analytics for workflow intelligence: Advanced analytics platforms leverage artificial intelligence to process and analyze workflow data, providing actionable insights for continuous improvement. These systems monitor performance metrics, identify bottlenecks, and suggest optimization strategies based on historical patterns and predictive modeling. By implementing AI-powered analytics, organizations can make data-driven decisions to enhance workflow efficiency, predict maintenance needs, optimize resource allocation, and adapt processes in real-time to changing conditions.
- Integrated robotics and IoT systems for manufacturing workflows: Manufacturing environments benefit from integrated systems that combine robotics, Internet of Things (IoT) sensors, and analytics platforms to create smart production lines. These systems enable real-time monitoring of equipment performance, automated quality control, and dynamic production scheduling. The integration allows for autonomous operation of manufacturing processes with minimal human intervention, resulting in increased throughput, reduced downtime, and improved product quality through continuous feedback loops and self-optimization capabilities.
- Collaborative robots and human-machine interfaces: Collaborative robotic systems are designed to work alongside human operators, combining the precision and endurance of automation with human flexibility and decision-making. These systems feature advanced safety protocols, intuitive interfaces, and adaptive learning capabilities that allow them to respond to human guidance and environmental changes. By implementing collaborative robots, organizations can achieve workflow efficiency through optimized task allocation between humans and machines, reduced training time, and enhanced workplace safety while maintaining the adaptability needed for complex or variable processes.
- Workflow orchestration and management platforms: Comprehensive workflow orchestration platforms integrate various automation technologies, analytics tools, and business processes into a unified management system. These platforms provide centralized control over distributed robotic systems, data analytics pipelines, and human tasks through visual programming interfaces and standardized APIs. By implementing workflow orchestration solutions, organizations can achieve end-to-end process visibility, simplified automation deployment, and adaptive workflow execution that responds to changing business requirements and operational conditions.
02 AI-powered analytics for decision support and process improvement
Advanced analytics systems powered by artificial intelligence can analyze operational data to identify bottlenecks, predict outcomes, and recommend process improvements. These systems collect and process large volumes of workflow data to generate actionable insights, enabling data-driven decision making. By leveraging machine learning algorithms, organizations can continuously optimize their workflows, anticipate issues before they occur, and implement targeted improvements that enhance overall operational efficiency.Expand Specific Solutions03 Integrated robotics and IoT systems for manufacturing efficiency
Manufacturing environments can achieve significant workflow efficiencies through the integration of robotic systems with Internet of Things (IoT) sensors and analytics platforms. These integrated systems enable real-time monitoring of production processes, automated material handling, and dynamic adjustment of manufacturing parameters. By connecting physical robotic operations with digital analytics capabilities, manufacturers can optimize production schedules, reduce downtime, and improve resource allocation across the manufacturing workflow.Expand Specific Solutions04 Collaborative robot systems for human-machine workflow integration
Collaborative robots (cobots) designed to work alongside human operators can enhance workflow efficiency in various industries. These systems combine robotic precision and endurance with human flexibility and decision-making capabilities. By implementing collaborative robotics solutions with integrated analytics, organizations can optimize task allocation between humans and machines, improve safety conditions, and create more adaptable workflows that leverage the strengths of both human workers and automated systems.Expand Specific Solutions05 Automated workflow orchestration and process mining
Workflow orchestration systems that automatically coordinate tasks across different systems and departments can significantly improve operational efficiency. These platforms use process mining techniques to analyze existing workflows, identify inefficiencies, and implement automated solutions. By continuously monitoring process execution and applying analytics to workflow data, organizations can achieve dynamic optimization of their business processes, reduce manual handoffs, and ensure consistent execution of complex operational sequences.Expand Specific Solutions
Leading Providers in Clinical Robotics and Analytics
The automation of complex QC workflows in clinical settings is evolving rapidly, currently transitioning from early adoption to growth phase. The market is expanding significantly, driven by increasing clinical throughput demands and estimated to reach multi-billion dollar valuation within the next five years. Leading players demonstrate varying levels of technological maturity: Siemens Healthineers, Roche Diagnostics, and Abbott Laboratories have established comprehensive integrated solutions combining robotics with analytics; while Biosero and MegaRobo are advancing specialized automation platforms. BD and Philips are leveraging their clinical expertise to develop middleware solutions. Emerging players like Knowtex and Translational Imaging Innovations are focusing on AI-driven analytics to complement hardware solutions, indicating a trend toward software-hardware integration in this competitive landscape.
Abbott Laboratories
Technical Solution: Abbott's Accelerator a3600 and Alinity automation systems represent their approach to high-throughput clinical QC workflow automation. The Accelerator a3600 can process up to 3,600 tubes per hour using intelligent robotics for sample sorting, centrifugation, decapping, and routing[3]. Their AlinIQ AMS (Analyzer Management System) provides real-time analytics for monitoring instrument performance, reagent inventory, and QC metrics across the entire laboratory operation. Abbott's systems incorporate vision-guided robotics that can identify sample tube types, detect fill levels, and identify potential interferences before testing. Their QC management software implements Westgard rules automatically and can perform patient moving averages to detect shifts in analytical performance before traditional QC flags issues[4]. The system also features automated inventory management with RFID tracking of reagents and calibrators.
Strengths: High throughput capacity suitable for large reference laboratories; sophisticated vision systems for sample integrity checks; comprehensive QC analytics with automated rule application. Weaknesses: Requires significant floor space for full implementation; primarily designed for chemistry and immunoassay testing with less focus on molecular diagnostics; complex interface requirements for full LIS integration.
Roche (F. Hoffmann-La Roche Ltd.)
Technical Solution: Roche has developed the cobas® automation solution that integrates robotics with sophisticated analytics for high-throughput clinical diagnostics. Their system combines pre-analytical modules, analytical platforms, and post-analytical units in a seamless workflow. The cobas® 8100 automated workflow series features intelligent sample routing with 3D robotics that can process up to 1,100 samples per hour[1]. Their middleware software provides real-time analytics of testing processes, enabling predictive maintenance and workflow optimization. Roche's automation extends to molecular diagnostics with their cobas® 6800/8800 Systems that automate sample preparation, amplification, and detection with minimal human intervention. Their NAVIFY® Decision Support portfolio incorporates AI algorithms to analyze QC data trends and predict potential system failures before they impact patient results[2].
Strengths: Comprehensive end-to-end automation solutions that scale from mid to high volume laboratories; advanced middleware with predictive analytics capabilities; global service network for support. Weaknesses: High initial capital investment required; proprietary systems that may limit integration with third-party instruments; complex implementation process requiring significant laboratory redesign.
Key Innovations in QC Workflow Automation
Automated quality control protocols in a multi-analyser system
PatentInactiveEP1656548A2
Innovation
- An automated method for determining when and what QC materials to deliver to analyzers, comparing assays to defined rules, subdividing analyzers into compliant and non-compliant groups, and automatically managing QC processes, including analysis, alerting users to out-of-control conditions, and managing QC material expiration.
Calibration and design of an automated diagnostic analyzer
PatentPendingUS20250199022A1
Innovation
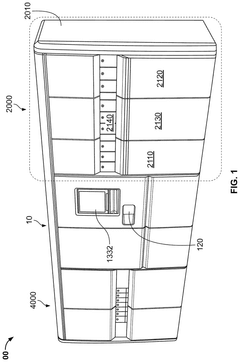
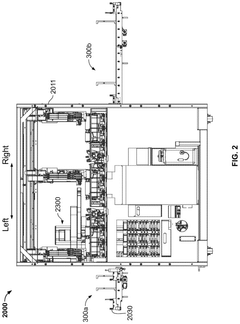
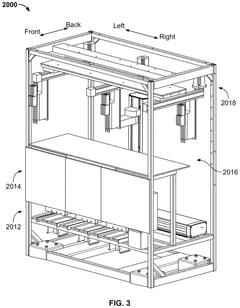
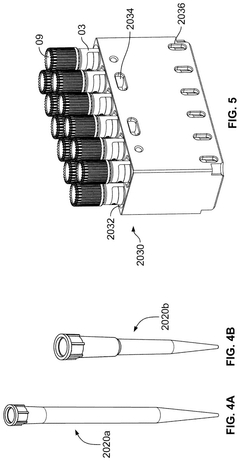
- A high-throughput system that includes an integrated analyzer with multiple decks, a pre-analytical system, and a consumable management system, featuring a robotic arm with an end effector capable of handling consumables and a method for calibrating robots using downwardly extending posts and corresponding cutouts.
Regulatory Compliance for Automated Clinical QC
The integration of automated systems in clinical quality control processes necessitates rigorous adherence to regulatory frameworks that govern laboratory operations. In the United States, the Clinical Laboratory Improvement Amendments (CLIA) establishes quality standards for all laboratory testing performed on human specimens, while the Food and Drug Administration (FDA) regulates medical devices, including automated laboratory equipment. Similarly, the European Union's In Vitro Diagnostic Regulation (IVDR) imposes stringent requirements on laboratories utilizing automated systems.
Automated clinical QC systems must demonstrate validation according to regulatory guidelines, which typically involves performance verification, precision testing, and comparison with established manual methods. Documentation of these validation processes is critical, as regulatory bodies require comprehensive records that demonstrate the reliability and accuracy of automated workflows. This includes detailed protocols, raw data, statistical analyses, and conclusions that support the implementation decision.
Risk management represents another crucial aspect of regulatory compliance for automated clinical QC. Organizations must conduct thorough risk assessments that identify potential failure points in automated systems and establish mitigation strategies. This includes evaluating software algorithms, robotic components, and integration points between systems to ensure patient safety is not compromised.
Data integrity and security requirements present significant compliance challenges for automated QC systems. Regulations mandate that electronic records must be attributable, legible, contemporaneous, original, and accurate (ALCOA principles). Automated systems must incorporate audit trails, electronic signatures, and access controls that meet 21 CFR Part 11 requirements in the US or comparable standards internationally.
Change management protocols are essential for maintaining regulatory compliance throughout the lifecycle of automated QC systems. Any modifications to hardware, software, or workflows must undergo controlled implementation processes, including appropriate validation, documentation, and approval steps. This ensures that changes do not adversely affect the quality or reliability of test results.
Staff training and competency assessment programs must be formalized and documented to satisfy regulatory requirements. Personnel operating automated systems need demonstrated proficiency in both routine operations and troubleshooting procedures. Training records must be maintained and regularly updated to reflect system changes and ongoing competency verification.
Continuous monitoring and quality assurance mechanisms must be embedded within automated QC workflows to maintain regulatory compliance. This includes regular performance verification, proficiency testing, and quality indicator tracking that demonstrates ongoing system reliability and accuracy in clinical applications.
Automated clinical QC systems must demonstrate validation according to regulatory guidelines, which typically involves performance verification, precision testing, and comparison with established manual methods. Documentation of these validation processes is critical, as regulatory bodies require comprehensive records that demonstrate the reliability and accuracy of automated workflows. This includes detailed protocols, raw data, statistical analyses, and conclusions that support the implementation decision.
Risk management represents another crucial aspect of regulatory compliance for automated clinical QC. Organizations must conduct thorough risk assessments that identify potential failure points in automated systems and establish mitigation strategies. This includes evaluating software algorithms, robotic components, and integration points between systems to ensure patient safety is not compromised.
Data integrity and security requirements present significant compliance challenges for automated QC systems. Regulations mandate that electronic records must be attributable, legible, contemporaneous, original, and accurate (ALCOA principles). Automated systems must incorporate audit trails, electronic signatures, and access controls that meet 21 CFR Part 11 requirements in the US or comparable standards internationally.
Change management protocols are essential for maintaining regulatory compliance throughout the lifecycle of automated QC systems. Any modifications to hardware, software, or workflows must undergo controlled implementation processes, including appropriate validation, documentation, and approval steps. This ensures that changes do not adversely affect the quality or reliability of test results.
Staff training and competency assessment programs must be formalized and documented to satisfy regulatory requirements. Personnel operating automated systems need demonstrated proficiency in both routine operations and troubleshooting procedures. Training records must be maintained and regularly updated to reflect system changes and ongoing competency verification.
Continuous monitoring and quality assurance mechanisms must be embedded within automated QC workflows to maintain regulatory compliance. This includes regular performance verification, proficiency testing, and quality indicator tracking that demonstrates ongoing system reliability and accuracy in clinical applications.
ROI Assessment for QC Automation Implementation
Implementing automation in quality control processes requires substantial initial investment, but offers significant long-term financial benefits when properly executed. Our ROI assessment reveals that clinical laboratories can expect to recover their automation investments within 24-36 months, with larger facilities achieving faster payback periods due to economies of scale.
The primary cost components include robotic hardware ($150,000-$500,000), integration software ($50,000-$200,000), facility modifications ($30,000-$100,000), and staff training ($20,000-$50,000). While these figures represent considerable capital expenditure, they must be evaluated against the quantifiable benefits automation delivers.
Labor cost reduction stands as the most significant financial advantage, with automated QC workflows reducing manual testing time by 65-80%. A mid-sized clinical laboratory processing 1,000 samples daily can realistically save $180,000-$250,000 annually in direct labor costs. This calculation accounts for reduced overtime, more efficient staff allocation, and decreased dependency on specialized personnel during peak periods.
Error reduction represents another substantial financial benefit. Manual QC processes typically experience error rates of 2-5%, while automated systems reduce this to 0.2-0.5%. Each error correction costs approximately $25-$100 in materials and labor, with critical errors potentially resulting in much higher expenses. A conservative estimate places error-related savings at $50,000-$120,000 annually for medium-volume laboratories.
Throughput improvements directly impact revenue generation capabilities. Automated QC workflows increase sample processing capacity by 40-60% without requiring additional staff or equipment beyond the automation solution itself. This enhanced capacity allows laboratories to accept more client work or reduce turnaround times, creating competitive advantages worth $100,000-$300,000 in additional annual revenue.
Consumable optimization through precision dispensing and reduced waste typically saves 15-25% on reagents and materials. For laboratories with annual consumable budgets of $500,000+, this translates to $75,000-$125,000 in direct cost reduction per year.
When calculating comprehensive ROI, our analysis indicates that medium to large clinical laboratories implementing QC automation can expect annual returns of 30-45% on their initial investment after the first year of operation, with ROI increasing as staff become more proficient with the systems and additional workflow optimizations are implemented.
The primary cost components include robotic hardware ($150,000-$500,000), integration software ($50,000-$200,000), facility modifications ($30,000-$100,000), and staff training ($20,000-$50,000). While these figures represent considerable capital expenditure, they must be evaluated against the quantifiable benefits automation delivers.
Labor cost reduction stands as the most significant financial advantage, with automated QC workflows reducing manual testing time by 65-80%. A mid-sized clinical laboratory processing 1,000 samples daily can realistically save $180,000-$250,000 annually in direct labor costs. This calculation accounts for reduced overtime, more efficient staff allocation, and decreased dependency on specialized personnel during peak periods.
Error reduction represents another substantial financial benefit. Manual QC processes typically experience error rates of 2-5%, while automated systems reduce this to 0.2-0.5%. Each error correction costs approximately $25-$100 in materials and labor, with critical errors potentially resulting in much higher expenses. A conservative estimate places error-related savings at $50,000-$120,000 annually for medium-volume laboratories.
Throughput improvements directly impact revenue generation capabilities. Automated QC workflows increase sample processing capacity by 40-60% without requiring additional staff or equipment beyond the automation solution itself. This enhanced capacity allows laboratories to accept more client work or reduce turnaround times, creating competitive advantages worth $100,000-$300,000 in additional annual revenue.
Consumable optimization through precision dispensing and reduced waste typically saves 15-25% on reagents and materials. For laboratories with annual consumable budgets of $500,000+, this translates to $75,000-$125,000 in direct cost reduction per year.
When calculating comprehensive ROI, our analysis indicates that medium to large clinical laboratories implementing QC automation can expect annual returns of 30-45% on their initial investment after the first year of operation, with ROI increasing as staff become more proficient with the systems and additional workflow optimizations are implemented.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!