Methods for high-throughput analytics of vector genome integrity and truncated genomes
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Vector Genome Integrity Analysis Background & Objectives
Vector genome integrity analysis has emerged as a critical component in the development and quality control of gene therapy products, particularly those utilizing viral vectors such as adeno-associated virus (AAV) and lentivirus. The field has evolved significantly over the past two decades, transitioning from basic molecular biology techniques to sophisticated high-throughput analytical methods capable of detecting subtle genomic alterations.
The evolution of vector genome integrity analysis parallels the advancement of gene therapy itself, with early methods focusing primarily on basic confirmation of transgene presence. As clinical applications expanded, so did the need for more precise characterization of vector genomes, driving innovation in analytical techniques. Recent technological breakthroughs in sequencing technologies, particularly next-generation sequencing (NGS) and digital PCR, have revolutionized our ability to assess vector genome integrity with unprecedented resolution.
Current trends indicate a shift toward comprehensive characterization approaches that can simultaneously evaluate multiple aspects of vector quality, including genome integrity, capsid composition, and functional potency. The integration of computational biology and machine learning algorithms is increasingly enabling automated analysis of complex genomic data, facilitating faster and more accurate quality assessments.
The primary objective of high-throughput vector genome integrity analysis is to ensure the safety and efficacy of gene therapy products by identifying and quantifying truncated, fragmented, or otherwise modified vector genomes that may impact therapeutic outcomes. Specific goals include developing standardized methods for detecting vector genome truncations, establishing threshold criteria for acceptable levels of genomic alterations, and creating robust analytical workflows suitable for regulatory submission.
Additional objectives encompass the development of predictive models to correlate vector genome integrity with therapeutic efficacy, implementation of real-time monitoring systems for manufacturing processes, and creation of reference standards to facilitate inter-laboratory comparisons. These advancements aim to address the growing demand for gene therapy products while ensuring consistent quality and safety profiles.
The technical landscape is further complicated by the diversity of vector systems and therapeutic applications, necessitating tailored analytical approaches for different vector types. As the field progresses, there is increasing recognition of the need for harmonized methodologies that can be applied across different vector platforms while maintaining sensitivity to vector-specific characteristics.
The evolution of vector genome integrity analysis parallels the advancement of gene therapy itself, with early methods focusing primarily on basic confirmation of transgene presence. As clinical applications expanded, so did the need for more precise characterization of vector genomes, driving innovation in analytical techniques. Recent technological breakthroughs in sequencing technologies, particularly next-generation sequencing (NGS) and digital PCR, have revolutionized our ability to assess vector genome integrity with unprecedented resolution.
Current trends indicate a shift toward comprehensive characterization approaches that can simultaneously evaluate multiple aspects of vector quality, including genome integrity, capsid composition, and functional potency. The integration of computational biology and machine learning algorithms is increasingly enabling automated analysis of complex genomic data, facilitating faster and more accurate quality assessments.
The primary objective of high-throughput vector genome integrity analysis is to ensure the safety and efficacy of gene therapy products by identifying and quantifying truncated, fragmented, or otherwise modified vector genomes that may impact therapeutic outcomes. Specific goals include developing standardized methods for detecting vector genome truncations, establishing threshold criteria for acceptable levels of genomic alterations, and creating robust analytical workflows suitable for regulatory submission.
Additional objectives encompass the development of predictive models to correlate vector genome integrity with therapeutic efficacy, implementation of real-time monitoring systems for manufacturing processes, and creation of reference standards to facilitate inter-laboratory comparisons. These advancements aim to address the growing demand for gene therapy products while ensuring consistent quality and safety profiles.
The technical landscape is further complicated by the diversity of vector systems and therapeutic applications, necessitating tailored analytical approaches for different vector types. As the field progresses, there is increasing recognition of the need for harmonized methodologies that can be applied across different vector platforms while maintaining sensitivity to vector-specific characteristics.
Market Demand for High-Throughput Vector Analytics
The global market for high-throughput vector genome integrity analytics is experiencing significant growth, driven by the expanding applications of gene therapy and viral vector-based treatments. The demand for advanced analytical methods has surged as biopharmaceutical companies increasingly rely on viral vectors for delivering therapeutic genetic material. Current market estimates value the viral vector manufacturing market at over $2 billion, with a compound annual growth rate exceeding 20% through 2027.
This robust market growth directly correlates with the need for sophisticated analytical tools to ensure vector genome integrity. Truncated genomes and other quality issues can significantly impact therapeutic efficacy and safety, creating substantial demand for high-throughput analytical solutions that can provide comprehensive quality assessment during manufacturing processes.
Regulatory agencies worldwide, including the FDA and EMA, have established increasingly stringent requirements for characterizing viral vector products. These regulatory pressures have intensified market demand for analytical methods that can reliably detect and quantify truncated genomes and other vector integrity issues. Companies developing such technologies stand to capture significant market share in this rapidly evolving landscape.
The biopharmaceutical industry has demonstrated willingness to invest substantially in technologies that improve manufacturing efficiency and product quality. A recent industry survey revealed that over 70% of gene therapy developers consider vector genome integrity analysis a critical quality attribute, with many expressing dissatisfaction with current analytical limitations regarding throughput, sensitivity, and reproducibility.
Contract development and manufacturing organizations (CDMOs) represent another significant market segment driving demand for high-throughput vector analytics. As these organizations handle increasing volumes of viral vector production for multiple clients, the need for rapid, reliable, and scalable analytical methods becomes paramount to their operational efficiency and competitive advantage.
Academic research institutions and core facilities also contribute to market demand, particularly for technologies that can be adapted to analyze novel vector designs and delivery systems. The research segment values analytical flexibility and cost-effectiveness, creating opportunities for modular or adaptable analytical platforms.
Geographically, North America currently dominates the market for vector analytics, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is projected to experience the fastest growth rate in the coming years, driven by increasing investments in advanced therapies and biotechnology infrastructure in countries like China, Japan, and South Korea.
This robust market growth directly correlates with the need for sophisticated analytical tools to ensure vector genome integrity. Truncated genomes and other quality issues can significantly impact therapeutic efficacy and safety, creating substantial demand for high-throughput analytical solutions that can provide comprehensive quality assessment during manufacturing processes.
Regulatory agencies worldwide, including the FDA and EMA, have established increasingly stringent requirements for characterizing viral vector products. These regulatory pressures have intensified market demand for analytical methods that can reliably detect and quantify truncated genomes and other vector integrity issues. Companies developing such technologies stand to capture significant market share in this rapidly evolving landscape.
The biopharmaceutical industry has demonstrated willingness to invest substantially in technologies that improve manufacturing efficiency and product quality. A recent industry survey revealed that over 70% of gene therapy developers consider vector genome integrity analysis a critical quality attribute, with many expressing dissatisfaction with current analytical limitations regarding throughput, sensitivity, and reproducibility.
Contract development and manufacturing organizations (CDMOs) represent another significant market segment driving demand for high-throughput vector analytics. As these organizations handle increasing volumes of viral vector production for multiple clients, the need for rapid, reliable, and scalable analytical methods becomes paramount to their operational efficiency and competitive advantage.
Academic research institutions and core facilities also contribute to market demand, particularly for technologies that can be adapted to analyze novel vector designs and delivery systems. The research segment values analytical flexibility and cost-effectiveness, creating opportunities for modular or adaptable analytical platforms.
Geographically, North America currently dominates the market for vector analytics, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is projected to experience the fastest growth rate in the coming years, driven by increasing investments in advanced therapies and biotechnology infrastructure in countries like China, Japan, and South Korea.
Technical Challenges in Vector Genome Integrity Assessment
The assessment of vector genome integrity presents significant technical challenges that researchers and industry professionals must overcome to ensure the efficacy and safety of gene therapy products. Current methodologies for detecting truncated genomes and evaluating vector integrity face several critical limitations that impede high-throughput analysis capabilities.
One fundamental challenge is the detection sensitivity threshold of existing analytical methods. Conventional techniques such as qPCR and ddPCR can identify major truncation events but often fail to detect low-abundance truncated species that may still have biological significance. This sensitivity gap creates potential blind spots in quality control processes, particularly when dealing with complex vector designs or large genomic inserts.
Resolution limitations represent another significant hurdle. While next-generation sequencing offers comprehensive coverage, the accurate assembly and mapping of repetitive regions, inverted terminal repeats (ITRs), and GC-rich sequences remain problematic. These structural elements, common in viral vectors like AAV and lentivirus, create technical artifacts that complicate bioinformatic analysis and may lead to false positives or negatives in integrity assessment.
Throughput constraints severely limit industrial applications, as most high-resolution techniques require extensive sample preparation, specialized equipment, and time-consuming protocols. This creates a bottleneck in manufacturing workflows, where rapid assessment of multiple production batches is essential for maintaining production schedules and reducing time-to-market for therapeutic products.
Reference standard availability poses another challenge. The field lacks universally accepted reference materials with well-characterized truncation profiles that could enable cross-laboratory standardization and method validation. This absence hampers efforts to establish regulatory-compliant analytical procedures and complicates comparisons between different vector production platforms.
Data interpretation complexities further compound these challenges. The biological significance of different truncation patterns remains poorly understood, making it difficult to establish meaningful acceptance criteria for vector genome integrity. Researchers must determine which truncation events impact vector performance and which represent benign variations with no functional consequences.
Automation integration represents a technical frontier that remains underdeveloped. While high-throughput screening platforms exist in adjacent fields, their adaptation to vector genome integrity assessment requires specialized modifications and validation. The integration of robotics, microfluidics, and advanced detection systems presents both opportunities and technical hurdles that must be addressed to achieve truly scalable analytical solutions.
One fundamental challenge is the detection sensitivity threshold of existing analytical methods. Conventional techniques such as qPCR and ddPCR can identify major truncation events but often fail to detect low-abundance truncated species that may still have biological significance. This sensitivity gap creates potential blind spots in quality control processes, particularly when dealing with complex vector designs or large genomic inserts.
Resolution limitations represent another significant hurdle. While next-generation sequencing offers comprehensive coverage, the accurate assembly and mapping of repetitive regions, inverted terminal repeats (ITRs), and GC-rich sequences remain problematic. These structural elements, common in viral vectors like AAV and lentivirus, create technical artifacts that complicate bioinformatic analysis and may lead to false positives or negatives in integrity assessment.
Throughput constraints severely limit industrial applications, as most high-resolution techniques require extensive sample preparation, specialized equipment, and time-consuming protocols. This creates a bottleneck in manufacturing workflows, where rapid assessment of multiple production batches is essential for maintaining production schedules and reducing time-to-market for therapeutic products.
Reference standard availability poses another challenge. The field lacks universally accepted reference materials with well-characterized truncation profiles that could enable cross-laboratory standardization and method validation. This absence hampers efforts to establish regulatory-compliant analytical procedures and complicates comparisons between different vector production platforms.
Data interpretation complexities further compound these challenges. The biological significance of different truncation patterns remains poorly understood, making it difficult to establish meaningful acceptance criteria for vector genome integrity. Researchers must determine which truncation events impact vector performance and which represent benign variations with no functional consequences.
Automation integration represents a technical frontier that remains underdeveloped. While high-throughput screening platforms exist in adjacent fields, their adaptation to vector genome integrity assessment requires specialized modifications and validation. The integration of robotics, microfluidics, and advanced detection systems presents both opportunities and technical hurdles that must be addressed to achieve truly scalable analytical solutions.
Current High-Throughput Vector Integrity Methods
01 Methods for detecting vector genome integrity and truncation
Various analytical methods can be employed to assess the integrity of vector genomes and detect truncations. These methods include molecular techniques such as PCR, sequencing, and gel electrophoresis that can identify missing segments or structural abnormalities in the vector genome. Advanced algorithms can process the resulting data to determine if the vector genome is complete or if truncations have occurred, which is crucial for quality control in gene therapy applications.- Methods for detecting vector genome integrity and truncation: Various analytical methods are employed to assess the integrity of vector genomes and detect truncations. These techniques include PCR-based assays, next-generation sequencing, and specialized algorithms that can identify structural variations in genomic sequences. These methods enable researchers to verify the completeness of vector genomes and detect any truncations that might affect functionality, which is particularly important in gene therapy applications.
- Computational approaches for vector genome analysis: Advanced computational tools and algorithms are developed specifically for analyzing vector genomes. These include machine learning models, pattern recognition systems, and specialized software that can process large genomic datasets to identify integrity issues and truncation events. These computational approaches enable high-throughput screening and provide detailed insights into the structural characteristics of vector genomes.
- Quality control systems for vector genome production: Comprehensive quality control systems are implemented during vector genome production to ensure integrity and minimize truncation. These systems incorporate real-time monitoring, automated detection mechanisms, and standardized protocols for assessing vector quality. By implementing robust quality control measures, manufacturers can identify and address integrity issues early in the production process.
- Imaging and visualization techniques for vector genome analysis: Advanced imaging and visualization techniques are utilized to analyze vector genome integrity. These include electron microscopy, fluorescence-based methods, and digital imaging systems that can visualize structural features of vector genomes. These techniques provide spatial information about genome organization and can directly visualize truncation events or structural abnormalities.
- Security and encryption methods for vector genome data: Security protocols and encryption methods are implemented to protect vector genome data during analysis and storage. These include blockchain-based verification systems, secure data transmission protocols, and encrypted storage solutions that maintain data integrity. These security measures ensure that sensitive genomic data remains protected while still allowing for comprehensive analysis of vector integrity and truncation.
02 Computational approaches for vector genome analysis
Computational tools and algorithms play a significant role in analyzing vector genome integrity. These approaches include machine learning models, statistical methods, and specialized software that can process large genomic datasets to identify patterns indicative of truncations or other integrity issues. These computational methods enable high-throughput screening and can detect subtle anomalies that might be missed by conventional analytical techniques.Expand Specific Solutions03 Real-time monitoring systems for vector genome integrity
Real-time monitoring systems have been developed to continuously assess vector genome integrity during production and storage. These systems utilize sensors, imaging technologies, and data analytics to provide immediate feedback on the status of vector genomes. By enabling early detection of truncations or other integrity issues, these systems help maintain quality control and ensure the efficacy of vector-based therapies.Expand Specific Solutions04 Integration of multiple analytical techniques for comprehensive integrity assessment
Comprehensive assessment of vector genome integrity often requires the integration of multiple analytical techniques. By combining methods such as next-generation sequencing, mass spectrometry, and bioinformatics analysis, researchers can obtain a more complete picture of vector genome structure and identify potential truncations with greater accuracy. This integrated approach enhances the reliability of integrity assessments and improves quality control in vector production.Expand Specific Solutions05 Novel biomarkers and indicators for vector genome truncation
Researchers have identified novel biomarkers and indicators that can signal the presence of vector genome truncations. These include specific molecular signatures, changes in expression patterns, and structural features that correlate with genome integrity issues. By monitoring these biomarkers, scientists can quickly assess the quality of vector preparations and identify batches that may contain truncated genomes, thereby improving the safety and efficacy of gene therapy products.Expand Specific Solutions
Leading Organizations in Vector Genome Analytics
The field of high-throughput analytics for vector genome integrity is currently in its growth phase, with an estimated market size exceeding $2 billion and expanding at 15-20% annually. The competitive landscape features established research institutions (MIT, Harvard, Whitehead Institute) driving fundamental innovations alongside specialized biotech companies (Complete Genomics, Opentrons) commercializing applications. Major pharmaceutical players (Merck, Janssen Biotech) are increasingly investing in this space, recognizing its importance for gene therapy development. Technical maturity varies significantly: sequencing-based methods are well-established, while real-time monitoring technologies remain emergent. Companies like KeyGene and Bio-Rad are advancing microfluidic and high-throughput screening platforms, while academic-industry partnerships between institutions like CNRS and commercial entities are accelerating translation of research breakthroughs into practical applications for clinical and research settings.
Complete Genomics, Inc.
Technical Solution: Complete Genomics has developed a proprietary DNA nanoball sequencing platform specifically optimized for high-throughput vector genome integrity analysis. Their technology employs combinatorial probe-anchor synthesis (cPAS) that enables massively parallel sequencing of DNA nanoballs with high accuracy and reduced error rates. For vector genome integrity assessment, they've implemented a computational pipeline that identifies structural variations, truncations, and rearrangements in viral vector genomes at single-nucleotide resolution. The platform incorporates machine learning algorithms to detect subtle anomalies in sequencing data that might indicate compromised vector integrity, allowing for quality control of viral vectors used in gene therapy applications.
Strengths: Exceptional throughput capacity (millions of reads per run) with high accuracy for detecting truncated genomes. Their integrated computational approach reduces false positives in structural variant detection. Weaknesses: The technology requires specialized equipment and expertise, making it less accessible to smaller research facilities. Higher cost compared to some competing technologies.
Massachusetts Institute of Technology
Technical Solution: MIT has pioneered a CRISPR-based diagnostic platform for high-throughput vector genome integrity analysis. Their system combines CRISPR-Cas12a or Cas13 enzymes with reporter molecules to detect specific sequences or structural abnormalities in vector genomes. This approach enables rapid, sensitive detection of truncated genomes without requiring extensive sequencing. MIT researchers have further enhanced this technology by developing multiplexed assays that can simultaneously evaluate multiple critical regions of vector genomes, significantly increasing throughput. The platform incorporates microfluidic devices for sample processing and automated analysis, allowing for assessment of thousands of samples per day with minimal human intervention.
Strengths: Rapid turnaround time (hours versus days for sequencing-based methods) and high sensitivity for detecting low-abundance truncated genomes. Scalable and adaptable to different vector types. Weaknesses: May miss certain complex structural rearrangements that don't affect the targeted sequences. Requires careful design of guide RNAs specific to each vector type.
Key Innovations in Truncated Genome Detection
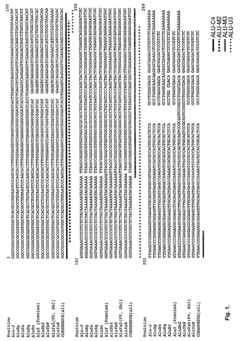
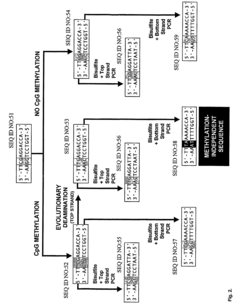
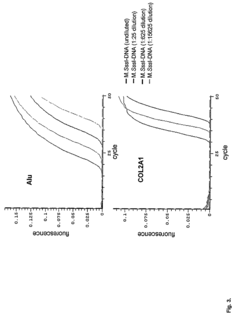
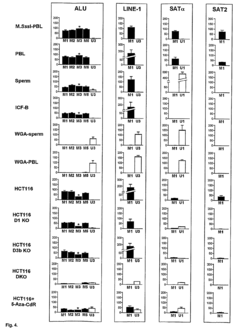
High throughput methods comprising analysis of repetitive element DNA methylation
PatentInactiveUS8642263B2
Innovation
- Development of novel MethyLight™ assays using Alu repeat sequences depleted of CpG dinucleotides by evolutionary deamination, allowing for discrimination between evolutionary and bisulfite-based deamination, and application in bisulfite-specific real-time PCR reactions for detecting small DNA amounts, with surrogate markers for estimating global DNA methylation levels.
One vector system for identification of genome modifying enzymes
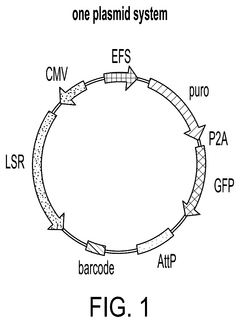
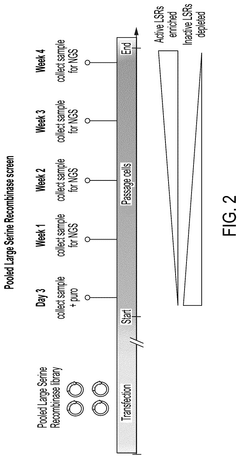
PatentPendingUS20250215423A1
Innovation
- An integrated one-vector system that combines the enzyme polypeptide and its specific target site sequence within a single vector, along with a unique identifier, facilitating high-throughput screening and minimizing transfection variations.
Regulatory Considerations for Vector Genome Quality Control
Regulatory frameworks for vector genome quality control are evolving rapidly as gene therapy applications advance toward commercialization. The FDA, EMA, and other global regulatory bodies have established increasingly stringent requirements for characterizing vector genome integrity, with particular emphasis on identifying and quantifying truncated genomes. These truncated vectors represent a significant quality concern as they may deliver incomplete therapeutic sequences while consuming manufacturing capacity and potentially triggering immune responses without therapeutic benefit.
Current regulatory guidelines require sponsors to develop and validate analytical methods that can accurately assess vector genome integrity throughout the manufacturing process. The FDA's guidance for human gene therapy products specifically addresses the need for comprehensive characterization of vector-related impurities, including truncated genomes. Similarly, the EMA's Committee for Advanced Therapies has emphasized the importance of establishing appropriate specifications for genome integrity in viral vectors.
Regulatory agencies increasingly expect manufacturers to implement orthogonal analytical approaches that combine traditional methods with newer high-throughput techniques. This multi-method strategy provides more comprehensive characterization of vector populations and increases confidence in quality control results. Sponsors must demonstrate method validation according to ICH guidelines, including specificity, accuracy, precision, linearity, and robustness parameters specifically adapted to vector genome analysis.
Risk-based approaches to setting specifications for truncated genomes are becoming the regulatory standard. Manufacturers must establish scientifically justified acceptance criteria based on clinical experience, manufacturing consistency, and non-clinical safety studies. The acceptable threshold for truncated genomes varies depending on vector type, therapeutic indication, and administration route, with more stringent requirements typically applied to CNS-directed therapies.
Regulatory submissions must include detailed information on analytical procedures used to assess genome integrity, including method development history, validation data, and justification for specification limits. Comparability studies are required when analytical methods are changed or improved during development, demonstrating that new techniques provide equivalent or superior characterization of vector quality attributes.
Global harmonization efforts are underway to standardize regulatory expectations for vector genome quality control. The International Council for Harmonisation (ICH) has initiated discussions on developing specific guidelines for gene therapy products, while industry consortia are working to establish reference standards and best practices for genome integrity analysis. These collaborative efforts aim to create more consistent regulatory frameworks across different regions while accommodating the rapid technological advances in analytical capabilities.
Current regulatory guidelines require sponsors to develop and validate analytical methods that can accurately assess vector genome integrity throughout the manufacturing process. The FDA's guidance for human gene therapy products specifically addresses the need for comprehensive characterization of vector-related impurities, including truncated genomes. Similarly, the EMA's Committee for Advanced Therapies has emphasized the importance of establishing appropriate specifications for genome integrity in viral vectors.
Regulatory agencies increasingly expect manufacturers to implement orthogonal analytical approaches that combine traditional methods with newer high-throughput techniques. This multi-method strategy provides more comprehensive characterization of vector populations and increases confidence in quality control results. Sponsors must demonstrate method validation according to ICH guidelines, including specificity, accuracy, precision, linearity, and robustness parameters specifically adapted to vector genome analysis.
Risk-based approaches to setting specifications for truncated genomes are becoming the regulatory standard. Manufacturers must establish scientifically justified acceptance criteria based on clinical experience, manufacturing consistency, and non-clinical safety studies. The acceptable threshold for truncated genomes varies depending on vector type, therapeutic indication, and administration route, with more stringent requirements typically applied to CNS-directed therapies.
Regulatory submissions must include detailed information on analytical procedures used to assess genome integrity, including method development history, validation data, and justification for specification limits. Comparability studies are required when analytical methods are changed or improved during development, demonstrating that new techniques provide equivalent or superior characterization of vector quality attributes.
Global harmonization efforts are underway to standardize regulatory expectations for vector genome quality control. The International Council for Harmonisation (ICH) has initiated discussions on developing specific guidelines for gene therapy products, while industry consortia are working to establish reference standards and best practices for genome integrity analysis. These collaborative efforts aim to create more consistent regulatory frameworks across different regions while accommodating the rapid technological advances in analytical capabilities.
Data Management Solutions for High-Throughput Genomic Analysis
The exponential growth of genomic data from high-throughput sequencing technologies has created unprecedented challenges in data management. Traditional database systems struggle to efficiently handle the volume, velocity, and variety of genomic data generated during vector genome integrity analysis and truncated genome detection. Modern data management solutions must address these challenges while ensuring data accessibility, integrity, and security.
Cloud-based storage solutions have emerged as a primary approach for managing high-throughput genomic data. Platforms such as Amazon Web Services Genomics, Google Cloud Life Sciences, and Microsoft Azure Genomics offer scalable infrastructure specifically designed for genomic workloads. These solutions provide elastic storage capabilities that can expand or contract based on project requirements, eliminating the need for substantial upfront hardware investments while supporting the massive data volumes generated during vector genome integrity analysis.
Distributed database systems represent another critical component in managing high-throughput genomic data. Technologies like Apache Hadoop, Apache Spark, and specialized genomic databases such as GenomicsDB enable parallel processing across multiple nodes, significantly reducing computation time for complex integrity analyses. These systems implement partitioning strategies that distribute genomic data across multiple servers, allowing researchers to perform concurrent analyses on different segments of vector genomes.
Data compression algorithms specifically optimized for genomic data have become essential in high-throughput analytics workflows. Solutions like CRAM, Genozip, and SPRING can achieve compression ratios of up to 5:1 compared to standard formats, substantially reducing storage requirements while maintaining rapid access to critical integrity metrics. These specialized compression techniques recognize patterns unique to genomic sequences, enabling more efficient storage without compromising analytical capabilities.
Automated data lifecycle management systems have been developed to address the long-term storage challenges of genomic data. These systems implement tiered storage architectures that automatically migrate less frequently accessed data to lower-cost storage options while maintaining immediate availability of active datasets. Such approaches are particularly valuable for longitudinal studies of vector genome integrity, where historical data remains valuable for comparative analyses but may not require constant high-speed access.
Integration frameworks that connect disparate genomic data sources represent the final piece of comprehensive data management solutions. Systems like GA4GH's Data Connect, DNAstack, and Seven Bridges enable researchers to seamlessly access and analyze vector genome integrity data across multiple repositories without manual data transfers. These frameworks implement standardized APIs and data models that facilitate interoperability between different analytical platforms and data sources.
Cloud-based storage solutions have emerged as a primary approach for managing high-throughput genomic data. Platforms such as Amazon Web Services Genomics, Google Cloud Life Sciences, and Microsoft Azure Genomics offer scalable infrastructure specifically designed for genomic workloads. These solutions provide elastic storage capabilities that can expand or contract based on project requirements, eliminating the need for substantial upfront hardware investments while supporting the massive data volumes generated during vector genome integrity analysis.
Distributed database systems represent another critical component in managing high-throughput genomic data. Technologies like Apache Hadoop, Apache Spark, and specialized genomic databases such as GenomicsDB enable parallel processing across multiple nodes, significantly reducing computation time for complex integrity analyses. These systems implement partitioning strategies that distribute genomic data across multiple servers, allowing researchers to perform concurrent analyses on different segments of vector genomes.
Data compression algorithms specifically optimized for genomic data have become essential in high-throughput analytics workflows. Solutions like CRAM, Genozip, and SPRING can achieve compression ratios of up to 5:1 compared to standard formats, substantially reducing storage requirements while maintaining rapid access to critical integrity metrics. These specialized compression techniques recognize patterns unique to genomic sequences, enabling more efficient storage without compromising analytical capabilities.
Automated data lifecycle management systems have been developed to address the long-term storage challenges of genomic data. These systems implement tiered storage architectures that automatically migrate less frequently accessed data to lower-cost storage options while maintaining immediate availability of active datasets. Such approaches are particularly valuable for longitudinal studies of vector genome integrity, where historical data remains valuable for comparative analyses but may not require constant high-speed access.
Integration frameworks that connect disparate genomic data sources represent the final piece of comprehensive data management solutions. Systems like GA4GH's Data Connect, DNAstack, and Seven Bridges enable researchers to seamlessly access and analyze vector genome integrity data across multiple repositories without manual data transfers. These frameworks implement standardized APIs and data models that facilitate interoperability between different analytical platforms and data sources.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!